A catalogue of spider mite (Prostigmata: Tetranychidae) fauna associated with agricultural ecosystems of Kerala, South India with a taxonomic key
Bhaskar, Haseena  1
; Mohan, Melvin
1
; Mohan, Melvin  2
; Gouthami, Desavath
2
; Gouthami, Desavath  3
; Swathi, Penuballi
3
; Swathi, Penuballi  4
; Poulose, Amal
4
; Poulose, Amal  5
; Sreelakshmi, Udaya Kumar
5
; Sreelakshmi, Udaya Kumar  6
; Gowda, Channegowda Chinnamade
6
; Gowda, Channegowda Chinnamade  7
; Vidya, Chengaloor Venugopalan
7
; Vidya, Chengaloor Venugopalan  8
and Mathew, Deepu
8
and Mathew, Deepu  9
9
1✉ All India Network Project on Agricultural Acarology, College of Agriculture, Kerala Agricultural University, Vellanikkara, Thrissur, Kerala - 680656, India.
2All India Network Project on Agricultural Acarology, College of Agriculture, Kerala Agricultural University, Vellanikkara, Thrissur, Kerala - 680656, India.
3Department of Agricultural Entomology, College of Agriculture, Kerala Agricultural University, Vellanikkara, Thrissur, Kerala - 680656, India.
4Department of Agricultural Entomology, College of Agriculture, Kerala Agricultural University, Vellanikkara, Thrissur, Kerala - 680656, India.
5All India Network Project on Agricultural Acarology, College of Agriculture, Kerala Agricultural University, Vellanikkara, Thrissur, Kerala - 680656, India.
6All India Network Project on Agricultural Acarology, College of Agriculture, Kerala Agricultural University, Vellanikkara, Thrissur, Kerala - 680656, India.
7All India Network Project on Agricultural Acarology, University of Agricultural Sciences, GKVK, Bengaluru, Karnataka - 560065, India.
8Department of Agricultural Entomology, College of Agriculture, Kerala Agricultural University, Vellanikkara, Thrissur, Kerala - 680656, India.
9Department of Biotechnology, College of Agriculture, Kerala Agricultural University, Vellanikkara, Thrissur, Kerala - 680656, India.
2025 - Volume: 65 Issue: 2 pages: 534-546
https://doi.org/10.24349/sx3u-ymwhOriginal research
Keywords
Abstract
Introduction
Spider mites (Acari: Tetranychidae) are distributed worldwide with about 1,358 described species (Migeon and Dorkeld, 2025). Globally, spider mites are known as very important pests in the agricultural production (Vacante, 2015). They are known for their capability to damage a variety of plants, including field crops, vegetables, fruits, ornamental plants, and medicinal and aromatic plants. Their short life cycle, polyphagous nature, and small size complicate management efforts at field level. Therefore, studying their diversity is critical for understanding their ecological roles, identifying invasive species, and developing effective management strategies.
The identification of spider mite species is particularly challenging due to their relatively small size, phenotypic plasticity, and unique morphological features, which complicate differentiation compared to the other arthropods with more distinct traits (Ros and Breeuwer, 2007). Accurate species identification requires both male and female specimens in spider mites, unlike many other organisms that can be identified from a single specimen (Majeed et al. 2022). In India, the first extensive documentation of spider mites were conducted by Gupta (1976) who reported 83 species under 18 genera. This was followed by further documentation of 100 species from 20 genera (Gupta, 1991) and a revision that reported 101 species in 1994 (Gupta & Gupta 1994). A recent compilation of the checklist of spider mite fauna in the country recorded 135 spider mite species (Tetranychidae) belonging to two subfamilies, six tribes and 20 genera (Chalil et al. 2024).
Studies on spider mite fauna on the vegetable crop ecosystems of Thrissur district, central Kerala reported Tetranychus as the predominant phytophagous mite genus in the region (Binisha and Bhaskar, 2013). Later, spider mites were reported as serious pests of vegetable crops grown under protected cultivation in Kerala (Lenin and Bhaskar, 2016). A study conducted in the Kasaragod district of Kerala (the northernmost district of the state) reported seven different species of spider mites infesting different vegetable crops in the region. Recently, eight species of spider mites belonging to three genera viz., Tetranychus truncatus, T. okinawanus (T. gloveri), T. urticae, T. fijiensis, T. neocaledonicus, T. marianae, Eutetranychus orientalis and Oligonychus biharensis were reported on ornamental plants in Thrissur and Ernakulam districts of Kerala. These mites were reported from fourteen different host plants namely, rose, marigold, chrysanthemum, balsam, cock's comb, gerbera, adenium, bauhinia, cairo morning glory, orchid (Vanda sp.), zinnia, cassia, crape jasmine and pinto peanut (Prakash et al. 2022).
Though the above studies were not carried out over a wide area covering the entire state, new host records have been reported for some mite species. Recently, several new pest problems involving spider mites have emerged on tissue culture banana saplings and tapioca in the state (Swathi and Bhaskar, 2023; Bhaskar et al. 2024). Considering the geography of Kerala bounded on one side with the biodiversity rich Western Ghats, the spider mite fauna in the state is believed to be more diverse. However, a comprehensive study on spider mite fauna covering various agro-ecological zones of Kerala is lacking, highlighting the significance of this study.
Materials and methods
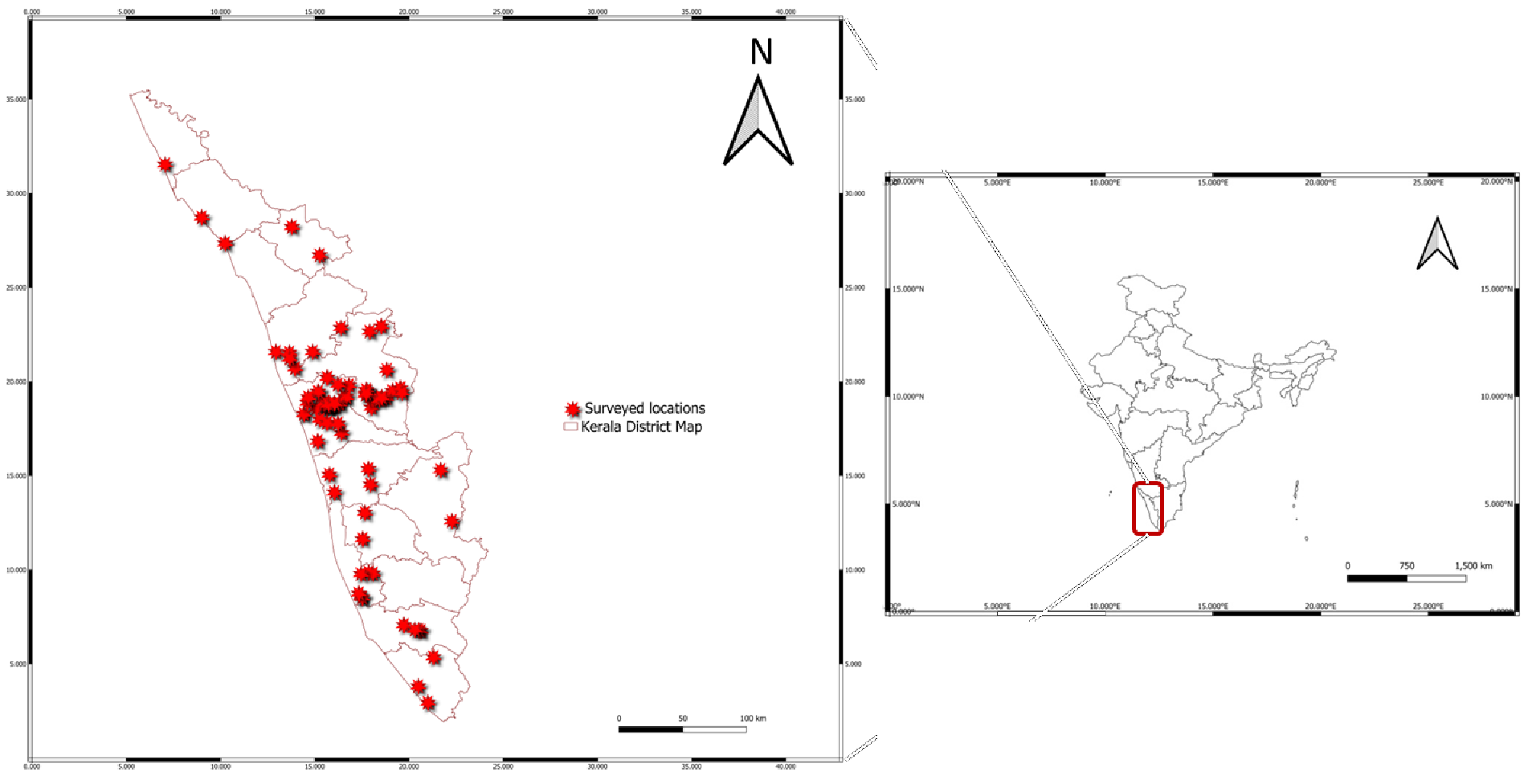
Purposive sampling surveys were conducted from 2021 to 2024 across 126 geographic locations in different agricultural ecosystems covering different districts across Kerala, to collect spider mites on field and plantation crops, ornamental, medicinal, aromatic and wild plants (Figure 1). Mite samples were collected from the randomly selected host plants showing obvious symptoms of mite infestation, from each location. Mite infested leaf samples were placed separately in sealed polythene bags, labelled, and brought to the laboratory for species identification. In the laboratory, a single gravid female mite from each sample was released onto mulberry leaf placed on wet sponge in plastic tray, to establish a pure line culture (isoline culture). Each culture was then assigned a unique accession number. Male (dorsal and lateral orientation) and female mites (dorsal orientation) from each accession were slide mounted on Hoyer's medium under a stereo binocular microscope (Leica ES2) to prepare permanent slides. Slide mounted specimens were examined under a phase contrast research microscope (RADICAL, RXLr-4) equipped with image analysis software, to study the taxonomic characters. For genus level identification, female mite characters such as chaetotaxy of the hysterosoma, number and position of duplex setae on tarsus I and the structure of empodium were studied. For species level identification, the characters such as chaetotaxy of tibia I and tarsus I, pattern of dorsal striae between dorsocentral hysterosomal setae in female and the structure of aedeagus and structure of terminal sensillum of pedipalp in male specimens were studied. Available literature sources and relevant taxonomic keys were used for species level identification (Pritchard and Baker, 1955; Baker and Pritchard, 1960; Gupta, 1985; Meyer, 1992; Gupta and Gupta, 1994; Ehara, 1995; Ehara, 1999; Bolland et al. 1998; Srinivasa et al. 2012; Zeity et al. 2016 and Migeon and Dorkeld, 2025). Taxonomic keys were prepared for the identification of genera and species of Tetranychidae collected during the study.


To study the host association of spider mites, the host plants on which each species was collected were recorded. The expertise of the scientists in the Department of Floriculture as well as All India Coordinated Research Project (AICRP) on Medicinal Plants in the College of Agriculture was sought for identification of those ornamental and medicinal plants which could not be identified at species level by the authors. Similarly, wild plants (mostly weeds) were identified by the Scientists in the AICRP on Weeds, Department of Agronomy. For identification, either the field photographs or the excised shoot with foliage and inflorescence of the host plants were used. The plant names are in accordance with the reference database World Flora Online (WFO, 2025).
Results
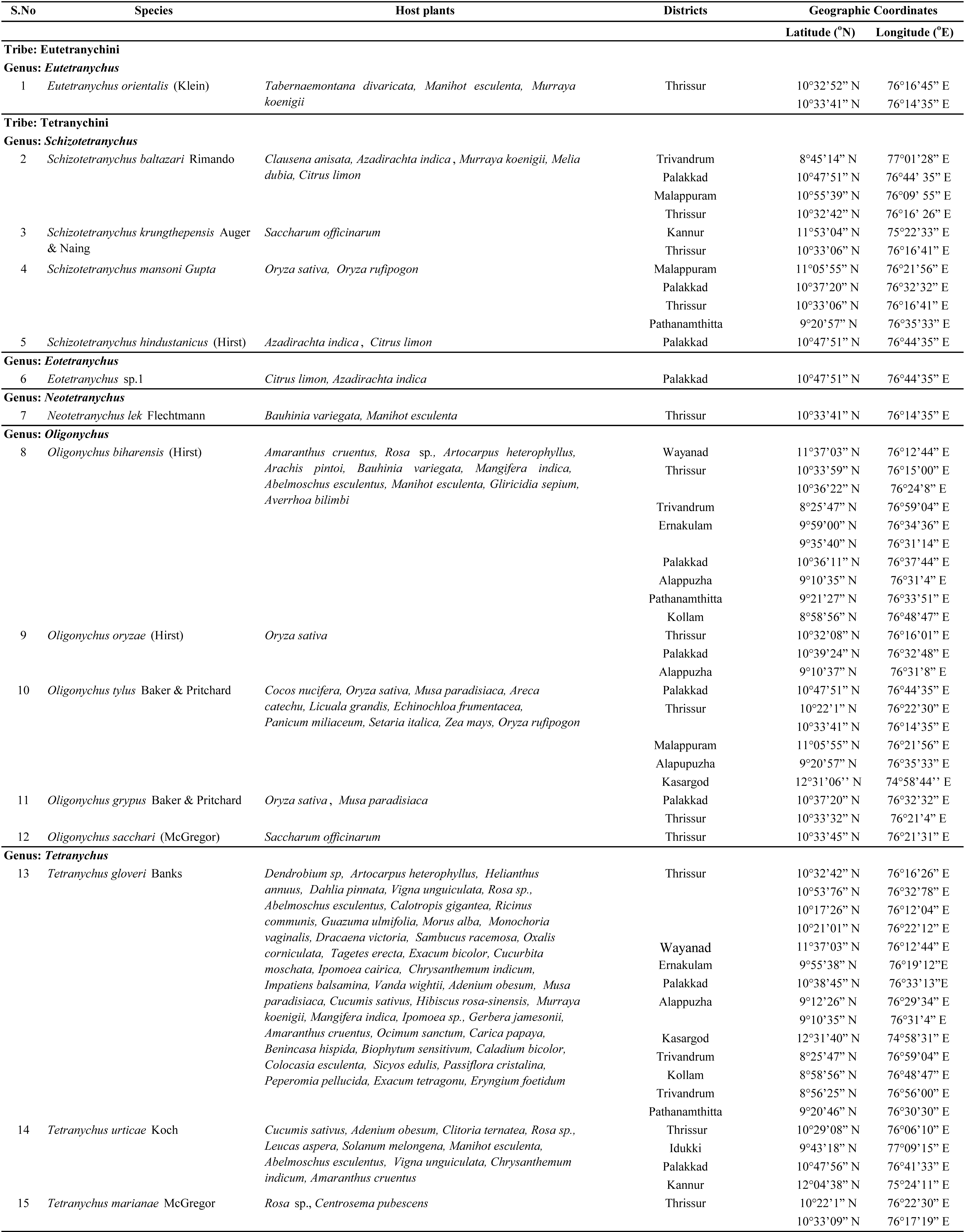


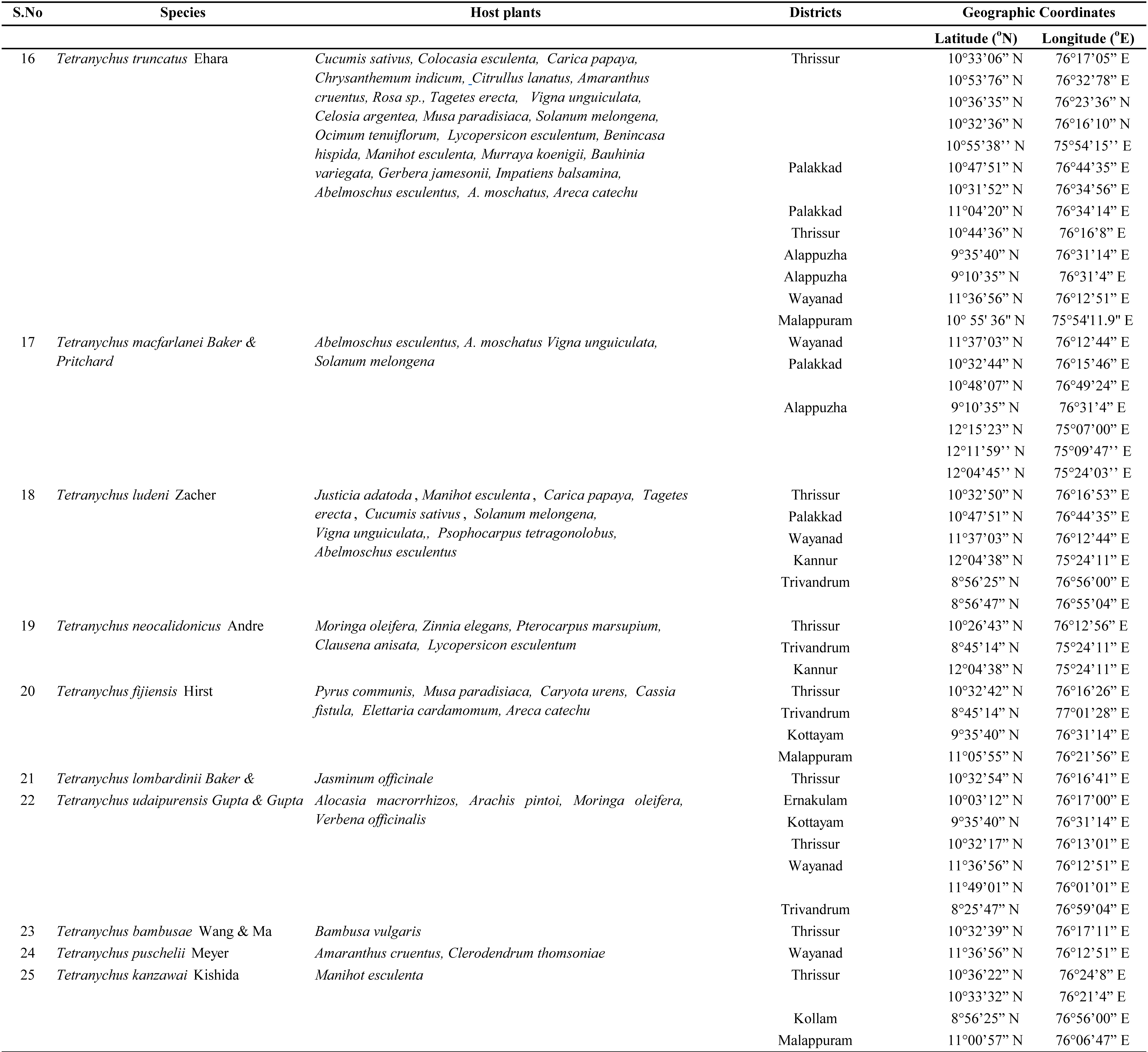
A total of 354 accessions of spider mites were collected from 126 geographic locations from 88 host plants, across different districts of Kerala during the study. The collection represented 25 species in a single subfamily Tetranychinae, under two tribes viz., Eurytetranychini and Tetranychini. Eurytetranychini was represented by only one species in the genus, Eutetranychus. Tetranychini was represented by 25 species within six genera: Tetranychus, Oligonychus, Schizotetranychus, Neotetranychus, Eutetranychus and Eotetranychus. The genus Tetranychus was diverse with 13 species viz., T. gloveri, T. truncatus, T. urticae, T. macfarlanei, T. ludeni, T. neocalidonicus, T. udaipurensis, T. marianae, T. fijiensis, T. lombardini, T. puschelli, T. bambusae and T. kanzawai. Four species of the genus Schizotetranychus recorded include: S. baltazari, S. hindustanicus, S. krungthipensis, and S. mansoni. The genus Oligonychus was represented by five species: O. oryzae, O. tylus, O. biharensis, O. grypus, and O. sacchari. The genera Eotetranychus, Eutetranychus and Neotetranychus were each represented by a single species. The spider mite species recorded in the study along with their host plants are furnished in Table 1.
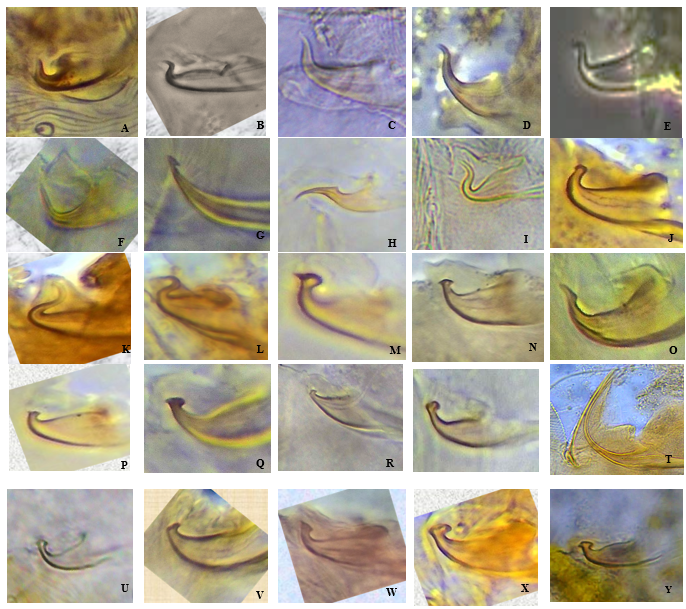
Taxonomic key for the identification of the tribes, genera and species of spider mites in agricultural ecosystems of Kerala is presented here. The structure of aedeagus of different species studied is furnished in Figure 2.


Key to the tribe and genera of Tetranychinae from Kerala
1a. Tarsus I dorsally with 0-1 set of duplex setae; empodium rudimentary
...... Eurytetranychini Reck; aedeagus hook like, dorsal margin of shaft slightly concave, aedeagus knob bluntly pointed without any projections (Fig. 2A) — E. orientalis (Klein)
1b. Tarsus I dorsally with 2 sets of duplex setae; empodium distinct
...... Tetranychini Reck — 2
2a. Para-anal setae 2 pairs (h2 and h3)
...... 3
2b. Para-anal setae 1 pair (h2)
...... 5
3a. Empodium split bilaterally into 2 claw-like structures, usually with appendent hairs
...... Schizotetranychus Trägårdh
3b. Empodium split distally into hairs
...... 4
4a. Dorsal body setae twice as long as the distance between bases of consecutive setae and set on tubercles
...... Neotetranychus Trägårdh; aedeagus knob axis at an acute angle with the shaft, knob with an acute anterior angulation, width of knob about twice the width of aedeagus neck (Fig. 2G) — N. lek Flechtmann
4b. Dorsal body setae at least as long as the distance between bases of consecutive setae, not borne on tubercles
...... Eotetranychus Oudemans; distal bent portion of aedeagus longer than the width of neck, knob pointed distally without projections (Fig. 2F) — Eotetranychus sp.1
5a. Tarsus I with two sets of duplex setae distal and adjacent; empodium of legs claw like with proximo ventral hairs
...... Oligonychus Berlese
5b. Tarsus I with two sets of duplex setae well separated, dividing segment into 3 more or less equal parts; empodium of legs claw like, splits distally into 3 pairs of hairs
...... Tetranychus Dufour
Key to the species of Schizotetranychus
1a. Dorso hysterosomal setae longer than longitudinal distance between bases of consecutive pair of setae; distal portion of aedeagus turns dorsal to form a sigmoid distal end, tip slightly hooked (Fig. 2E)
...... S. hindustanicus (Hirst)
1b. Dorso hysterosomal setae shorter or approximately as long as the longitudinal distance between bases of consecutive pair of setae; aedeagus not as above
...... 2
2a. Tibia I with 8 tactile and 2 sensory setae; distal end of peritreme bulb like; aedeagus dorsally directed but not sigmoid (Fig. 2D)
...... S. mansoni Gupta
2b. Tibia I with 7 tactile and 1 sensory seta; distal end of peritreme hooked
...... 3
3a. Dorsal striae between third pair of dorso central hysterosomal setae longitudinal; aedeagus bent dorsad and dorsal margin of the tip pointed posteriorly at an angle (Fig. 2B)
...... S. baltazari Rimando
3b. Dorsal striae between third pair of dorso central hysterosomal setae transverse; aedeagus bent dorsad at right angle with shaft dorsal margin, with slender distal part, slightly curved caudad (Fig. 2C)
...... S. krungthepensis Auger & Naing
Key to the species of Oligonychus
1a. Male aedeagus with distinct knob
...... 2
1b. Male aedeagus without distinct knob
...... 3
2a. Aedeagus with knob very prominent, curved downwards with long slender tapered posterior projection and flat dorsal margin (Fig. 2H)
...... O. biharensis (Hirst)
2b. Aedeagus with knob small, spear like with broad base; with dorsal margin of shaft forming almost right angle to anterior margin of upturned part (Fig. 2J)
...... O. tylus Baker and Pritchard
3a. Dorsally directed aedeagal part longer, strongly angulate medially, with long and slender posterior projection (Fig. 2K)
...... O. grypus Pritchard and Baker
3b. Dorsally directed aedeagal part comparatively short, weakly angulate medially, with small posterior projection forming slender, blunt or finger like tip
...... 4
4a. Palp with terminal sensillum about thrice as long as wide and dorsal sensillum slender; dorsal idiosomal setae slightly longer than the interval between their longitudinal base; dorsal projection of aedeagus narrow, finger-like, projecting posteriorly (Fig. 2I)
...... O. oryzae (Hirst)
4b. Palpus with terminal sensillum two times as long as wide and slender, dorsal sensillum slender; dorsal idiosomal setae twice longer than the interval between their longitudinal bases; aedeagus with distal part strongly sigmoid; upturned part recurved distally, forming strong and comparatively thick downturned tip (Fig. 2L)
...... O. sacchari (McGregor)
Keys to species of Tetranychus
1a. Spur on empodium I of female stout, 2/3rd the length of proximoventral hairs; aedeagus knob forming an acute angle with the shaft, posterior angulation acute (Fig. 2W)
...... T. bambusae Wang & Ma
1b. Spur on empodium I when present short, never exceeding 1/3rd the length of proximoventral hairs; aedeagus not as above
...... 2
2a. Aedeagus long, slender, tapering; without a distinct terminal knob (Fig. 2T)
...... T. fijiensis Hirst
2b. Aedeagus not long, slender and tapering; with a terminal knob
...... 3
3a. Female with proximal set of duplex setae more or less in line with the proximal tactile setae
...... 4
3b. Female with proximal set of duplex setae well beyond the proximal four tactile setae
...... 5
4a. Aedeagal knob with very small anterior and posterior projections; empodium II of male with proximoventral tridigitate spurs (Fig. 2Q)
...... T. macfarlanei Baker & Pritchard
4b. Aedeagal knob with acute anterior projection and without posterior projections; empodium II of male with 3 pairs of proximoventral hairs and with a small distinct medio dorsal spur (Fig. 2R)
...... T. ludeni Zacher
5a. Aedeagus with a tiny knob; knob with slight anterior and posterior angulations (Fig. 2V)
...... T. udaipurensis Gupta & Gupta
5b. Aedeagus with a well-developed knob; knob not as above
...... 6
6a. Dorsum of aedeagal knob with median indentation, berry like
...... 7
6b. Dorsum of aedeagal knob slightly rounded without median indentation, not berry like
...... 8
7a. Aedeagal neck not conspicuous, shaft shorter, anterior projection of the knob rounded and much bigger than the posterior projection (Fig. 2X)
...... T. puschelii Meyer
7b. Aedeagal neck conspicuous, shaft longer, anterior and posterior projections of the knob subequal (Fig. 2S)
...... T. neocaledonicus André
8a. Female empodium with strong mediodorsal spur; female terminal sensillum slightly longer than wide; aedeagal knob looks like a bird's head with posterior projection beak like (Fig. 2M)
...... T. gloveri Banks
8b. Female empodium with mediodorsal spur inconspicuous or absent, female terminal sensillum approximately twice as long as wide; aedeagal knob not as above
...... 9
9a. Dorsal surface of aedeagal knob at an angle to the axis of the shaft
...... 10
9b. Dorsal surface of knob parallel to the axis of the shaft
...... 11
10a. Posterior angulation of knob elongated, width of the knob more than twice the width of the aedeagal neck (Fig. 2O)
...... T. marianae McGregor
10b. Posteror angulation of knob shorter, width of the knob one and a half times the width of the aedeagus neck (Fig. 2U)
...... T. lombardinii Baker & Pritchard
11a. Dorsal surface of the aedeagal knob not convex completely, at least posterior half of the knob with a median indentation (Fig. 2P)
...... T. truncatus Ehara
11b. Dorsal surface of the aedeagal knob convex or most part of it is
...... 12
12a. Terminal sensillum of male palpus about 3-4 times as long as broad, aedeagal knob about 1/5th to 1/4th the length of the dorsal margin of the shaft (Fig. 2W)
...... T. urticae Koch
12b. Terminal sensillum of male palpus about 3 times as long as broad, aedeagus knob about 1/3rd the length of the dorsal margin of the shaft (Fig. 2Y)
...... T. kanzawai Kishida
Host range of spider mite fauna in Kerala
In this study, spider mites have been recorded on 83 different host plants. Tetranychus gloveri was recorded on the widest host range of 44 host plants in 28 plant families (Table 1; Figure 3). This mite was distributed in eight districts covering northern, central and southern regions: Kasargod, Wayanad, Malappuram, Thrissur, Palakkad, Ernakulam, Alappuzha, Kollam, Pathanamthitta and Thiruvananthapuram districts of Kerala. Tetranychus truncatus was recorded on 23 host plants followed by T. urticae (11 hosts), Oligonychus tylus and O. biharensis (10 hosts each), T. ludeni (9 hosts), T. fijiensis (6 hosts), T. neocalidonychus and Schizotetranychus baltazari (5 hosts each), T. udaipurensis and T. macfarlanei (4 hosts each), Eutetranychus orientalis (3 hosts), Tetranychus marianae, T. puschelii, Oligonychus grypus, Schizotetranychus mansoni, S. hindustanicus, Eotetranychus sp. 1, and Neotetranychus lek (2 hosts each). All other species recorded only single host each.
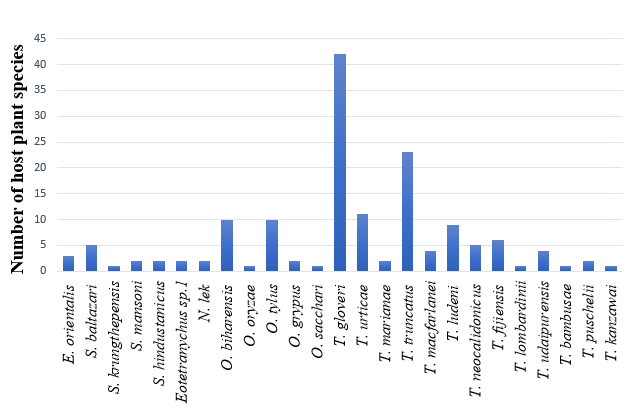


Discussion
From the 25 species of spider mites recorded in the study, many were found to be serious pests of field crops and ornamental plants in Kerala. The most predominant mite species was T. gloveri, followed by T. truncatus and T. urticae, which recorded wider host range compared to other species.
Tetranychus gloveri was first recorded in India on Adenium obesum from Kerala, which was then reported as Tetranychus okinawanus Ehara (Zeity et al. 2016). However, recently, Sharkey et al. (2022) reported T. okinawanus as a junior synonym of T. gloveri. Subsequently, it emerged as a predominant pest of major agricultural and horticultural crops in Kerala (Arunima et al. 2018; Prakash et al. 2022). This study reports 21 new host plants for this mite species as: orchid (Dendrobium sp.); jackfruit (Artocarpus heterophyllus); West Indian elm (Guazuma ulmifolia); curry leaf (Murraya koenigii), country kreat (Exacum bicolor), persian violet (Exacum tetragonum), sunflower (Helianthus annuus), victoria corn plant (Dracaena victoria), creeping wood sorrel (Oxalis corniculata) pumpkin (Cucumis moschata), red amaranth (Amaranthus cruentus), giant milk weed (Calotropis gigantean), little tree plant (Biophytum sensitivum), holy basil (Ocimum sanctum), aquatic pickerel weed (Monochoria vaginalis), angelwings (Caladium bicolor), betel (Piper betle), pepper elder (Peperomia pellucida), chowchow (Sicyos edulis), passion vine (Passiflora cristalina) and spiny coriander (Eryngium foetidum). Hence, it can be inferred that T. gloveri has established and spread across Kerala by widening its host range and expanding its geographical area of distribution.
Tetranychus truncatus has a broad distribution, with records on 104 host plants in 34 plant families. It was first recorded in India from Jammu and Kashmir and Himachal Pradesh on Dahlia sp. (Rather, 1983) and later, from Karnataka on mulberry (Srinivasa et al. 2012). In Kerala, it was recorded on cowpea, amaranthus, and cucumber (Bennur et al., 2015) under open cultivation and cucumber and amaranthus under protected cultivation (Lenin and Bhaskar, 2016). Subsequently, it was identified as a serious banana pest (Bhaskar & Lenin, 2018) and reported on brinjal (Lekha & Kinathi, 2019) as well as ornamental plants, including marigold, cock's comb (Celosia sp.), and rose (Prakash et al., 2022). This study adds new host records: tomato (Lycopersicon esculentum), orchid tree (Bauhinia variegata), ash gourd (Benincasa hispida), and holy basil (Ocimum tenuiflorum).
Tetranychus urticae is the most polyphagous spider mite species with 1577 different host plants species from the 132 different plant families, worldwide (Migeon and Dorkeld, 2025). It is very interesting that our study recorded only 11 different host plants for T. urticae. Thumba (Leucas aspera) and desert rose (Adenium obesum) are new hosts recorded for T. urticae in the study.
Neotetranychus lek is reported for the first time from India, while S. mansoni for the first time from south India. Flechtmann (2013) described N. lek based on specimens collected on cassava from Thailand. No further record was made on the mite species. In our study the species was collected on cassava and bauhinia. Schizotetranychus mansoni was described by Gupta (1980) from the specimens collected on paddy from south Andaman Island. In the Indian mainland though it was reported from the state of Arunachal Pradesh, the host plant was not mentioned (Gupta, 1991). In this study, brownbeard rice (Oryza rufipogon) is reported as new host for S. mansoni.
Oligonychus grypus, O. tylus, O. sacchari and Tetranychus bambusae are new distribution records for Kerala. Oligonychus grypus was earlier recorded from India on rice from Tamil Nadu (Sivakumar and Kunchithapatham, 2014) and from Karnataka on Apluda mutica and Pennisutum glaucum (Majeed et al. 2022). Oligonychus tylus was recorded earlier in India on coconut from Tamil Nadu (Dhyamanagouda et al. 2021), on date palm from Gujarat (Muralidharan et al. 2020) and on banana and arecanut from Karnataka (Majeed et al. 2022). Rice, arecanut, the ruffled fan palm (Licuala grandis), proso millet (Panicum miliaceum), barnyard millet (Echinochloa esculenta) and foxtail millet (Setaria italica) are reported here as new host plants for the mite species. Oligonychus sacchari was earlier reported in India from West Bengal (Gupta 1992) and from Tamil Nadu (Gupta and Gupta, 1994). Tetranychus bambusae was reported in India earlier from Karnataka on Bambusa bambos by Zeity et al. (2016). In this study, it was collected on Bambusa vulgaris, which is a new host plant from India.
This study records three new host plants for T. udaipurensis viz., Alocasia macrorrhizos (Araceae), Arachis pintoi (Arachidaceae), and Verbena officinalis (Verbenaceae). Horsewood (Clausina anisata) and Malabar neem (Melia dubia) are new hosts recorded for S. baltazari. In Kerala, T. udaipurensis was earlier reported on okra, cassava and banana (Arunima, 2017), while S. baltazari is a major pest of curry leaf (Nilamudheen, 2021).
Other new host records made in the study are: red amaranth (Amaranthus cruentus), okra, (Abelmoschus esculentus), Mexican lilac (Gliricidia sepium) and bilimbi (Averrhoa bilimbi) for Oligonychus biharensis; red amaranth (A. cruentus) and bleeding heart vine (Clerodendrum thomsoniae) for T. puschelii; Malabar kino (Pterocarpus marsupium) and horsewood (Clausena anisata) for T. neocalidonychus; banana (Musa paradisiaca) and jaggery palm (Caryota urens) for Tetranychus fijiensis; winged bean (Psophocarpus tetragonolobus) for T. ludeni and cassava (Manihot esculenta) for E. orientalis.
Oligonychus oryzae and T. kanzawai are serious pests of rice and cassava, respectively in Kerala (Bhaskar and Thomas, 2011; Bhaskar et al. 2024). Schizotetranychus krungthepensis was collected in the study only on sugarcane. It was first described by Naing et al. (2014) from specimens collected on sugarcane from Thailand. This exotic mite pest was later reported from different states of India including Kerala on sugarcane (Mahesh et al. 2021; Poojar et al. 2021), which is now considered as an emerging threat to sugarcane crop in India.
The results of the study indicates that the fauna of spider mites in Kerala is more diverse than documented till date. Two exotic mite pest species, T. truncatus and T. gloveri, reported recently in the state have established and turned invasive by expanding the geographical area of distribution and widening their host range. However, the most polyphagous spider mite species T. urticae recorded only fewer number of host plants in Kerala, compared to the above two species. The study also reports new geographical distribution and host records of spider mite species for the country, India and the state, Kerala.
Conflict of interest statement
The authors have no conflicts of interest to declare in relation to the subject matter of this paper.
Acknowledgements
The authors extend sincere thanks to Science and Engineering Research Board (SERB), Government of India for funding the project and All India Network Project on Agricultural Acarology (AINPAA) for providing facilities to conduct the work.
References
- Arunima V. 2017. DNA barcoding of spider mites (Prostigmata: Tetranychidae) on major crop plants of Kerala [M.Sc (Ag) thesis]. KAU: Thrissur. pp. 59.
- Arunima V., Bhaskar H., Abida P.S., Shylaja, M.R. 2018. Tetranychus okinawanus Ehara (Prostigmata: Tetranychidae) emerging as a potential invasive pest in Kerala, India. Abstract Book. In XV International Congress of Acarology; September 2-8, 2018. Antalya, Turkey, pp.72.
- Baker E.W., Pritchard A. E. 1960. The tetranychoid mites of Africa. p. 455-574. https://doi.org/10.3733/hilg.v29n11p455
- Bennur S., Abida P.S., Valsala P.A., Mathew D., Bhaskar, H. 2015. DNA barcoding of spider mites (Prostigmata: Tetranychidae) in vegetables using COI and ITS2 markers. In Genome 65 Auriga DR, Suite 203, Ottawa, on K2E 7W6, Canada: Canadian Science Publishing, NRC Research Press. 58 (5): pp. 195-195.
- Bhaskar H., Lenin N. 2018. Management of banana leaf mite, Tetranychus truncatus (Prostigmata: Tetranychidae), a new pest of banana. Book of Abstracts, National Symposium on Entomology. Pp. 1-9.
- Bhaskar H., Swathi P., Gowda C., Mohan M., Miraj A.S., Venugopalan, V.C., Mathew D. 2024. New Record of Tetranychus kanzawai (Acari: Tetranychidae) on Cassava in India. J. Entomol. Sci., 60 (1): 154-156. https://doi.org/10.18474/JES24-04
- Bhaskar H., Thomas J. 2011. Sporadic incidence of paddy leaf mite Oligonychus oryzae (Tetranychidae: Prostigmata) in Palakkad, Kerala. Insect Environ, 17 (2): 55-56.
- Binisha K.V., Bhaskar H. 2013. Mite fauna associated with major vegetable crops of Thrissur district, Kerala. Entomon, 38 (1): 47-52. https://entomon.in/index.php/Entomon/article/view/20
- Bolland H.R., Gutierrez J., Flechtmann C.H.W. 1998. World catalogue of the spider mite family (Acari: Tetranychidae). Brill, Netherlands. pp. 392.
- Chalil S.P., Kunnathattil M., Kaimal S.G., Punathil T. 2024. A checklist of spider mites (Acari: Tetranychidae) of India. Persian J. Acarol., 13 (1): 29-75. https://doi.org/10.22073/pja.v13i1.78022
- Dyamanagouda P., Vishnupriya, R., Gowda, C.C. 2022. Two new records of spider mites (Acari: Tetranychidae) with new host plant from Coimbatore district, Tamil Nadu, India. Persian J. Acarol., 11 (1): 153-157. https://doi.org/10.22073/pja.v11i1.70382
- Ehara S. 1995. A New Species of Tetranychus (Acari, Tetranychidae) from the Ryukyu Islands. Japan. J. Entomol., 63 (1): 229-233.
- Ehara S. 1999. Revision of the spider mite family Tetranychidae of Japan (Acari, Prostigmata). Species diversity, 4 (1): 63-141. https://doi.org/10.12782/specdiv.4.63
- Flechtmann C.H. 2013. A new species of Neotetranychus Trägårdh (Acari: Prostigmata: Tetranychidae) from Thailand with a key to world species. Persn. J. Acarol., 2 (1). https://doi.org/10.22073/pja.v2i1.9947
- Gupta S.K. 1976. Contribution to our knowledge of tetranychid mites (Acarina) with descriptions of three new species from India. Orient. Insects, 10: 327-351. https://doi.org/10.1080/00305316.1976.10432332
- Gupta S.K. 1985. Hand book of Plant mites of India. Zoological Survey of India, Calcutta, pp. 520.
- Gupta S.K. 1991. The mites of agricultural importance in India with remarks on their economic status. In: Modern Acarology: Proceedings of the VIII International Congress of Acarology, Dusbábek, F. and V. Bukva (Eds.) Ceske Budejovice, Czechoslovakia, 6-11 August, SPB Academic Publishing: The Hague. 1: 509-522.
- Gupta S.K., Gupta Y.N. 1994. A taxonomic review of Indian Tetranychidae (Acari: Prostigmata) with description of new species and redescriptions of known species, keys to genera and species. Memoirs Zool. Survey India. 18: 1-196.
- Gupta Y.N. 1980. Some spider mites (Acarina: Tetranychidae) from Andaman and Nicobar Islands with descriptions of three new species. Records of the Zoological Survey of India. pp. 111-117. https://doi.org/10.26515/rzsi/v77/i1-4/1979/161847
- Gupta. 1992. Arachnida: plant mites (Acari). Zoological Survey of India, State Fauna Series 3: Fauna of West Bengal, Part 3: pp. 61-211.
- Lekha K., Kinathi P. 2019. First report of Tetranychus truncatus on brinjal in Kerala. Indian J. Entomol., 81 (3): 456-457.
- Lenin J.A., Bhaskar H. 2016. Mite pests of vegetable crops under protected cultivation in Kerala. Entomon, 41 (4): 218-224. https://doi.org/10.33307/entomon.v41i4.218
- Mahesh P., Srikanth J., Mahendran B., Chandran K., Singaravelu B., Salin, K.P. 2021. Occurrence of the exotic mite Schizotetranychus krungthepensis (Acarina: Tetranychidae) in sugarcane germplasm in India. Crop Prot., 144: 105556. https://doi.org/10.1016/j.cropro.2021.105556
- Majeed S.A.A., Rajashekharappa K., Srinivasa N., Hegde J.N., Chinnamadegowda C.C. 2022. Diversity and Abundance of Spider Mites and Associated Predatory Mites of Shivamogga Region. Biol. Forum - An Int. J., 14 (2): 1303-1307.
- Migeon A., Dorkeld F. 2025. Spider Mites Web: a comprehensive database for the Tetranychidae. [18 January 2025]. Available from: https://www1.montpellier.inrae.fr/CBGP/spmweb
- Muralidharan C.M., Baidiyavadra D.A., Sharma K.M., Srinivasa, N. 2020. First incidence of a spider mite, Oligonychus tylus (Baker & Pritchard), in date palm (Phoenix dactylifera L.) groves of Kachchh in Gujarat, India. J. Plantation Crops, 48 (2): 137-41. https://doi.org/10.25081/jpc.2020.v48.i2.6373
- Naing H.H., Chandrapatya A., Navajas M., Auger P. 2014. Know more about spider mites (Acari: Tetranychidae) in Myanmar. Proceedings of 90th YAU (Yezin Agricultural University) Conference held at: Yezin, Nay Pyi Taw, Myanmar. pp. 257-275.
- Nilamudeen M. 2021. The occurrence of Citrus green mite, Schizotetranychus baltazari Rimando (Acari: Tetranychidae) on curry leaf (Murraya koeningii F: Rutaceae) in Kerala. Insect Environ., 24 (2): 247-248.
- Poojar D., Ramakrishnan V., Gowda, C. 2021. Two new records of spider mites (Acari: Tetranychidae) with new host plant from Coimbatore district, Tamil Nadu, India. Persian J. Acarol., 11 (1): 153-157. https://doi.org/10.22073/pja.v11i1.70382
- Prakash J., Bhaskar H., Mathew D., Vidya C.V., Gowda C.C., Shylaja, M.R. 2022. Diversity of spider mites (Tetranychidae) on ornamental plants in Central Kerala Indian J. Entomol., 84 (1): 82-87. https://doi.org/10.55446/IJE.2021.255
- Pritchard A.E., Baker E.W. 1955. A revision of the spider mite family Tetranychidae. Memoirs Series, San Francisco, Pacific Coast Entomological Society. pp. 472. https://doi.org/10.5962/bhl.title.150852
- Rather A.Q. 1983. New records of five genera and eighteen species of phytophagous mites (Acarina) from India with notes on their host range, distribution and economic importance. In: Abstracts of 2nd All India Symposium on Acarology, Pune, pp. 25-26.
- Ros V.I.D., Breeuwer J.A.J. 2007. Spider mite (Acari: Tetranychidae) mitochondrial COI phylogeny reviewed: host plant relationships, phylo-geography, reproductive parasites and barcoding. Exp. Appl. Acarol., 42 (4): 239-262. https://doi.org/10.1007/s10493-007-9092-z
- Sharkey E.R., Beaulieu F., Moore M.R., Bolton S.J. 2022. Morphological and molecular data reveal the conspecificity of the spider mites Tetranychus gloveri and T. okinawanus (Acari: Trombidiformes: Tetranychidae). Syst. Appl. Acarol., 27 (2): 250-268. https://doi.org/10.11158/saa.27.2.7
- Sivakumar K., Kunchithapatham R. 2014. Phytophagous mite diversity of Tetranychus spp. and Oligonychus sp. (Acari: Tetranychidae) found on different host plants in Coimbatore district and surrounding regions. Trends Biosci., 7 (24): 4113-4117.
- Meyer M.K.P.S. 1992. Four new species of Bryobia Koch (Acari: Tetranychidae) from South Africa, with a revised key to the African species. Phytophylactica, 24 (1): 1-8.
- Srinivasa N., Gowda C.C., Mallik B., Raghavendra P. 2012. New record of Tetranychus truncatus Ehara (Acari: Tetranychidae) as a potential pest from Karnataka. Indian J. Entomol., 74: 379-383.
- Swathi P., Bhaskar H. 2023. Tetranychus gloveri (Acari: Tetranychidea): an emerging threat to tissue culture banana plantlets in nurseries. Pest Manage. Hortic Ecosyst., 29 (2): 299-301. https://doi.org/10.5859.0974-4543.2023.00046.7
- Vacante V. 2015. The handbook of mites of economic plants: identification, bio-ecology and control, CABI, Croydon, UK. pp. 1-865. https://doi.org/10.1079/9781845939946.0001
- WFO 2025. World Flora Online. Accessed on: [19 May 2025]. Available from https://www.worldfloraonline.org.
- Zeity M., Srinivasa N., Gowda C.C. 2016. New species, new records and re-description of spider mites (Acari: Tetranychidae) from India. Zootaxa. 4085 (3): 416-430. https://doi.org/10.11646/zootaxa.4085.3.5



2025-02-06
Date accepted:
2025-05-22
Date published:
2025-05-26
Edited by:
Migeon, Alain

This work is licensed under a Creative Commons Attribution 4.0 International License
2025 Bhaskar, Haseena; Mohan, Melvin; Gouthami, Desavath; Swathi, Penuballi; Poulose, Amal; Sreelakshmi, Udaya Kumar; Gowda, Channegowda Chinnamade; Vidya, Chengaloor Venugopalan and Mathew, Deepu
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)