New faunistic data on oribatid mites (Acari, Oribatida) from western, southern and southeastern Ethiopia, with description of two new species of the family Carabodidae
Ermilov, Sergey G.  1
and Rybalov, Leonid B.
1
and Rybalov, Leonid B.  2
2
1✉ University of Tyumen State University, Institute of Environmental and Agricultural Biology (X-BIO), Tyumen, Russia.
2Institute of Ecology and Evolution, Russian Academy of Sciences, Laboratory of Soil Zoology and General Entomology, Moscow, Russia.
2025 - Volume: 65 Issue: 2 pages: 559-574
https://doi.org/10.24349/ikjq-9q2dZooBank LSID: BD53878B-EA2E-4B80-9806-CCB67A658060
Original research
Keywords
Abstract
Introduction
Oribatid mites (Acari, Oribatida) of Ethiopia have been actively studied in recent years (e.g, Ermilov and Rybalov 2024; Ermilov et al. 2024). The present study is based on materials collected from the Oromia region (western and southeastern Ethiopia) and South Ethiopia Regional State (southern Ethiopia) during 2015–2018. The main goals of the paper are: to present a list of the identified taxa and to describe two new carabodid species (Carabodidae) belonging to Carabodes (Klapperiches) Mahunka, 1978 and Congocepheus Balogh, 1958.
Carabodes (Klapperiches) was proposed by Mahunka (1978) as an independent genus, with Klapperiches nigrosetosus Mahunka, 1978 as type species. Its main subgeneric traits can be found in Mahunka (1978, 1986), Subías and Shtanchaeva (2023). Presently, the subgenus comprises about 50 species, which collectively have a cosmopolitan distribution (Subías 2022, online version 2024; including personal data of the first author). An identification key to the known species of Carabodes (Klapperiches) from the Afrotropical region has been presented by Ermilov and Kontschán (2022).
Congocepheus was proposed by Balogh (1958), with Congocepheus heterotrichus Balogh, 1958 as type species. Its main subgeneric traits can be found in Mahunka (1986), Fernandez et al. (2014), Subías and Shtanchaeva (2023). Presently, the genus comprises 13 species, which collectively distributed in the Paleotropical region (Subías 2022, online version 2024). Within the genus there are groups of species with distinctive morphological characters in the presence/absence of the specific notogastral ridges as well as the presence/absence, size and deep of the depressions in anterior and posterior parts of the notogaster, so the genus seems heterogeneous, and additional phylogenetic and molecular investigations are necessary.
Prior to this study, two species of Carabodes (Klapperiches) and three species of Congocepheus have been registered in Ethiopia (Ermilov et al. 2010, 2012a, b; Ermilov and Rybalov 2024a, b): C. (K.) dilatatus Ermilov, Sidorchuk and Rybalov, 2010; C. (K.) pocsi Mahunka, 1983 (in 1983b); Co. ornatus Mahunka, 1983 (in 1983a); Co. rwandensis Fernández, Theron and Leiva, 2016; Co. taurus Balogh, 1961.
Materials and methods



Sampling — Substrate samples containing oribatid mites were collected using a stainless-steel frame (50 × 50 cm) with a sieve (mesh size 2 × 2 cm) (Fig. 1c) in the following locations (Figs 1a–d):
# 12–13. Western Ethiopia, Oromia region, West Wollega Zone, Dhati Walal National Park, 9°23′54.7″N, 34°40′10.7″E, mountains, 1636 m a.s.l., litter (#12) and green mosses on the tree (#13) in forest with Ficus sp., Schefflera abyssinica and Coffea arabica on mollic Nitisols, 02.XI.2017, end of wet season (L.B. Rybalov).
# 14. Western Ethiopia, Oromia region, West Wollega Zone, near Dhati Walal National Park, 9°25′01.2″N, 34°45′21.3″E, mountains, 1761 m a.s.l., litter in a mixed forest on brown eutrophic soil, 02.XI.2017, end of wet season (L.B. Rybalov).
# 15. Southeastern Ethiopia, Oromia Region, Bale Zone, 6°35′24.0″N, 39°43′29.5″E, 1790 m a.s.l., Bale Mountains National Park, southern macroslope, Harenna Forest, broadleaved forest (first layer of forest: Syzygium guineense dominates, singly Polyscias fulva; second layer of forest: Teclea nobilis, Galiniera saxifraga, Pandanus sp.; among the shrubs the most common is Maytenus sp.), litter, 31.X.2015, beginning of dry season (O.G. Gorbunov).
# 16–17 Southern Ethiopia, South Ethiopia Regional State, Gamo Zone, 6°01′28.7″N, 37°35′49.7″E, 1179 m a.s.l., 5 km East from the town Arba Minch, 20–40 m from the lake Abai, litter (#16) and mineral soil (0–5 cm) (#17) in a mixed deciduous forest on alluvial organic soil, wet place, around water spots, 8.XII.2018, dry season (L.B. Rybalov).
# 18–19. Southern Ethiopia, South Ethiopia Regional State, Gamo Zone, 6°01′36.7″N, 37°35′42.7″E, 1170 m a.s.l., 5 km East from the town Arba Minch, 1 km from the lake Abai, litter (#18) and mineral soil (0–5 cm) (#19) in a mixed forest on the terrace (first layer of forest: Celtis sp., Ficus sycomorus, Schefflera sp., Acacia sp.; second layer of forest: Lemon sp., Coffea arabica) on brown (black-brown) soil with light-medium loam soil texture, 7.XII.2018, dry season (L.B. Rybalov).
Mites were extracted into 75% ethanol using Berlese's funnels with electric lamps in laboratory conditions.
Observation and documentation — For measurement and illustration, specimens were mounted in lactic acid on temporary cavity slides. Some specimens preliminarily were placed for one day in a 5% potassium hydroxide solution to lighten the body cuticle, dissolve cerotegument, and release adherent debris. All body measurements are presented in micrometers (µm); body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the notogaster; body width refers to the maximum width of the notogaster in dorsal view; body setae were measured in lateral aspect. Formulas for leg setation are given in parentheses according to the sequence trochanter-femur-genu-tibia-tarsus (famulus of tarsus I included); formulas for leg solenidia are given in square brackets, according to the sequence genu-tibia-tarsus. Drawings were made with a camera lucida using a Leica DM 2500 light microscope.
Terminology — Morphological terminology is mostly that of F. Grandjean (references in Travé and Vachon 1975). Fundamentals of leg setation were reviewed by Norton (1977).
Abbreviations — Prodorsum: lam = lamella; tlam = translamella; hs = hump-like structure; dep = depression; ro, le, in, bs = rostral, lamellar, interlamellar, and bothridial setae, respectively; tu = tutorium; lpr = lateral prodorsal ridge. Notogaster: c, da, la, dm, lm, dp, lp, h1–h3, p1–p3 = setae; ia, im, ip, ih, ips = lyrifissures; gla = opisthonotal gland opening. Gnathosoma: a, m, h = subcapitular setae; d, l, sup, inf, acm, ul, su, lt, vt = palp setae; ω = solenidion; cha, chb = cheliceral setae; Tg = Trägårdh's organ. Epimeral and lateral podosomal regions: 1a–1c, 2a, 3a–3c, 4a–4c = epimeral setae; PdI, PdII = pedotecta I, II, respectively; dis = discidium. Anogenital region: g, ag, an, ad = genital, aggenital, anal, and adanal setae, respectively; iad = adanal lyrifissure; po = preanal organ. Legs: ω, φ, σ = solenidia; e = famulus; d, l, v, bv, ev, ft, tc, it, p, u, a, s, pv = setae; pa = porose area.
Notes — References to the original descriptions of taxa in the List of identified taxa section are not included in the References section. Paired structures are described in the singular, unless otherwise noted.
List of identified taxa
Distribution: mostly from Subías (2022, online version 2024). Unidentified species are not included. The number of the collected specimens is in parentheses.
The list includes 70 species belonging to 54 genera and 33 families. Eight species (Annectacarus insculptus, Neoliodes terrestris, Zetorchestes ornatus, Oxyoppia (Oxyoppiella) bituberculata, Eupelops forsslundi, Protoribates ziemowiti, Transoribates agricola, Benoibates rugosus), five genera (Annectacarus, Zetorchestes, Transoribates, Benoibates, Punctoribates) and two families (Zetorchestidae, Oripodidae) are reported in Ethiopia for the first time. One species (Punctoribates zachvatkini) is reported for the first time in the Afrotropical region. Seventeen species are known only from Ethiopia, 27 species are known from the Afrotropical region and 26 species (including six cosmopolitan/semicosmopolitan species) have broad distributions (more than one geographical region).
Lohmanniidae
Annectacarus insculptus Wallwork, 1962: location 19 (3 exs.). Distribution: Ghana. New record of the genus and species in Ethiopia.
Cosmochthoniidae
Cosmochthonius lanatus (Michael, 1885): location 15 (1 ex.). Distribution: Semicosmopolitan.
Sphaerochthoniidae
Sphaerochthonius splendidus (Berlese, 1904): locations 17 (5 exs.), 18 (1 ex.). Distribution: Tropical and Subtropical regions.
Epilohmanniidae
Epilohmannia pallida Wallwork, 1962: locations 18 (1 ex.), 19 (4 exs.). Distribution: Tropical and Subtropical regions.
Nanhermanniidae
Masthermannia mammillaris (Berlese, 1904): location 12 (1 ex.). Distribution: Tropical and Subtropical regions.
Nothridae
Nothrus crassisetus Mahunka, 1982: locations 18 (1 ex.), 19 (3 exs.). Distribution: Afrotropical region.
Malaconothridae
Malaconothrus ensifer Mahunka, 1982: locations 12 (1 ex.), 18 (1 ex.), 19 (3 exs.). Distribution: Afrotropical region.
Hermanniellidae
Hermanniella dubiosa Mahunka & Mahunka-Papp, 2007: locations 16 (9 exs.), 17 (8 exs.), 18 (2 exs.), 19 (7 exs.). Distribution: Afrotropical region.
Plasmobatidae
Plasmobates foveolatus Ermilov, Sidorchuk & Rybalov, 2010: location 12 (1 ex.). Distribution: Afrotropical region.
Neoliodidae
Neoliodes terrestris (Wallwork, 1963): locations 15 (1 ex.), 18 (1 ex.). Distribution: Tropical and southern Palaearctic regions. New record of the species in Ethiopia.
Aleurodamaeidae
Aleurodamaeus recenfesevpi Ermilov and Rybalov, 2012: location 15 (3 exs.). Distribution: Ethiopia.
Damaeidae
Metabelba (Pateribelba) glabriseta Mahunka, 1982: locations 16 (5 exs.), 18 (1 ex.), 19 (1 ex.). Distribution: Afrotropical region.
Microzetidae
Berlesezetes ornatissimus (Berlese, 1913): locations 15 (4 exs.), 18 (4 exs.), 19 (4 exs.). Distribution: Tropical and Subtropical regions.
Microzetes (Megazetes) eckeri (Mahunka, 1982): location 16 (1 ex.). Distribution: Ethiopia.
Basilobelbidae
Basilobelba gigantea Ermilov, Sidorchuk & Rybalov, 2011: locations 12 (1 ex.), 14 (1 ex.), 18 (5 exs.), 19 (4 exs.). Distribution: Ethiopia.
Damaeolidae
Fosseremus laciniatus (Berlese, 1905): locations 12 (1 ex.), 19 (7 exs.). Distribution: Cosmopolitan.
Eremobelbidae
Eremobelba tuberculata Mahunka, 1982: location 14 (6 exs.). Distribution: Ethiopia.
Eremulidae
Eremulus flagellifer Berlese, 1908: locations 15 (1 ex.), 19 (4 exs.). Distribution: Cosmopolitan.
Zetorchestidae
Zetorchestes ornatus Mahunka, 1985: locations 16 (5 exs.), 18 (4 exs.). Distribution: Afrotropical region. New record of the family, genus and species in Ethiopia.
Carabodidae
Austrocarabodes (Austrocarabodes) glabrus Mahunka, 1982: location 18 (12 exs.). Distribution: Ethiopia.
Austrocarabodes (Austrocarabodes) heterosetosus Ermilov, Sidorchuk and Rybalov, 2010: location 16 (3 exs.). Distribution: Afrotropical region.
Austrocarabodes (Uluguroides) arboreus Ermilov, Sidorchuk and Rybalov, 2010: location 15 (3 exs.). Distribution: Ethiopia.
Carabodes (Klapperiches) paradilatatus Ermilov n. sp.: locations 18 (4 exs.), 19 (2 exs.). Distribution: Ethiopia.
Congocepheus setiformis Ermilov n. sp.: location 18 (7 exs.). Distribution: Ethiopia.
Dampfiellidae
Beckiella opposita Mahunka, 1982: location 12 (1 ex.). Distribution: Ethiopia.
Machadobelbidae
Machadobelba shtanchaevae Ermilov, Sidorchuk & Rybalov, 2010: location 12 (5 exs.). Distribution: Ethiopia.
Oppiidae
Antennoppia capilligera (Berlese, 1916): locations 16 (1 ex.), 17 (4 exs.), 19 (3 exs.). Distribution: Afrotropical region.
Afroppia brevipila (Mahunka, 1982): location 12 (7 exs.). Distribution: Ethiopia.
Arcoppia rugosa (Mahunka, 1974): location 14 (11 exs.). Distribution: Afrotropical region, Hawaii.
Neoamerioppia polygonata (Mahunka, 1982): locations 12 (21 exs.), 13 (13 exs.), 14 (16 exs.), 16 (3 exs.). Distribution: Afrotropical region.
Oppia kuehnelti Csiszár, 1961: location 18 (3 exs.). Distribution: Tropical and southern Palaearctic regions.
Oxyoppia (Oxyoppiella) bituberculata (Balogh, 1958): location 19 (2 exs.). Distribution: Afrotropical and Sub-Antarctic regions. New record of the species in Ethiopia.
Pseudoamerioppia barrancensis (Hammer, 1961): location 15 (1 ex.). Distribution: Neotropical, Oriental and Afrotropical regions, Canary Is.
Ramusella (Insculptoppia) seniczakae Hugo-Coetzee, 2016: locations 15 (6 exs.), 17 (1 ex.), 19 (10 exs.). Distribution: South Africa.
Suctobelbidae
Suctobelbella (Flagrosuctobelba) kontschani (Mahunka & Mahunka-Papp, 2007): locations 12 (2 exs.), 17 (1 ex.), 19 (3 exs.). Distribution: Afrotropical region.
Suctobelbella (Ussuribata) spirochaeta Mahunka, 1983: locations 12 (1 ex.), 15 (1 ex.). Distribution: Afrotropical region.
Tectocepheidae
Tectocepheus sarekensis Trägårdh, 1910: locations 14 (5 exs.), 17 (24 exs.), 19 (11 exs.). Distribution: Cosmopolitan.
Tegeozetes tunicatus Berlese, 1913: location 16 (1 ex.). Distribution: Tropical region, Hungary.
Phenopelopidae
Eupelops forsslundi (Balogh, 1959): location 15 (9 exs.). Distribution: Afrotropical region, Vietnam. New record of the species in Ethiopia.
Caloppiidae
Zetorchella arsiensis Ermilov, 2023: location 16 (1 ех.). Distribution: Ethiopia.
Zetorchella nortoni Ermilov, Sidorchuk & Rybalov, 2010: location 15 (2 exs.). Distribution: Ethiopia.
Haplozetidae
Pilobatella dhatiensis Ermilov, 2019: location 14 (3 exs.). Distribution: Ethiopia.
Protoribates aethiopicus Ermilov & Rybalov, 2013: locations 16 (4 exs.), 17 (1 ex.), 18 (5 exs.), 19 (8 exs.). Distribution: Afrotropical region.
Protoribates paracapucinus (Mahunka, 1988): locations 18 (1 ex.), 19 (7 exs.). Distribution: Tropical and Subtropical regions.
Protoribates ziemowiti Ermilov & Starý, 2020: locations 16 (4 exs.), 17 (2 exs.), 18 (7 exs.), 19 (4 exs.). Distribution: Madagascar. New record of the species in Ethiopia.
Transoribates agricola (Nakamura & Aoki, 1989): location 16 (7 exs.). Distribution: eastern Palaearctic region, Vietnam, South Africa. New record of the genus and species in Ethiopia.
Mochlozetidae
Unguizetes atypicus (Mahunka, 1982): location 12 (1 ex.). Distribution: Afrotropical region.
Oripodidae
Benoibates rugosus Mahunka, 2001: location 16 (3 exs.). Distribution: Kenya. New record of the family, genus and species in Ethiopia.
Scheloribatidae
Muliercula walalensis Ermilov, 2019: location 16 (4 exs.). Distribution: Ethiopia.
Scheloribates aethiopicus Mahunka, 1982: locations 12 (5 exs.), 14 (47 exs.). Distribution: Afrotropical region, Canary Islands.
Scheloribates fimbriatus Thor, 1930: location 14 (1 ex.). Distribution: Pantropical and Subtropical regions.
Scheloribates leleupi Balogh, 1959: location 12 (50 exs.). Distribution: Afrotropical region.
Scheloribates pallidulus (Koch, 1841): location 13 (3 exs.). Distribution: Cosmopolitan.
Scheloribates perisi Pérez-Íñigo, 1982: location 14 (21 exs.). Distribution: Afrotropical region.
Scheloribates praeincisus (Berlese, 1910): locations 12 (1 ex.), 16 (10 exs.). Distribution: Tropical and Subtropical regions.
Scheloribates (Bischeloribates) munesaensis Ermilov, 2016: location 15 (4 exs.). Distribution: Afrotropical region.
Scheloribates (Perscheloribates) paratranslamellatus (Ermilov & Rybalov, 2014): location 15 (1 ex.). Distribution: Ethiopia.
Punctoribatidae
Afroleius valerieae Coetzee, 2014: location 18 (16 exs.). Distribution: Afrotropical region.
Lamellobates molecula (Berlese, 1916): locations 16 (6 exs.), 17 (5 exs.), 18 (9 exs.). Distribution: Tropical and Subtropical regions.
Punctoribates zachvatkini Shaldybina, 1969: location 18 (2 exs.). Distribution: southern Palaearctic region. New record of the species in the Afrotropical region. New record of the genus in Ethiopia.
Galumnidae
Allogalumna machadoi (Balogh, 1960): location 12 (1 ex.). Distribution: Paleotropical region.
Galumna flabellifera Hammer, 1958: locations 12 (1 ex.), 16 (45 exs.), 17 (17 exs.), 18 (15 exs.), 19 (12 exs.). Distribution: Tropical and Subtropical regions.
Galumna incisa Mahunka, 1982: location 12 (7 exs.). Distribution: Afrotropical region.
Pergalumna pocsi Mahunka, 1984: locations 16 (11 exs.), 17 (10 exs.), 18 (1 ex.). Distribution: Afrotropical region.
Pilizetes anufrievi Ermilov, Sidorchuk & Rybalov, 2010: location 12 (1 ex.). Distribution: Ethiopia.
Trichogalumna africana Ermilov, Sidorchuk & Rybalov, 2011: locations 12 (8 exs.), 13 (12 exs.), 14 (33 exs.). Distribution: Afrotropical region.
Trichogalumna nipponica (Aoki, 1966): location 15 (18 exs.). Distribution: Semicosmopolitan.
Galumnellidae
Galumnella apiculata Mahunka, 1992: location 12 (1 ex.). Distribution: Afrotropical region.
Galumnella subareolata Mahunka, 1969: location 15 (7 exs.). Distribution: Afrotropical region.
Galumnopsis ruginervis Balogh, 1962: location 15 (1 ex.). Distribution: Afrotropical region.
Descriptions
Carabodes (Klapperiches) paradilatatus Ermilov n. sp.
ZOOBANK: 1FEEB9AE-E513-459C-9B27-D2CFB0F9C746 ![]()
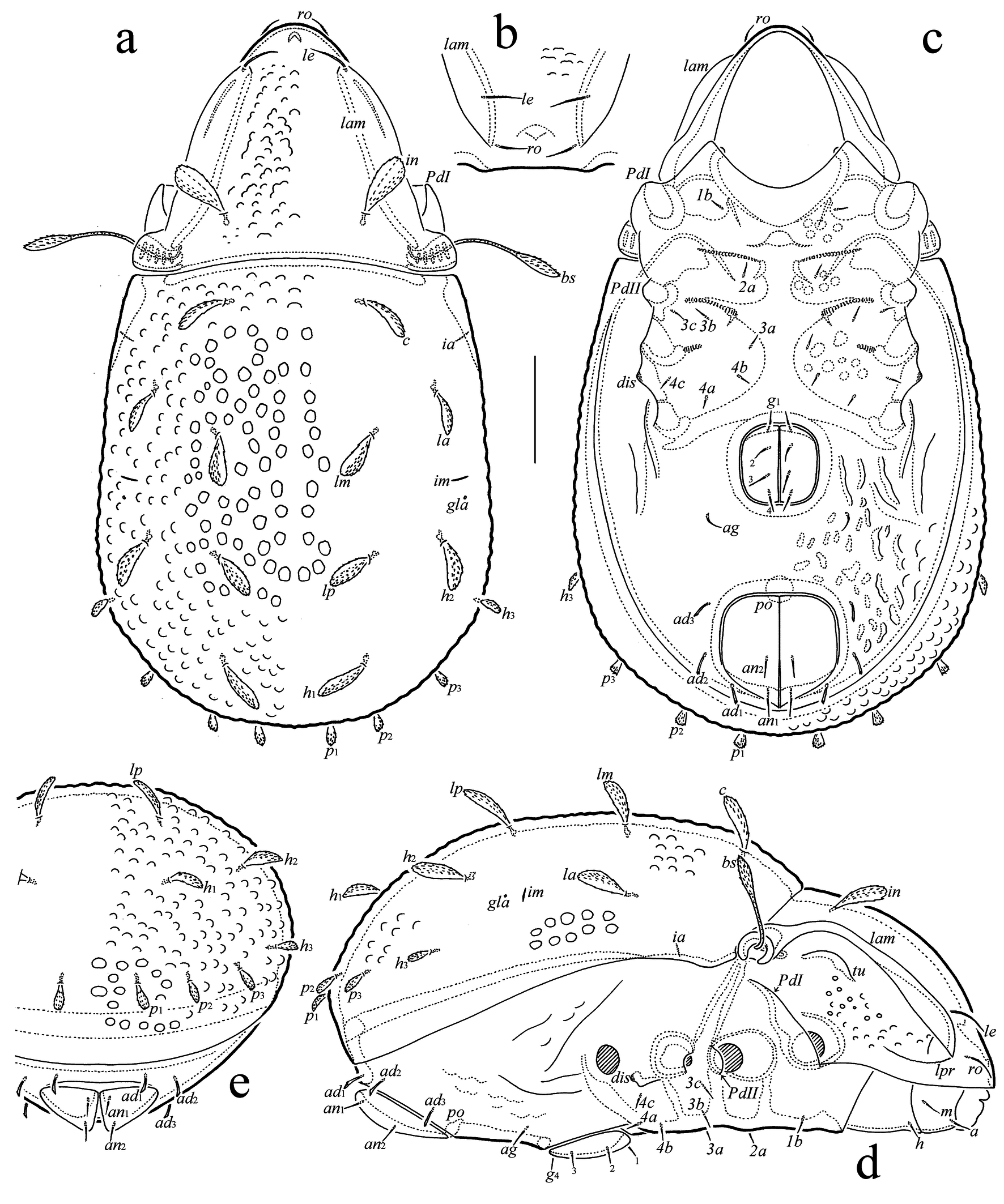
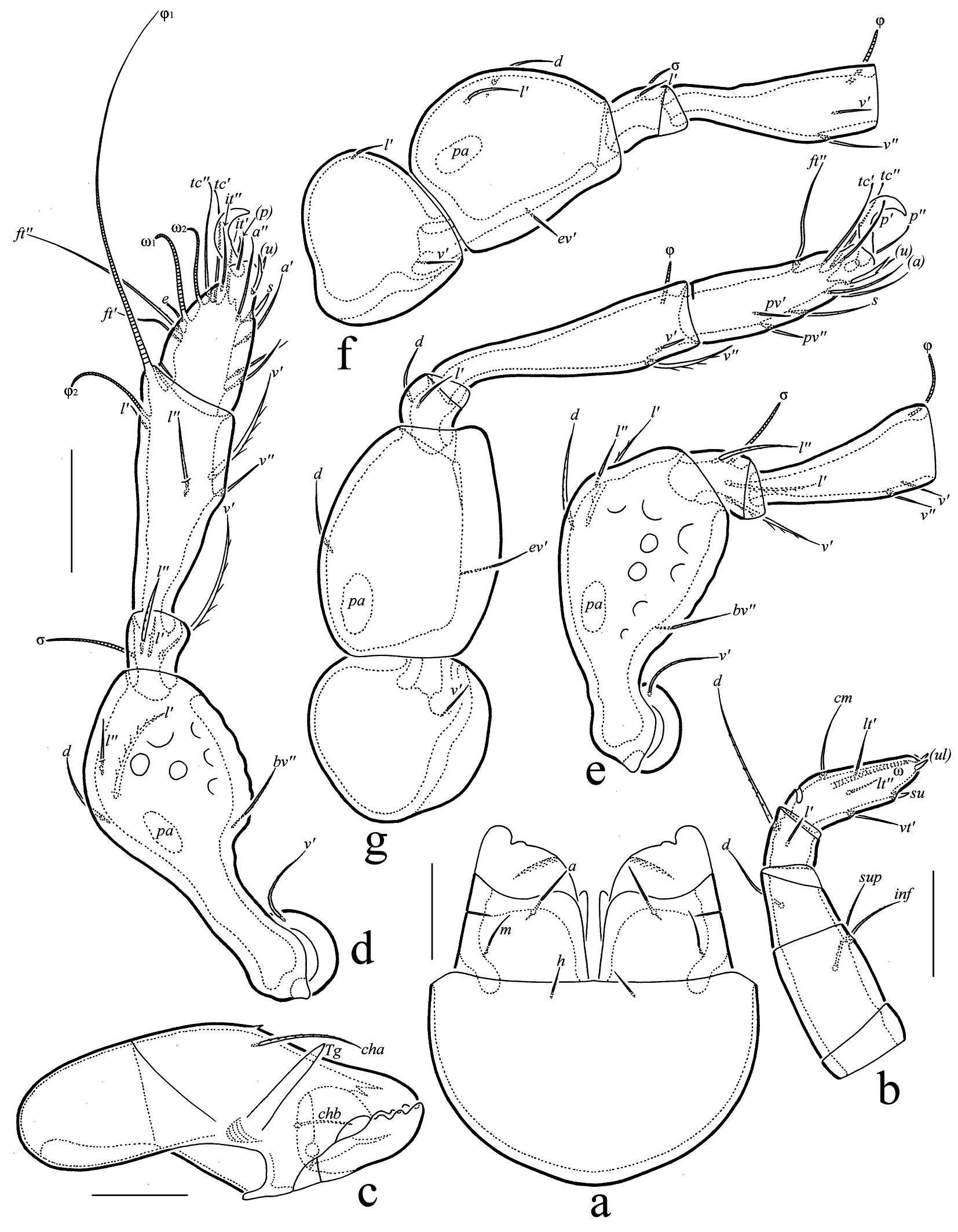
(Figures 2, 3)




Type material — Holotype (female) and three paratypes (three females): Southern Ethiopia, South Ethiopia Regional State, Gamo Zone, 6°01′36.7″N, 37°35′42.7″E, 1170 m a.s.l., 5 km East from the town Arba Minch, 1 km from the lake Abai, litter in a mixed forest on the terrace (first layer of forest: Celtis sp., Ficus sycomorus, Schefflera sp., Acacia sp.; second layer of forest: Lemon sp., Coffea arabica) on brown (black-brown) soil with light-medium loam soil texture, 7.XII.2018, dry season (L.B. Rybalov). Two paratypes (two females): same as the holotype, but mineral soil (0–5 cm).
The holotype is deposited in the collection of the Senckenberg Museum of Natural History, Görlitz, Germany; five paratypes are in the collection of the University of Tyumen, Museum of Zoology, Tyumen, Russia. All specimens are preserved in 70% solution of ethanol with a drop of glycerol.
Diagnosis — Body length: 300–345. Prodorsum between lamellae with unevenly spaced tubercles, frequently fused and forming short tuberculate ridges. Lamella triangular distally; translamella absent. Rostral and lamellar setae short, setiform, slightly barbed; interlamellar seta comparatively short, phylliform, dilated mediodistally, barbed; bothridial seta long, fusiform, with barbed head. Notogaster with unevenly spaced tubercles (dorsocentral part with some areas without tubercles). All notogastral setae comparatively short (posterior setae shorter than others), phylliform, dilated mediodistally, barbed. Subcapitular mentum and epimeral region without sculpture; epimere I with one pair of setae; all epimeral setae short, simple. Anogenital region with slightly developed unevenly spaced tuberculate ridges, simple ridges and some small depressions. Genital, aggenital and anal setae short, simple; all adanal setae short, slightly dilated and barbed mediodistally. Leg tarsus IV with 12 setae.
Description — Measurements. Body length: 345 (holotype), 300–345 (female paratypes); notogaster width: 180 (holotype), 165–180 (female paratypes). Body ratio (length/width): ≈ 1.8–1.9.
Integument (Figs 2(a–e), 3(e, d)) — Body color light brown. Body covered by thin layer of gel-like cerotegument. Prodorsum between lamellae with unevenly spaced tubercles (diameter up to 7), frequently fused and forming short tuberculate ridges; lateral part of prodorsum partially with foveolae (diameter up to 5); notogaster with unevenly spaced tubercles (diameter up to 7; dorsocentral part with some areas without tubercles); anogenital region with slightly developed unevenly spaced tuberculate ridges, simple ridges and some depressions; subcapitular mentum, epimeral region, genital and anal plates without sculpture; antiaxial side of leg femora I, II partially with large, poorly developed foveolae.
Prodorsum (Figs 2(a, b, d)) — Rostrum broadly rounded. Lamella long and broad, triangular distally; translamella absent; tutorium short, ridge-like; lateral part of prodorsum with slight ridge (lpr) directed to end of lamella and bearing indistinct teeth. Rostral (13–15) and lamellar (17–19) setae setiform, slightly barbed; ro inserted dorsally on rostrum; le inserted dorsomedially in distal part of lamella; interlamellar seta (26–30) phylliform, dilated mediodistally, barbed; bothridial seta (45–55) fusiform, with long, roughened stalk and short, barbed head; exobothridial seta not observed. Bothridium interrupted ventrally.
Notogaster (Figs 2(a, d, e)) — Anterior notogastral margin slightly convex medially. Humeral shoulder small, visible in dorsolateral and lateral aspects. Ten pairs of notogastral setae (p1, p2, p3, h3: 13–15; others: 22–30) phylliform, dilated mediodistally, barbed. Opisthonotal gland opening and lyrifissures ia, im visible; lyrifissures ip, ih and ips not observed.
Gnathosoma (Figs 3(a–c)) — Subcapitulum size: 75–77 × 60–64; subcapitular seta a (11) setiform, roughened; m (9–11) and h (7) setiform, nearly smooth. Palp length: 37; setation: 0-2-1-2-7(+ω); setae acm and v′ on tarsus and l″ on tibia absent; postpalpal seta (4) spiniform, smooth. Chelicera length: 82–86; seta cha (22–26) setiform, barbed; seta chb (11–13) setiform, roughened.
Epimeral and lateral podosomal regions (Figs 2(c, d)) — Epimeral setation: 1-1-3-3; all setae (7) setiform, nearly smooth. Discidium tubercle-like.
Anogenital region (Figs 2(c–e)) — Anogenital formula: 4-1-2-3; genital (7), aggenital (11) and anal (11) setae setiform, nearly smooth; all adanal setae (11) slightly dilated and barbed mediodistally. Adanal lyrifissure not observed.
Legs (Figs 3(d–g)) — Claw of each leg strong, slightly roughened dorsally, with ventrobasal indistinct microtubercle. Dorsoparaxial porose area on femora I–IV elongate oval. Formulas of leg setation and solenidia: I (1-4-3-4-16) [1-2-2], II (1-4-3-2-15) [1-1-2], III (2-3-1-2-15) [1-1-0], IV (1-2-2-2-12) [0-1-0]; homology of setae and solenidia indicated in Table 1; seta s of tarsus I spiniform; phylliform seta absent on segments; solenidion φ1 of tibia I long, subflagellate versus other solenidia medium-sized or short, thickened, rounded distally.
Download as Note: Tr, Fe, Ge, Ti, Ta = trochanter, femur, genu, tibia, and tarsus, respectively; Roman letters refer to normal setae; Greek letters to solenidia; single prime (‘) marks setae on the anterior and double prime (’’) setae on the posterior side of a given leg segment; parentheses refer to a pair of setae.
Leg
Tr
Fe
Ge
Ti
Ta
I
v’
d, (l), bv’’
(l), v’, σ
(l), (v), φ1, φ2
(ft), (tc), (it), (p), (u), (a), s, (pv), e*, ω1,ω2
II
v’
d, (l), bv’’
(l), v’, σ
(v), φ
(ft), (tc), (it), (p), (u), (a), *s**, (pv), ω1, ω2
III
l’, v’
d, l’, ev’*
l’, σ
(v), φ
(ft), (tc), (it), (p), (u), (a), s, (pv)
IV
v’
d, ev’
d, l’
(v), φ
ft’’, (tc), (p), (u), (a), s, (pv)
Remarks — Carabodes (Klapperiches) paradilatatus Ermilov n. sp. is most similar to C. (K.) dilatatus Ermilov, Sidorchuk and Rybalov, 2010 from Ethiopia in having tuberculate notogaster and dorsal part of prodorsum, long bothridial seta with well-developed head, and short, phylliform interlamellar and notogastral setae. However, the new species differs from the latter by smaller body size (length: 300–345 versus 365–464), the morphology of lamellar, bothridial and adanal setae (le setiform; bs fusiform, with barbed head; ad1–ad3 dilated mediodistally versus le dilated in median part; bs clavate, nearly smooth; ad1–ad3 setiform), the density of arrangement of the tubercles in dorsocentral part of the notogaster (unevenly spaced, with some comparatively large areas without tubercles versus evenly and densely spaced, without large free areas between tubercles), and the absence (versus presence) of foveolae on the subcapitular mentum.
Etymology — The species name paradilatatus refers to the similarity of C. (K.) paradilatatus to C. (K.) dilatatus.
Congocepheus setiformis Ermilov n. sp.
ZOOBANK: 1B2C9206-2CCE-4309-AF17-5D9B43984037 ![]()
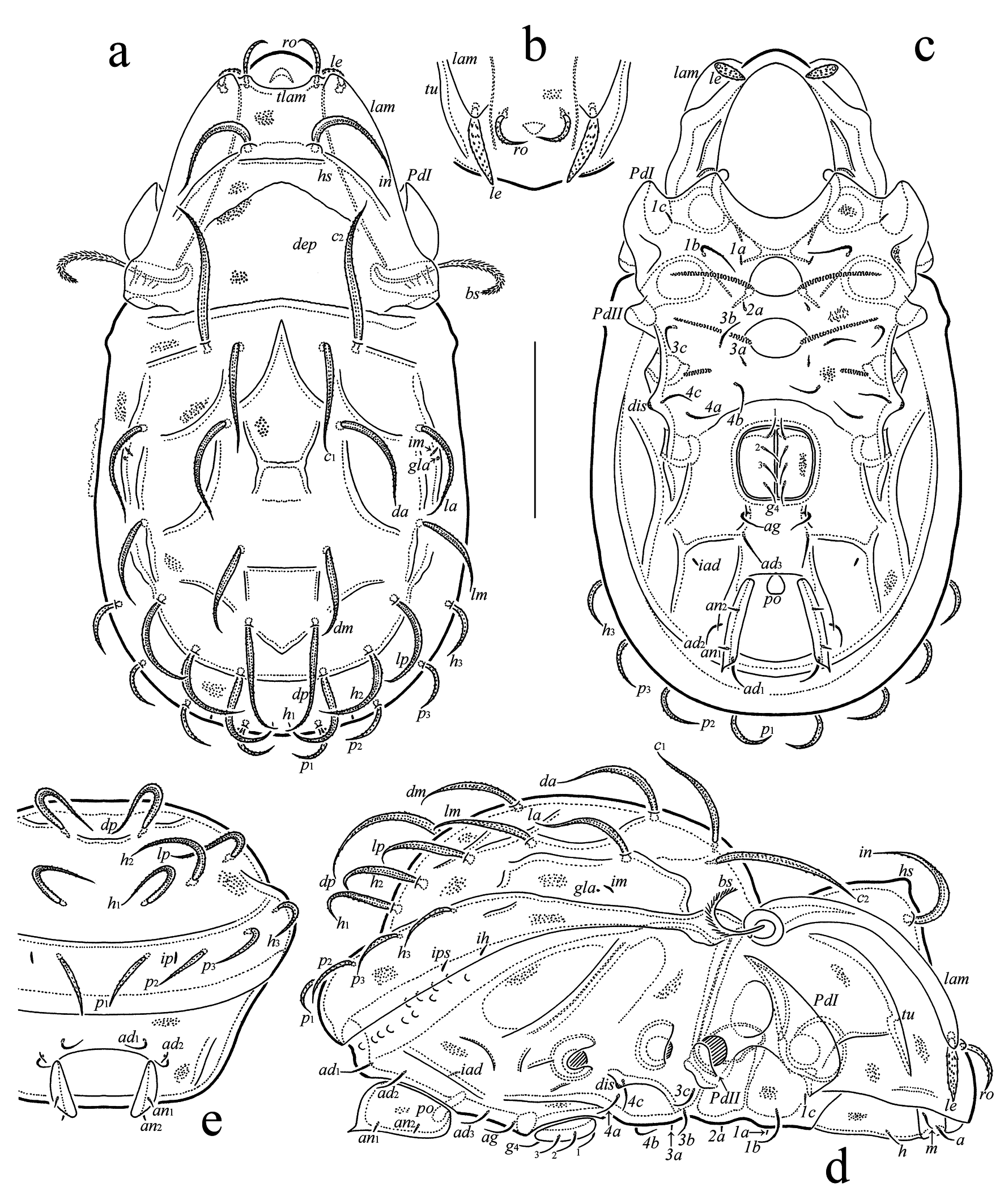
(Figures 4, 5)


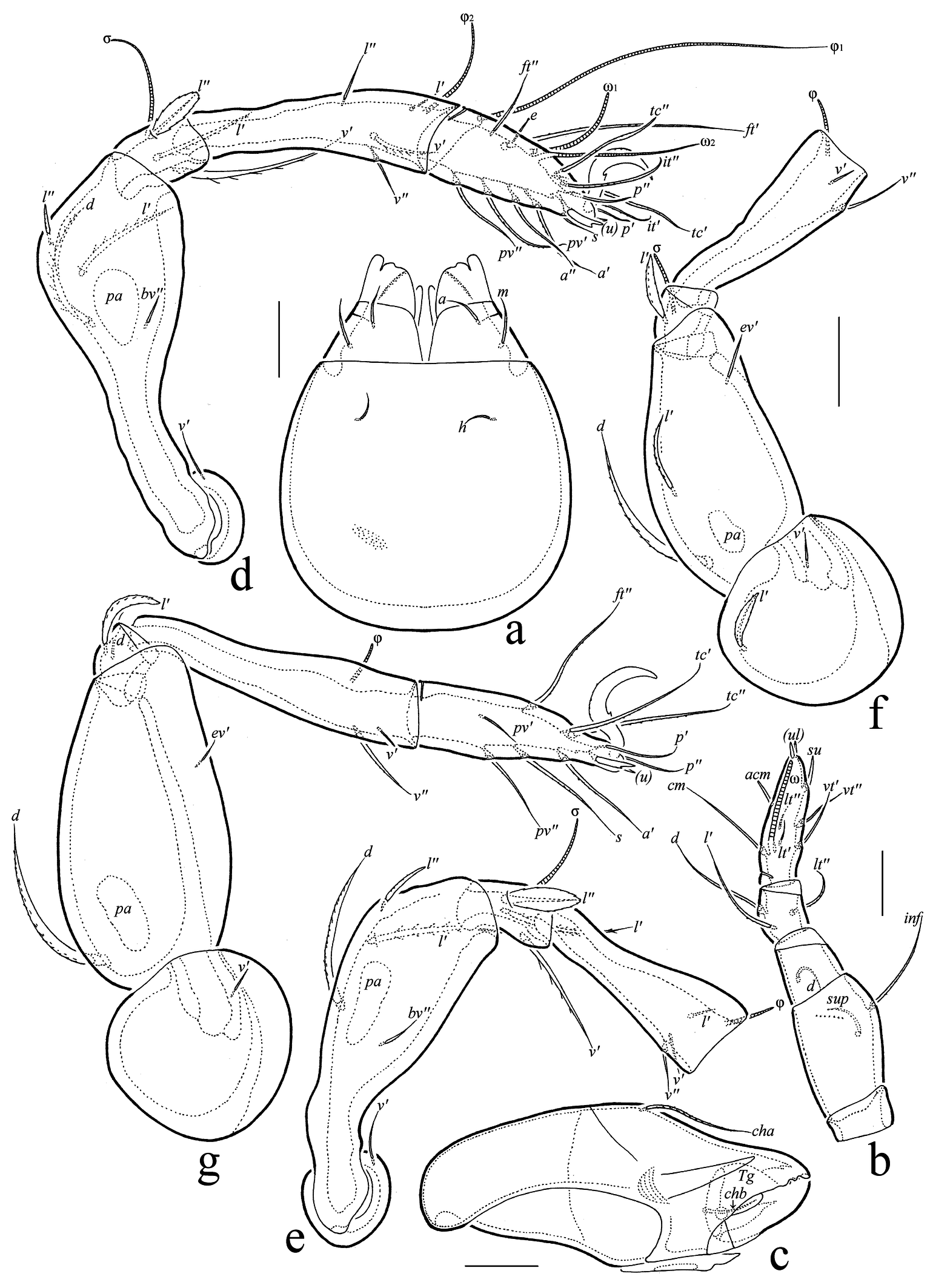


Type material — Holotype (male) and six paratypes (three males and three females): Southern Ethiopia, South Ethiopia Regional State, Gamo Zone, 6°01′36.7″N, 37°35′42.7″E, 1170 m a.s.l., 5 km East from the town Arba Minch, 1 km from the lake Abai, litter in a mixed forest on the terrace (first layer of forest: Celtis sp., Ficus sycomorus, Schefflera sp., Acacia sp.; second layer of forest: Lemon sp., Coffea arabica) on brown (black-brown) soil with light-medium loam soil texture, 7.XII.2018, dry season (L.B. Rybalov).
The holotype is deposited in the collection of the Senckenberg Museum of Natural History, Görlitz, Germany; six paratypes are in the collection of the University of Tyumen, Museum of Zoology, Tyumen, Russia. All specimens are preserved in 70% solution of ethanol with a drop of glycerol.
Diagnosis — Body length: 375–450. Notogaster with specific system of comparatively sparse ridges and depressions; two anteromedial ridges fused distally. Lamella rounded distally; translamella present. Dorsocentral prodorsal region distinctly hump-like; mediobasal prodorsal part with large depression. Rostral seta medium-sized, phylliform, barbed, inserted on translamella; lamellar seta medium-sized phylliform, covered by strong spines, inserted in ventrodistal part of lamella; interlamellar seta long, phylliform, barbed; bothridial seta medium-sized, setiform, heavily barbed. Fourteen pairs of phylliform, barbed notogastral setae: c2, dp longest; p1, p2, p3, h3 shortest; other setae medium-sized; c2 directed forward. Epimeral setae 1a, 1c, 2a, 3a and anal setae short, spiniform; genital setae short, setiform, nearly smooth; other epimeral setae well as aggenital and adanal setae short, setiform, slightly barbed; seta ad3 in preanal position. Leg tarsus IV with 12 setae; seta l″ of genua I, II and l′ of genua III, IV phylliform.
Description — Measurements. Body length: 375 (holotype), 375–450 (male and female paratypes); notogaster width: 210 (holotype), 210–255 (male and female paratypes). Body ratio (length/width): ≈ 1.7–1.9. No difference between males and females in size.
Integument (Figs 4(a–e)) — Body color light grey to dark brown. Body surface nearly smooth, covered by thin layer of dense microgranulate cerotegument. Notogaster and anogenital region with specific system of ridges and depressions; two oblique anteromedial notogastral ridges fused distally.
Prodorsum (Figs 4(a, b, d)) — Rostrum broadly rounded. Lamella long and broad, rounded distally; translamella present (observed in dorsal aspect); tutorium long (slightly shorter than lamella), ridge-like. Dorsocentral region distinctly hump-like; mediobasal part with large depression. Rostral seta (37–41) phylliform, dilated in median part, barbed, inserted on distinct tubercle (tubercle located on translamella); lamellar seta (37–41) phylliform, dilated in median part, covered by strong spines, inserted in ventrodistal part of lamella; interlamellar seta (75–82) phylliform, dilated mediobasally or in median part, barbed; bothridial seta (52–56) setiform, heavily barbed, curved mediodistally; exobothridial seta not observed. Bothridium interrupted ventrally.
Notogaster (Figs 4(a, d, e)) — Anterior margin slightly convex medially; lateral and posterior parts slightly depressed. Humeral shoulder small, well visible. Fourteen pairs of notogastral setae (c3 absent; c2, dp: 82–90; p1, p2, p3, h3: 37–41; others: 67–71) phylliform, dilated mediobasally or in median part, barbed; c2 directed forward; c1 located medial to c2. Opisthonotal gland opening and all lyrifissures visible.
Gnathosoma (Figs 5(a–c)) — Subcapitulum size: 94–97 × 75–79; subcapitular seta h (9–11) setiform, slightly barbed; a (11–15) and m (11–15) narrowly phylliform, slightly dilated in median part, slightly barbed. Palp length: 60–64; setation: 0-2-1-3-9(+ω); postpalpal seta (7) spiniform, roughened. Chelicera length: 109–112; seta cha (30–34) setiform, barbed; seta chb (15–17) setiform, roughened.
Epimeral and lateral podosomal regions (Figs 4(c, d)) — Epimeral setation: 3-1-3-3; setae 1a, 1c, 2a, 3a (4) spiniform, smooth; other setae (1b: 30; others: 19–22) setiform, slightly barbed. Discidium tubercle-like.
Anogenital region (Figs 4(c–e)) — Anogenital formula: 4-1-2-3; all genital setae (11–15) setiform, nearly smooth; aggenital and adanal setae (19–22) setiform, slightly barbed; seta ad3 in preanal position, posterior to aggenital seta; both anal setae (4) spiniform, smooth. Adanal lyrifissure distanced from anal plate.
Legs (Figs 5(d–g)) — Claw of each leg strong, barbed dorsally, with small ventrobasal tooth. Dorsoparaxial porose area on femora I–IV elongate oval. Formulas of leg setation and solenidia: I (1-4-3-4-16) [1-2-2], II (1-4-3-3-15) [1-1-2], III (2-3-1-2-15) [1-1-0], IV (1-2-2-2-11) [0-1-0]; homology of setae and solenidia indicated in Table 2; seta s of tarsus I eupathidial; seta l″ of genua I, II and l′ of genua III, IV phylliform, dilated in median part; solenidion φ1 of tibia I long, subflagellate; ω2 long, rod-like; other solenidia medium-sized or short, thickened, rounded distally.
Download as Note: See Table 1 for explanations.
Leg
Tr
Fe
Ge
Ti
Ta
I
v’
d, (l), bv’’
(l), v’, σ
(l), (v), φ1, φ2
(ft), (tc), (it), (p), (u), (a), s, (pv), e, ω1, ω2
II
v’
d, (l), bv’’
(l), v’, σ
(v), l’, φ
(ft), (tc), (it), (p), (u), (a), s, (pv), ω1, ω2
III
l’, v’
d, l’, ev’
l’, σ
(v), φ
(ft), (tc), (it), (p), (u), (a), s, (pv)
IV
v’
d, ev’
d, l’
(v), φ
ft’‘, (tc), (p), (u), a’, s, (pv)
Remarks — Congocepheus setiformis Ermilov n. sp. is most similar to C. rwandensis Fernández, Theron and Leiva, 2016 from the Afrotropical region in having setiform bothridial seta and the presence of one pair of long notogastral setae of c-row directed forward. However, the new species differs from the latter by the seta c2 (versus c1) directed forward and a significantly smaller number of ridges and depressions on the notogaster.
Etymology — The species name setiformis refers to the morphology of the bothridial seta (setiform).
General remark
This section discusses taxonomic status of some supraspecies taxa within Carabodidae based on the direction of notogastral setae in anterior part of the notogaster. The subgenus Austrocarabodes (Austroflexa) was proposed by Subías (2019), with Austrocarabodes brasiliensis Ermilov and Tolstikov, 2015 as type species. The genus Flexa was proposed by Kulijev (1977), with Carabodes dubius Kulijev, 1968 as type species; Subías (2004) considered Flexa as the subgenus within Carabodes Koch, 1835. Tanzaniacepheus was proposed by Fernández et al. (2017), with Tanzaniacepheus ornatus (Mahunka 1983) (in Mahunka 1983a) as type species; Subías (2022) considered Tanzaniacepheus as a subgenus within Congocepheus. Austrocarabodes (Austroflexa), Flexa and Tanzaniacepheus are morphologically similar to Austrocarabodes (Austrocarabodes), Carabodes (Carabodes) and Congocepheus, respectively, but differs from them mainly by the direction of notogastral setae of the c-row (usually one or two setae directed forward).
In our opinion, the direction of notogastral setae of the c-row in the cases listed above is a specific morphological character and is sufficient only to distinguish groups of species within the genera (see Balogh 1988; Ermilov et al. 2015), but not enough to support the generic or subgeneric status. Moreover, other important morphological characters are ignored in justifying these genera (for example, body sculpture/ornamentation; presence/absence, size and deep of notogastral depressions). Therefore, we consider Austrocarabodes (Austroflexa), Flexa and Tanzaniacepheus as junior subjective synonyms of Austrocarabodes (Austrocarabodes), Carabodes (Carabodes) and Congocepheus, respectively.
Acknowledgements
We thank Dr. Gezahegn Degefe for supporting our field studies and organizing laboratory work, Dr. Oleg G. Gorbunov for sampling assistance and two anonymous reviewers for valuable comments. The collection of materials was conducted under the Agreement between the Russian Academy of Sciences and the Ministry of Science and Technology (Ministry of Innovation and Technology) of the Federal Democratic Republic of Ethiopia. The work was performed within the framework of the Joint Russian-Ethiopian Biological Expedition, financially supported by the Russian Academy of Sciences.
References
- Balogh J. 1958. Oribatides nouvelles de l′Afrique tropicale. Revue de Zoologie et de Botanique Africaines, 58: 1-34.
- Balogh J. 1961. The scientific results of the first Hungarian Zoological Expedition to East Africa. Annales Historico-Naturales Musei Nationalis Hungarici, 53: 517-524.
- Balogh P. 1988. Oribatid mites (Acari) from Sri Lanka. Acta Zoologica Hungarica, 34: 171-189.
- Ermilov S.G., Kontschán J. 2022. A new species of Carabodes (Klapperiches) (Acari, Oribatida, Carabodidae) from Malawi, with a key to known species of the subgenus from the Afrotropical region. Persian Journal of Acarology, 11: 387-395. https://doi.org/10.11158/saa.27.9.4
- Ermilov S.G., Rybalov L.B. 2024a. Faunistic and taxonomic data on oribatid mites (Acari, Oribatida) from Central Ethiopia. Acarina, 32: 3-11. https://doi.org/10.21684/0132-8077-2024-32-1-3-11
- Ermilov S.G., Rybalov L.B. 2024b. New faunistic and taxonomic data on oribatid mites (Acari, Oribatida) from the Gambela region, Western Ethiopia. Persian Journal of Acarology, 13: 395-407. https://doi.org/10.11158/saa.30.5.2
- Ermilov S.G., Tolstikov A.V. 2015. A new species of Austrocarabodes (Austrocarabodes) from Brazil, including keys to known species of the subgenus from the Neotropical Region and to the agressor-group (Acari, Oribatida, Carabodidae). Neotropical Entomology, 44: 264-269. https://doi.org/10.1007/s13744-015-0283-8
- Ermilov S.G., Rybalov L.B., Kuzmicheva E.A. 2024. New species and record of oribatid mites (Acari, Oribatida) from highlands bamboo location in Ethiopia. Systematic and Applied Acarology, 29: 1297-1309. https://doi.org/10.11158/saa.29.9.7
- Ermilov S.G., Sidorchuk E.A., Rybalov L.B. 2010. Two new species of oribatid mites of the family Carabodidae from Ethiopia (Acari: Oribatida). Genus, 21, 659-671. https://doi.org/10.11646/zootaxa.2646.1.3
- Ermilov S.G., Sidorchuk E.A., Rybalov L.B. 2012a. Oribatid mites (Acari: Oribatida) of Ethiopia. Zootaxa, 3208: 27-40. https://doi.org/10.11646/zootaxa.3208.1.2
- Ermilov S.G., Winchester N.N., Lowman M.M., Wassie A. 2012b. Two new species of oribatid mites (Acari: Oribatida) from Ethiopia, including a key to species of Pilobatella. Systematic and Applied Acarology, 17: 301-317. https://doi.org/10.11158/saa.17.3.9
- Fernandez N., Theron P., Leiva S. 2016. Revision of the family Carabodidae (Acari, Oribatida) V. Fourth part. Two new species of the genus Congocepheus from the Republic of Rwanda: Congocepheus rwandensis sp. n., and Congocepheus kayoveae sp. n. ZooKeys, 556: 19-41. https://doi.org/10.3897/zookeys.556.7011
- Fernandez N., Theron P., Leiva S., Jordaan A. 2017. Revision of the family Carabodidae (Acari: Oribatida) V. Redescription of Congocepheus latilamellatus Mahunka, 1984, with complementary studies of C. ornatus, Mahunka, 1983. Descriptions of Tanzaniacepheus gen. nov. and Zimbabwecepheus gen. nov. Zootaxa, 4324: 315-330. https://doi.org/10.11646/zootaxa.4324.2.5
- Fernandez N., Theron P., Rollard C., Castillo E.R. 2014. Revision of the family Carabodidae V (third part). Redefinition of Congocepheus, definition of Cavaecarabodes gen. n., and descriptions of three new species, Congocepheus germani sp. n., Cavaecarabodes pulchritude gen. n., sp. n., and Cavaecarabodes anouchkae gen. nov., sp. nov. International Journal of Acarology, 40: 535-555. https://doi.org/10.1080/01647954.2014.959050
- Koch C.L. 1835. Deutschlands Crustaceen, Myriapoden und Arachniden. Regensburg, Heft, 1-3.
- Kulijev K.A. 1968. New species and subspecies of oribatid mites from the forests of Azerbaijan. Uchenye zapiski Azerbaijanskogo gosudarstvennogo universiteta. Series Biology, 2: 84-101. [In Russian]
- Kulijev K.A. 1977. Flexa Kulijev nov. gen., with the type species Carabodes dubius Kulijev, 1968, family Carabodidae C.L. Koch, 1837. Doklady Academii Nauk Azerbaijanskoy SSR, 33: 64-69. [In Russian]
- Mahunka S. 1978. Neue und interessante Milben aus dem Genfer Museum XXIV. First contribution to the fauna of the Dominican Republic (Acari: Oribatida). Redia, 61: 551-564.
- Mahunka S. 1983a. Oribatids from the Eastern Part of the Ethiopian Region II. Acta Zoologica Academiae Scientiarum Hungaricae, 29: 151-180.
- Mahunka S. 1983b. Oribatids from the Eastern Part of the Ethiopian Region (Acari) III. Acta Zoologica Academiae Scientiarum Hungaricae, 29: 397-440.
- Mahunka S. 1986. A survey of the family Carabodidae C.L. Koch, 1836 (Acari: Oribatida). Acta Zoologica Hungarica, 32: 73-135.
- Norton R.A. 1977. A review of F. Grandjean's system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae. In: Dindal D.L. (Ed.). Biology of Oribatid Mites. Syracuse: SUNY College of Environmental Science and Forestry. pp. 33-61.
- Subías L.S. 2004. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (1758-2002). Graellsia, 60: 3-305. https://doi.org/10.3989/graellsia.2004.v60.iExtra.218
- Subías L.S. 2019. Nuevas adiciones al listado mundial de ácaros oribátidos (Acari, Oribatida) (14ª actualización). Revista Ibérica de Aracnología, 34: 76-80.
- Subías L.S. 2022. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles). Monografías Electrónicas Sociedad Entomológica Aragonesa, 12: 1-538.
- Subías L.S. 2024. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles), 19ª actualización, 1-545. Available from: http://bba.bioucm.es/cont/docs/RO_1.pdf (accessed January 2024).
- Subías L.S., Shtanchaeva U.Ya. 2023. Claves de familias, géneros y subgéneros de ácaros oribátidos del mundo (Acari, Oribatida). Monografías Electrónicas Sociedad Entomológica Aragonesa, 13: 1-290.
- Travé J., Vachon M. 1975. François Grandjean. 1882-1975 (Notice biographique & bibliographique). Acarologia, 17: 1-19.



2025-02-09
Date accepted:
2025-05-23
Date published:
2025-05-27
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Ermilov, Sergey G. and Rybalov, Leonid B.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



