Mite species of kiwi vines in Türkiye
Akyazı, Rana  1
; Soysal, Mete
1
; Soysal, Mete  2
and Ueckermann, Edward A.
2
and Ueckermann, Edward A.  3
3
1✉ Ordu University, Faculty of Agriculture, Plant Protection Department, Ordu, Türkiye.
2Ordu University, Faculty of Agriculture, Plant Protection Department, Ordu, Türkiye.
3Unit for Environmental Sciences and Management, Potchefstroom Campus, North-West University, Private Bag X6001, Potchefstroom, 2520, South Africa.
2024 - Volume: 64 Issue: 4 pages: 1030-1051
https://doi.org/10.24349/9lvs-4bzyOriginal research
Keywords
Abstract
Introduction
Kiwi is the common name for fruits acquired from the hybridization of Actinidia deliciosa (A. Chev.) C. F. Liang & A. R. Ferguson (Actinidiaceae) and other Actinidia species (Gökdoğan 2022). The kiwi was originally cultivated in China, and due to its delicious taste, various health benefits, and versatile usage, it has gained popularity worldwide. As a result, many countries have started producing and exporting kiwis, with China, Italy, and New Zealand leading the way in production. Türkiye is ranked 7th (FAO 2022) among the top countries in kiwi production. A total of 89.000 tons of kiwi are produced annually on 23.941,709 hectares in Türkiye (TUIK 2023).
Kiwi cultivation in Türkiye started later than in other countries. In the 1990s, the Atatürk Central Horticultural Research Institute in Yalova initiated kiwifruit cultivation (Atak 2015). Over time, the profitability of kiwifruit in Türkiye has led to an increase in production and cultivation every year (Atak 2018).
Kiwifruit production in Türkiye is spread across different regions, with 56% of the production areas located in the Black Sea Region. The Marmara Region follows with 41% while the Mediterranean and Aegean Regions have a combined share of 3% (Alp 2017). Ordu province (Black Sea Region, Türkiye) is the seventh-largest producer of kiwifruit in Türkiye, with a production of 5,469 tons (TUIK 2023).
Kiwi is a highly beneficial fruit for human health due to its high content of vitamin C, proteins, and mineral salts. Its juice has been found to prevent cancer. Moreover, kiwi can be used to treat asthma and coughs, as it helps to improve breathing. Additionally, consuming kiwi can increase resistance to winter illnesses (Gökdoğan 2022). Additionally, the growth in kiwi exports has also contributed significantly to the national economy, reaching almost 800 million Turkish Liras in 2022 (TRG 2021).
Despite its countless benefits and popularity, few studies have been conducted on mite species found in kiwifruit vines. Lay-Yee and Whiting (1996), Chandurkar (2003), CKC and CMCC Report (2003), Maughan and Black (2015), and Satyagopal et al. (2015) stated that Tetrancychus urticae Koch (Trombidiformes: Tetranychidae) is an important pest mite species on Kiwifruit. According to Steven et al. (1997), Brevipalpus chilensis Baker (Trobidiformes: Tenuipalpidae) is the most prevalent mite species on Kiwifruit in Chile. The researchers also identified three species of spider mites, two species of tydeid mites, and unidentified phytoseiid, stigmaeid, bdellid, rhaphignathid, Oribatei and Czenspinskiinae mites on the plant. Childers et al. (2013) found a Brevipalpus sp. on kiwi leaves at the Kearney Research and Extension Center in Parlier, California. Han et al. (2020) identified a new mite species, Leipothrix argutae Han, Wang & Ai (Trombidiformes: Eriophyidae) in China (Jilin province) on the hardy kiwi, Actinidia arguta (Siebold & Zucc.) Planch. ex Miq. (Actinidiaceae). Tshikhudo et al. (2021) investigated South Africa's import of kiwifruit (Actinidia spp.) as a pathway for the introduction of mites in the genus Brevipalpus from 2009 to 2019. They noted the presence of B. chilensis, Brevipalpus cf. hondurani Evans, Brevipalpus obovatus Donnadieu, Brevipalpus lewisi McGregor (Trombidiformes: Tenuipalpidae) and an undescribed Brevipalpus species (Brevipalpus sp. nov.). Recently, Saccaggi and Ueckermann (2024) reported that the unknown Brevipalpus sp. on imported kiwis is B. gramani Baker. In Türkiye, just Gençer Gökçe et al. (2022) found a predatory mite, Phytoseius finitimus Ribaga (Parasitiformes: Phytoseiidae) on Actinidia chinensis Planch. (Actinidiaceae) in Tekirdağ. To our knowledge, a detailed survey of the mite fauna associated with kiwi vines has not been conducted in Türkiye or anywhere else in the world, despite the expanding kiwifruit industry worldwide. So, the overall goal of this work was to determine leaf-inhabiting mite species in kiwi vines in Türkiye.
Material and methods
Sampling areas
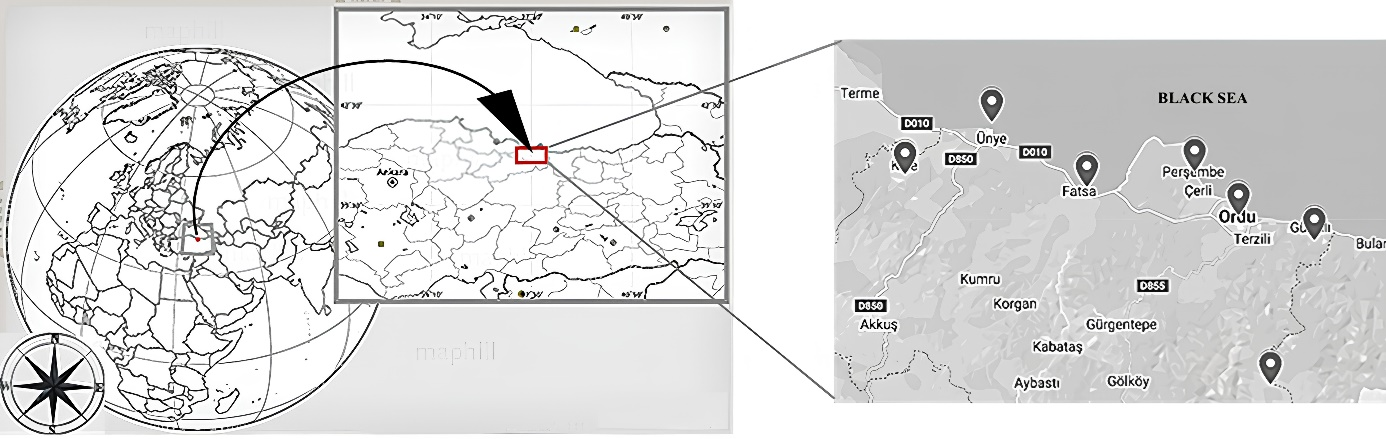
The surveys were conducted in kiwi vines in seven different areas in Ordu, Black Sea Region, Türkiye where kiwis are cultivated: Altınordu, Fatsa, Gülyalı, İkizce, Kabadüz, Perşembe, and Ünye (Figure 1). The sampling site's geographical coordinates were recorded using a GPS device (Garmin Etrex 30).


Survey studies
Samplings were carried out from June to November in 2018 and 2019. Leaf samples were collected at an interval of 15-20 days. Leaves were sampled from the lower, middle, and upper parts of the plant canopy on each sampling date. The samples were placed in labeled paper bags, then enclosed in plastic bags and transferred to the laboratory.
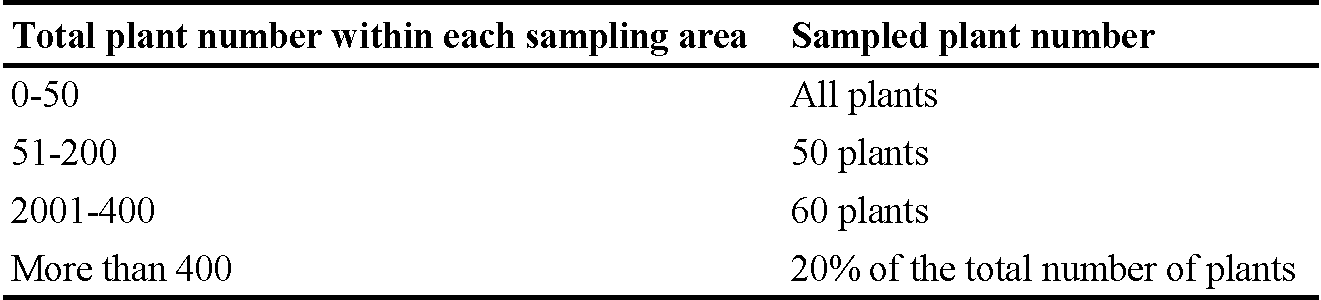
The number of plants sampled per kiwi vine was determined based on the total number of plants in each sampling kiwi vine (Table 1). Approximately 20 leaves were collected from each plant.


Identification of mite specimens
Mites were collected from upper and lower leaf surfaces under a stereomicroscope (Leica S8 APO, Heerbrugg, Switzerland) using a fine sable brush (N: 5/0). Specimens were kept in Eppendorf tubes filled with 70% ethanol for preservation purposes. All mites were cleared in lactophenol solution, mounted in Hoyer's medium, and dried at 50 °C for 7-10 days (Krantz and Walter 2009). The mites were identified at the species level using appropriate identification keys such as Zhang (2000) for the family Tarsonemidae, Çobanoğlu et al. (2016) and Mesa et al. (2019) for the family Tenuipalpidae, Migeon and Dorkeld (2024) for the family Tetranychidae, Volgin (1987) and Yeşilayer and Çobanoğlu (2012) for the family Cheyletidae, Tseng (1982) and Gonzalez-Rodriguez (1965) for the family Stigmaeidae, Ueckermann et al. (2019) for the family Iolinidae, Ueckermann et al. (2019) and Da Silva et al. (2016) for the family Tydeidae, Döker et al. (2016), Akyazı et al. (2016b), Bas et al. (2022) and Denmark and Evans (2019) for the family Phytoseiidae. The mite species were identified using a light microscope equipped with a phase-contrast feature (Leica DM 2500, Heerbrugg, Switzerland). Confirmation of species identification and some identifications were made by the third author at North-West University, South Africa.
Results and discussion
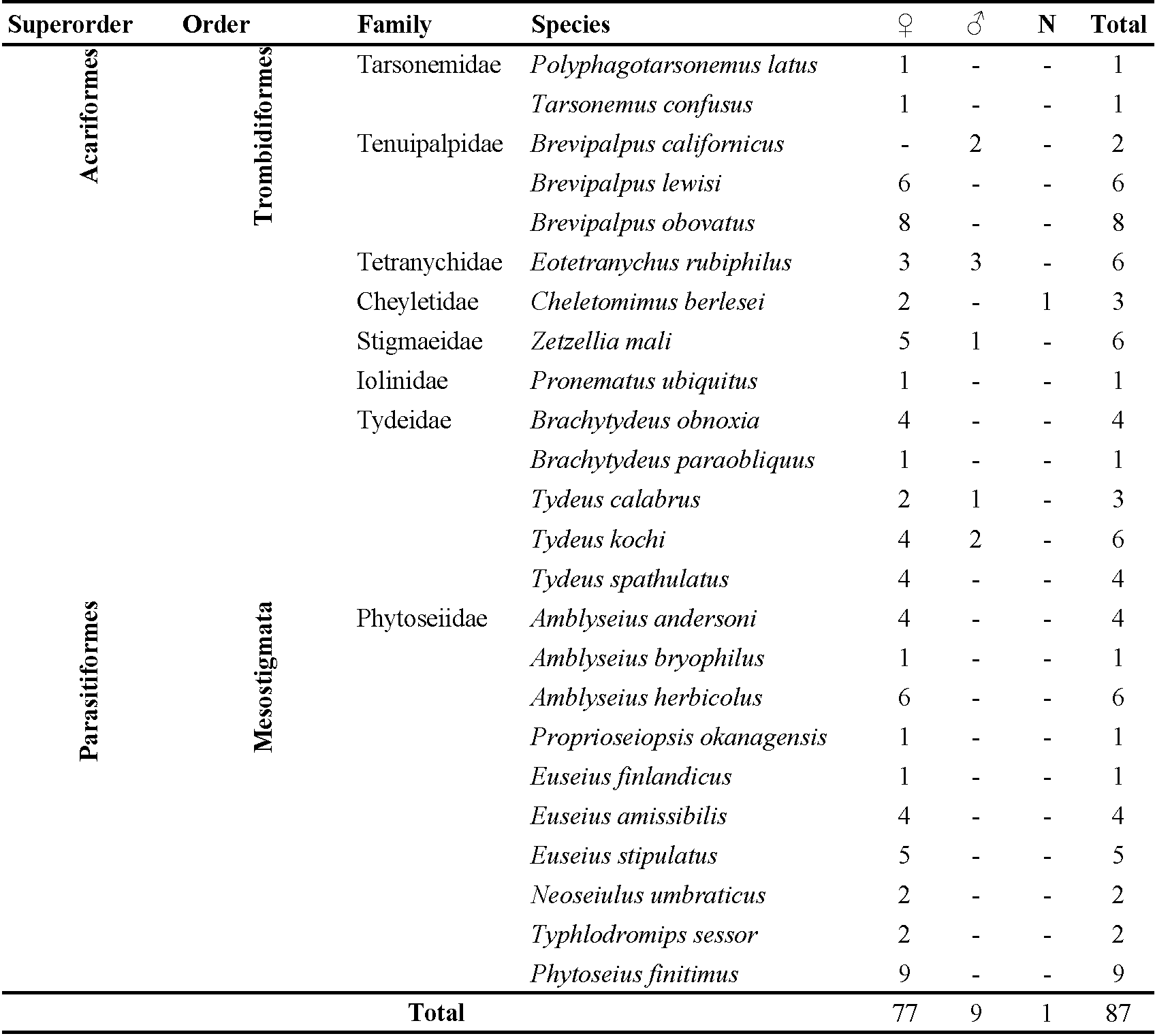
In the study conducted in Kiwi vines located in Tukey's Black Sea region (Ordu), 24 species of mites were identified from 2 orders, 8 families, and 15 genera during the years 2018-2019 as listed in Table 2. The number of species of the eight families: Phytoseiidae 10, Tydeidae 5, Tenuipalpidae 3, Tarsonemidae 2, Cheyletidae 1, Iolinidae 1, Stigmaeidae 1 and, Tetranychidae 1.


A comprehensive survey on mite species in kiwi vines worldwide has not been conducted. Additionally, there is no available information on the damage symptoms caused by pest mite species on kiwi plants. Therefore, the study discusses the injury symptoms of detected pest mite species on various host plants. Additionally, only B. lewisi and B. obovatus in all determined species have been identified on imported kiwi fruit in previous studies. None of the other species have been previously detected in kiwi vines, so other hosts/habitats of determined species were discussed.
Order Trombidiformes
Superfamily Tarsonemoidea
Family Tarsonemidae Canestrini & Fanzago 1877
Polyphagotarsonemus latus (Banks 1904)
Material examined — 1 ♀ (Ünye, N41°5′3.21″ E37°11′12.07″, 241 m, 18.VII.2018)
Remarks — Banks (1904) first described this species as Tarsonemus latus from mango buds in Washington D.C., USA (Denmark 1980). This phytophagous mite is distributed worldwide (Fasulo 2004). It has been reported in various regions including Australia, Asia, Africa, Europe, North and South America, and the Pacific Islands. The species is commonly found in tropical and subtropical regions but can also occur in temperate areas. It is a globally significant agricultural pest with the ability to feed on plants from over 60 botanical families including Solanaceae, Cucurbitaceae, and Malvaceae (Grinberg et al. 2005; Ovando-Garay 2022). In Türkiye, it was first recorded from citrus and Morus alba L. (Moraceae) leaves in Antalya by Çobanoğlu (1995). It was also collected from vineyards, mulberry, cotton, rubber, peach (Uygun et al. 1995), vegetables (Uygun et al. 1995; Bulut and Göçmen 2000; Can and Çobanoğlu 2010; Soysal and Akyazı 2018), tea plantations (Özman Sullivan et al. 2006, 2007; Diler et al. 2022) and persimmon (Akyazı et al. 2016a, 2017). This pest targets the young parts of plants and can inject toxic saliva into their tissues. Although there is no record of its damage symptoms on kiwi, feeding by this pest causes twisted, hardened, and distorted growth in the terminal tips of a plant. Damaged leaves turn coppery in color and curl. Heavily infested plants will drop their leaves, fail to flower, and produce bronze, cracked, or blemished fruits. Since these mites are microscopic, they can only be detected when the symptoms become visible (Akyazı et al. 2022a). Due to its high reproductive potential, it can quickly reach damaging densities (CABI 2021). Before 1992, no specific natural enemies of P. latus were known. Gerson (1992) first summarized the control of P. latus by predatory mites belonging to Amblyseius, Euseius, and Typhlodromus species on various crops worldwide. Among the phytoseiid mites found in this study, Amblyseius herbicolus (Chant) (Mesostigmata: Phytoseiidae) was found as associated with P. latus (Luypaert 2015).
Tarsonemus confusus Ewing 1939
Material examined — 1 ♀ (Altınordu, N40°56′48.96″ E37°59′45.86″, 20 m, 02.IX.2019)
Remarks — Tarsonemus confusus was first reported on Delphinium belladonna (Ranunculaceae) by Ewing (1939). It is a cosmopolitan species and has been recorded on various continents including North America (USA; Canada), South America (Brazil), Europe (Türkiye, Portugal, Italy, Ireland, Germany, Poland, Belarus, Ukraine, Russia), East Asia (Japan, Korea, China), and Africa (Egypt) (Zhang 2003; Santos 2011; Andrade-Bertolo et al. 2013; Akyazı et al. 2021). This species has been found worldwide on many plants, in soil, filters, house dust, and bird's nests (Zhang 2003; Demite et al. 2022). In Türkiye, this mite was first identified on Pyracantha coccinea Roem (Rosaceae) in Edirne (Çobanoğlu 1995). Later, it was collected from many other plants, such as Cucumis sativus L. (Cucurbitaceae), Solanum esculentum L., Capsicum annuum L., Solanum nigrum L. (Solanaceae), Convolvulus arvensis L. (Convolvulaceae) Stellaria media L. (Caryophyllaceae), Amaranthus retroflexus L. (Amaranthaceae), Chenopodium album L. (Amaranthaceae) (Tokkamış 2011), S. esculentum (Çobanoğlu and Kumral 2014), Solanum melongena L. (Solanaceae) (Kumral and Çobanoğlu 2015), persimmon (Akyazı et al. 2017), vegetables (Soysal and Akyazı 2018), and pome fruits (Akyol and Akyazı 2022). This species has been found in both thelytokous and sexual populations (Lindquist 1986; Wrensch and Ebbert 1993; Hernandes and Feres 2006). Tarsonemus confusus was declared primarily a fungivorous mite (Garga et al. 1997; Zhang 2003). In addition, it is considered a minor pest for some ornamentals in greenhouses in Europe and tomatoes in North America (Zhang 2003). However, Zhang (2003) noted that T. confusus can cause significant damage to plants when its population is high. Recently, it has become an economically important pest in orchards in China (Li et al. 2022). Although no reports regarding its damage to kiwi fruit have been identified, Wang et al. (1999) suggested that it is a major contributor to black dot symptoms on apple and peach fruit. Further evidence by Hao et al. (2007, 2010) also indicates that symptoms on bagged apples may be attributed to T. confusus (Li et al. 2022). It was also found strongly associated with core rot diseases in apple orchards (Michailides et al. 1994; Van der Walt et al. 2011; Li et al. 2022). Li et al. (2018) demonstrated that Neoseiulus barkeri Hughes (Mesostigmata: Phytoseiidae) has the potential to be a highly effective biological control agent for T. confusus. There is no record of its relationship with the predatory mite detected in the current study.
Superfamily Tetranychoidea Donnadieu 1876
Family Tenuipalpidae Berlese 1913
Brevipalpus californicus (Banks 1904)
(Probably group of species Brevipalpus californicus, Beard et al. 2015)
Material examined — 2 ♂♂: 1 ♂ (Altınordu, N40°57′1.99″ E38°0′15.43″, 13 m, 02.IX.2019), 1 ♂ (İkizce, N41°2′58.80″ E37°4′23.71″, 147 m, 31.VII.2019)
Remarks — Beard et al. (2015) identified 4 morpho-type groups in this species that are still due for further examination. According to confirmed specimens, this species was detected in Australia, Ecuador, Italy, Mexico, Nepal, Palestine, South Africa, Spain, Sri Lanka, The Philippines, USA (MD). It was collected from Abies sp. (Pinaceae), Begonia sp. (Bignoniaceae); Chirimoya sp. (Annonaceae); Choisya ternate Kunth, Citrus sp. Citrus aurtantifolium (Christm.), Citrus nobilis Lour. (Rutaceae), coconut Cocos nucifera L. (Areaceae); Gardenia sp. (Rubiaceae); lemon (Rutaceae); orchids (Orchidaceae) and tea Camelia sinensis L. (Theaceae) in these countries (Beard et al. 2015). In Türkiye, it was collected from Philadelphus coronarius L. (Hydrangeaceae) in Erzurum by Çobanoğlu et al. (2020). Brevipalpus species can damage plants by causing symptoms such as chlorosis, blistering, bronzing, or necrotic areas at high population densities. Their most significant impact is the ability to transmit plant viruses (Tshikhudo et al. 2021). Although there is no record of B. californicus's damage to kiwi, it is an economically important Brevipalpus species that feed on twigs, petioles, leaf surfaces, and developing buds of seedlings. Its feeding causes silvering of affected tissue and brown speckling of orange rinds. Due to feeding, Grevilla robust Cunningham's leaves become chlorotic, turn brown and drop prematurely (Childers et al. 2003). Phytoseiulus persimilis Athias-Henriot, Amblyseius largoensis (Muma), Euseius scutalis (Athias-Henriot), Neoseiulus cucumeris (Oudemans), Proprioseiopsis sp. (Mesostigmata: Phytoseiidae) and Cheletomimus sp. (Trombidiformes: Cheyletidae) were given among natural enemies associated with B. californisus by Cortez- Mondaca et al. (2022). Among them, Proprioseiopsis okanagensis (Chant) (Mesostigmata: Phytoseiidae) was found in kiwifruit orchards in the current study.
Brevipalpus lewisi McGregor 1949
Material examined — 6 ♀♀: 3 ♀♀ (Altınordu, N40°56′31.25″ E37°58′59.40″, 58 m, 02.IX.2019), 3 ♀♀ (Gülyalı, N40°58′16.97″ E37°59′52.57″, 7 m, 02.IX.2019)
Remarks — Brevipalpus lewisi, a phytophagous mite, was recorded in Australia, China, Egypt, Georgia, Greece, Hungary, India, Iran, Israel, Italy, Japon, Mexico, Portugal, Saudi Arabia, South Africa, South Korea, Spain, Taiwan, Tunisia, Türkiye, Ukraine and USA (Alaska, Arizona, California). It was found on various plants, mainly belonging to the families Rutaceae, Vitaceae, Rosaceae, and Oleaceae from around the world (Beard et al. 2015; Castro et al. 2024). In Türkiye, it was collected from Aegean vineyards (Bayram and Çobanoğlu 2007; Göven et al. 2009), S. melongena, Phaseolus vulgaris L. (Fabaceae) and C. annuum in Ordu (Soysal and Akyazı 2018) and Campis radicans Seem. (Bignoniaceae) in Tekirdağ (Gençer Gökçe et al. 2022). This mite is a major problem for oranges, tangerines, and lemons in California, USA, and Japan. The population of this pest reaches its peak during the warmest months. Mites feeding on fruit create scab-like scars that can cover most of the fruit, reducing its quality. The pest caused severe leaf chlorosis in ''blue Serbian'' vineyards in Israel. It is a pest of citrus, grapes, and pomegranates in Greece. However, it is currently unknown whether it can transmit viruses (Ueckermann et al. 2018). Brevipalpus lewisi was detected on kiwifruit imported to South Africa from Greece and Italy (Tshikhudo et al. 2021). Homeopronematus anconai (Baker) (Prostigmata: Iolinidae), E. scutalis, Metaseiulus (Metaseiulus) flumenis (Chant) (mentioned as Typhlodromus flumenis Chant), Typhlodormus reticulatus Oudemans (Mesostigmata: Phytoseiidae) were given as natural enemies of B. californicus, but none of them were found in the Kiwi vines in the present study (CABI, 2019).
Brevipalpus obovatus Donnadieu 1875
Material examined — 8 ♀♀: 2 ♀♀ (Fatsa, N41°0′23.60″ E37°31′20.10″, 15 m, 06.VIII.2018), 1 ♀ (Fatsa, N41°0′56.71″ E37°31′27.23″, 7 m, 06.VIII.2018), 1 ♀ (Fatsa, N40°55′57.68″ E37°37′14.16″, 372 m, 06.VIII.2018), 1 ♀ (Perşembe, N41°1′12.14″ E37°49′28.42″, 6 m, 26.VII.2018), 3 ♀♀ (Ünye, N41°4′11.40″ E37°13′35.34″, 281 m, 18.VII.2018)
Remarks — Brevipalpus obovatus was found on a wide range of hosts worldwide (Beard et al. 2015; Castro et al. 2024). In Türkiye, the species was first discovered on lemon trees in Mersin by Düzgüneş (1952). Later, it was found on various plants such as Citrus limonium L. (Rutaceae), Fragaria sp. (Rosaceae), Hedera helix L. (Araliaceae), ornamental plants, weeds (Düzgüneş 1965, 1977), chestnut (Önuçar and Ulu 1988) Coleus sp. (Lamiaceae), H. helix, Mikania sp. (Asteraceae) and Spathiphyllum wallisii Regel (Araceae) (Bozkurt 1994), tea (Özman- Sullivan et al. 2007), persimmon (Akyazı et al. 2016a, 2017), vegetables (Soysal and Akyazı 2018), and Cornus mas L. (Cornaceae) (Altunç and Akyazı 2019) over the years. This phytophagous mite may cause chlorosis or necrotic areas on citrus leaves. In addition, it is strongly associated with the nuclear citrus leprosis viruses; citrus leprosis virus N (CiLV-N) and citrus necrotic spot virus (CiNSV) in the New World (Ueckerman et al. 2018). The species was detected on just kiwifruit imported to South Africa from New Zealand, China, Greece and Italy (Tshikhudo et al. 2021). It was found in association with the phytoseiids, Amblyseius swirskii Athias & Henriot, Typhlodromus (Anthoseius) recki Wainstein, P. finitimus, E. scutalis, Euseius stipulatus (Athias & Henriot) on solanaceous plants in the Syrian coastal region (Dayoub and Boubou 2023). Among them, P. finitimus and E. stipulatus were detected in Ordu's kiwi vines in the current study.
Family Tetranychidae Donnadieu 1875
Eotetranychus rubiphilus Reck 1948
Material examined — 3 ♀♀, 3 ♂♂: 2 ♀♀ (Kabadüz, N40°49′33.83″ E37°52′43.94″, 698 m, 14.VI.2018), 1 ♀ 3 ♂♂ (Perşembe, N41°4′29.02″ E37°43′52.77″, 455 m, 26.VII.2018)
Remarks — Eotetranychus rubiphilus was first discovered on Rubus sp. (Rosaceae) in Georgia by Reck (1948). So far, this phytophagous mite has been recorded in several Palearctic countries such as Armenia, Azerbaijan, France, Georgia, Italy, Korea, Portugal, the Russian Federation, Serbia, Spain, Syria, Türkiye, and Ukraine. According to Migeon and Dorkeld (2024), it has been found on various host plants worldwide, including those from the families Brassicaceae, Geraniaceae, Rosaceae, and Vitaceae. In Türkiye, it was first reported on Prunus domestica L. and Prunus cerasus L. (Rosaceae) in Ordu by Altunç and Akyazı (2020). Little is known about the biology of E. rubiphilus. It was considered as the most important phytophagous mite affecting blackberries, particularly in greenhouses (Naves et al. 2021). Altunç and Akyazı (2020) found this mite along with some predators such as P. finitimus, Euseius finlandicus (Oudemans), Typhlodromus (Typhlodromus) tiliae Oudemans (Mesostigmata; Phytoseiidae), Cunaxoides lootsi Den Heyer & Castro (Trombidiformes: Cunaxidae) and Tydeus californicus (Banks), T. goetzi Schruft (Trombidiformes: Tydeidae) (predator tydeoid mites according to Baker and Wharton 1952; Gerson et al. 2003; Walter and Proctor 2013). Among phytoseiid predators, P. finitimus and E. finlandicus were also found in the kiwi vines of Ordu in the present study.
Superfamily: Cheyletoidea, Leach 1815
Family Cheyletidae, Leach 1815
Cheletomimus (s.str.) berlesei (Oudemans 1904)
Material examined — 2 ♀♀, 1 nymph: 1 ♀, 1 nymph (Altınordu, N40°56′13.73″ E38°0′20.40″, 345 m, 02.IX.2019), 1 ♀ (Gülyalı, N40°56′26.84″ E38°1′12.29″, 312 m, 18.IX.2019)
Remarks — Cheletomimus berlesei is a predatory mite species that has been reported in different parts of the world including Italy, France, Israel, USA, New Zealand, Russia, Iran, and Türkiye (Summer and Price 1970; Volgin 1987; Jalilirad et al. 2013; Salarzehi et al. 2018; Doğan 2022). In Türkiye, this species was first reported on Cedrus atlantica (Endl.) Manetti ex Carrière (Pinaceae), Cupressus arizonica Greene (Cupressaceae), and Pittosporum tobira (Thunb.) W.T.Aiton (Pittosporaceae), C. arizonica, and P. tobira in Istanbul by Yeşilayaer and Çobanoğlu (2012). Later, it was collected from S. melongena (Soysal and Akyazı 2018) and Eriobotrya japonica (Thunberg) Lindley (Rosaceae) (Akyol and Akyazı 2022) in Ordu. It has been declared as a natural enemy of Cenopalpus lineola (Canestrini and Fanzago) (Trombidiformes: Tenuipalpidae) and Hemiberlesia lataniae (Signoret) (Hemiptera: Diaspididae) (CABI 2004).
Superfamily Raphignathoidea Kramer 1877
Family Stigmaeidae Oudemans 1931
Zetzellia mali (Ewing 1917)
Material examined — 5 ♀♀ 1 ♂: 4 ♀, 1 ♂ (Altınordu, N40°54′36.26″ E37°50′22.97″, 140 m, 02.IX.2019), 1 ♀ (Ünye, N41°5′3.21″ E37°11′12.07″, 241 m, 18.VII.2018)
Remarks — Zetzellia mali was originally classified under the genus Caligonus. Later, Ewing (1921) transferred the species to the genus Syncaligus. However, Oudemans (1929) transferred it to the genus Zetzellia (Summers 1960). The species was recorded in Austria, Bulgaria, Canada, China, Czech, France, Germany, Hungary, India, Iran, Italy (including Sardinia), Lebanon, Lithuanian SSR, Moldavia, Netherlands, Poland, Serbia, Slovenia, Spain, Switzerland, Tunisia, Türkiye, UK, USA and Yugoslavia (Fan et al. 2016). In Türkiye, this species was first reported as Mediolata mali (Ewing) (Trombidiformes: Raphignathidae) on apple leaves in Bilecik by Düzgüneş (1963). It has since been recorded from various plants including hazelnut (Samsun), Juglans regia L. (Juglandaceae) (Ankara, Van), ornamental trees and shrubs (Ankara, Istanbul), fruit trees (Van, Bursa, Tokat, Diyarbakır), S. esculentum and S. melongena (Bursa, Ankara) (Akyazı and Ecevit 2003; Çobanoğlu et al. 2003; Kumral 2005; Doğan 2007; Kasap et al. 2007; Kasap and Çobanoğlu 2007; Denizhan and Çobanoğlu 2008, 2009; Sağlam and Çobanoğlu 2010; Yeşilayer and Çobanoğlu 2013; Çobanoğlu and Kumral 2014; Kumral and Çobanoğlu 2015; Miroğlu and Çıkman 2022). In previous studies in Ordu, which is the field of this study, it was collected from various plants such as Diospyros kaki Thunb. (Ebenaceae) (Akyazı et al. 2016a, 2017), P. vulgaris, S. melongena, Solanum lycopersicum L. (Solanaceae), Cucurbita sp. (Cucurbitaceae) (Soysal and Akyazı 2018), Malus domestica Borkhausen, Pyrus communis L., Cydonia oblonga Miller (Rosaceae), E. japonica (Akyol and Akyazı 2022), and Prunus laurocerasus L. (Rosaceae) (Akyazı et al. 2022b). Croft (1994) stated that Z. mali feeds on eggs and immature stages of the European red mite, as well as the active stages of the apple rust mite. Khanjani and Ueckermann (2002) also reported that it prefers to feed on eriophyid mites rather than adult tetranychid mites. Santos (1976) and Clements and Harmsen (1993) found that it has a high reproduction rate on Panonychus ulmi (Koch) (Trombidiformes: Tetranychidae). Denizhan and Çobanoğlu (2009) collected it from eriophyid galls. Kasap and Çobanoğlu (2007) also found it in apple orchards, along with Bryobia rubrioculus Scheuten, Amphitetranychus viennensis (Zacher) (Trombidiformes: Tetranychidae), P. ulmi, tydeid and phytoseiid species. Additionally, Dönel and Doğan (2013) have even collected it from bird nests. Moreover, it is known that Z. mali can feed on the eggs of other predator mites, as mentioned by Kain and Nyrop (1995). Akyol and Akyazı (2022) found that Z. mali and Transeius wainsteini (Gomelauri) (Mesostigmata: Phytoseiidae) had the highest tendency to occur together in a predatory species complex. The pair E. finlandicus- Typhlodromus (Anthoseius) rapidus Wainstein & Arutunjan (Mesostigmata: Phytoseiidae), and T. rapidus-Z. mali also showed a highly positive association.
Superfamily: Tydeoidea Kramer 1877
Family: Iolinidae Pritchard 1956
Pronematus ubiquitus (McGregor 1932)
Material examined — 1 ♀ (Ünye, N41°4′11.40″ E37°13′35.34″, 281 m, 18.VII.2018)
Remarks — Pronematus ubiquitus was originally reported as Tydeus ubiquitus in Lindsay, California from foliage of citrus trees (McGregor 1932). This species is distributed across all continents except for Antarctica. It was found in North America (Acuna-Soto et al. 2017), South America (Sousa et al. 2015), Europe (Vela et al. 2017), Africa (Ueckermann and Grout 2007), Asia (Baradaran and Arabi 2009), and Oceania (Maynard et al. 2018). The following locations in Türkiye have reported the presence of this species on various plants: tomato in İzmir (Yaşarakıncı and Hıncal 1997); vineyards in İzmir, Manisa, and Denizli (Göven et al. 2009); eggplant, melon, and zucchini in Antalya (Can and Çobanoğlu 2010); tomato in Ankara (Çobanoğlu and Kumral 2014) and Bursa (Çobanoğlu and Kumral 2014; Aysan and Kumral 2018), Solanum dulcamara L. (Solanaceae) in Ankara, S. nigrum in Ankara and Bursa (Kumral and Çobanoğlu 2015); pear (Akyol and Akyazı 2022); and P. laurocerasus (Akyazı et al. 2022b) in Ordu. This mite is a predator species that feeds on small prey such as eriophyid and tetranychid mites, as well as plant-based food sources like plant sap, pollen, and fungi (Pijnakker et al. 2022a, b). In addition, it was noted that P. ubiquitus is an effective solution to combat two main problems in tomato crops: Aculops lycopersici (Massee) (Tormbidiformes: Eriophyidae) and powdery mildew (Oidium neolycopersici L. Kiss). It is possible to pre-establish and build up large populations of this mite by supplementing tomato plants with pollen (Duarte et al. 2021; Pijnakker et al. 2022b; Moerkens et al. 2023). Because of its importance as potential bio-control agent of Aculops lycopersici but with morphological variability between specimens of different origins and type material, which is in a bad state, Ueckermann et al. (2024) undertook a thorough revision of this species based on material from the type locality compared with populations of other regions.
Family: Tydeidae Kramer 1877
Brachytydeus obnoxious Kuznetzov et Zapletina 1972
Brachytydeus obnoxia, incorrect subsequent spelling (gender) used by Da Silva et al. (2016: 20)
Brachytydeus obnoxius subsequent combination used by Da Silva et al. (2016: 20) corrected under art. 34.2 of ICZN
Material examined — 4 ♀♀: 1 ♀ (Altınordu, N40°56′33.03″ E37°56′20.31″, 11 m, 02.IX.2019), 2 ♀♀ (Fatsa, N40°58′39.92″ E37°36′41.00″, 178 m, 06.VIII.2018), 1 ♀ (İkizce, N41°2′1.16″ E36°59′18.41″, 605 m, 31.VII.2019), 1 ♀ (İkizce, N41°5′8.42″ E37°1′38.94″, 484 m, 31.VII.2019)
Remarks — Brachytydeus obnoxius was first reported on nuts from Azerbaijan (Livshitz et al. 1972). It was later recorded from a birch tree stand in Poland (Laniecki et al. 2021) and grassy plant leaves in Russia (Khaustov 2023). In Türkiye, this species was found in a survey of hazelnut orchards throughout the growing areas in the Black Sea Region by Özman-Sullivan et al. (2005).
Brachytydeus paraobliquus (Panou & Emmanuel 1996)
Brachytydeus paraobliqua: Incorrect subsequent spelling (gender) used by Tempfli et al. (2015:946) and adopted by Da Silva et al. (2016: 21)
Brachytydeus paraobliquus, subsequent combination used by Tempfli et al. (2015: 946) and adopted by Da Silva et al. (2016: 21) corrected under art. 34.2 of ICZN.
Material examined — 1 ♀ (Ünye, N41°6′18.31″ E37°19′39.58″, 9 m, 18.VII.2018)
Remarks — Brachytydeus paraobliquus was first described by Panou and Emmanouel in 1996 from P. cerasus and Cornus sp. in Greece. In Hungary, Ripka et al. (2002) were the first to identify this species on Tilia tomentosa Moench (Malvaceae) leaves. Later, Tempfli et al. (2015) found it in Hungarian vineyards. In Türkiye, Özman-Sullivan et al. (2005) first reported it as Lorryia paraobliqua in the hazelnut orchards of Samsun. In a recent study in Ordu Province of Türkiye, Akyazı et al. (2017) found the species on D. kaki and Diospyros latus L. (Ebenaceae) leaves and re-described it. They also described the deutonymph and tritonymph for the first time. The species was found on various other plants in Ordu, including P. domestica, Prunus avium L. (Rosaceae), P. cerasus, C. mas (Altunç and Akyazı 2019), M. domestica, P. communis, Cydonia ablonga Mill. (Rosaceae) (Akyol and Akyazı 2022) and P. laurocerasus (Akyazı et al. 2022b).
Tydeus calabrus (Castagnoli 1984)
Material examined — 2 ♀♀ 1 ♂: 1 ♀ (İkizce, N41°5′31.02″ E37°3′0.60″, 525 m, 31.VII.2019), 1 ♀ (İkizce, N41° 0′38.45″ E37°1′36.16″, 527 m, 31.VII.2019), 1 ♂ (Ünye, N41°5′3.21″ E37°11′12.07″, 241 m, 18.VII.2018)
Remarks — Tydeus calabrus is a rare species. Castagnoli (1984) first reported the species from olive trees in Italy. Momen and Lundqvist (1996) collected it from the bark of apple trees in Sweden, while Sadeghi et al. (2012) found this species on citrus trees in Iran. In Türkiye, it was recorded on Fagus orientalis Lipsky (Fagaceae) in Kırklareli and Citrus sp. in İzmir by Çobanoğlu and Kazmierski (1999) and on P. laurocerasus in Ordu by Akyazı et al. (2022b).
Tydeus kochi Oudemans 1928
Material examined — 4 ♀♀ 2 ♂♂: 1 ♀ (Gülyalı, N40°56′54.57″ E38° 2′6.78″, 380 m, 18.IX.2019), 3 ♀♀ 2 ♂♂ (İkizce, N41°5′8.42″ E37°1′38.94″, 484 m, 31.VII.2019)
Remarks — Tydeus kochi has a worldwide distribution. It was found in all types of climatic zones on moss, lichens and Vitis sp. (Tempfli et al. 2015; Da Silva et al. 2016). In Türkiye, the species was found on pome fruits and weeds in Balıkesir and Çanakkale (Kasap et al. 2013), tomato in Ankara and Bursa (Çobanoğlu and Kumral 2014), eggplant in Bursa (Kumral and Çobanoğlu 2015), grape leave litter in Ankara (İnak and Çobanoğlu 2018), Syringa vulgaris L. (Oleaceae) and Robinia pseudoacacia L. (Fabaceae) in Erzurum (Çobanoğlu et al. 2020). Rasmy (1971) identified this species as an important predatory species for the biological control of Citrus Brown mite, Eutetranychus orientalis (Klein) (Trombidiformes: Tetranychidae) in two citrus orchards in Giza and Taheer, Egypt. Aysan and Kumral (2018) found this species to be associated with A. lycopersici on tomato in Türkiye (Bursa).
Tydeus spathulatus Oudemans 1928 sensu Andre (2005)
Material examined — 4 ♀♀. 1 ♀ (Gülyalı, N40°58′37.75″ E37°59′56.64″, 4 m, 02.IX.2019), 1 ♀ (İkizce, N41°2′58.80″ E37°4′23.71″, 147 m, 31.VII.2019), 1 ♀ (İkizce, N41°2′17.67″ E37°3′13.69″, 180 m, 31.VII.2019), 1 ♀ (Perşembe, N41°4′29.02″ E37°43′52.77″, 455 m, 26.VII.2018)
Remarks — It has been observed that some specimens previously identified as Tydeus californicus (Banks 1904) sensu Baker and Wharton (1952), or sensu Baker (1970) might be Tydeus spathulatus Oudemans (Trombidiformes: Tydeidae) (Oudemans 1928; Baker and Wharton 1952; Baker 1970; Andre 2005). Both T. californicus and T. spathulatus have spatulate-fusiform and distally tapered posterior opisthosomal setae (f1, f2, h1, h2, ps1, ps2). There are transverse or subtly bent striae between their setae h1 as well. Moreover, a third species, namely Tydeus laudatus Tseng (Trombidiformes: Tydeidae) is potentially a junior synonym of the above-mentioned two species (Tseng 1985). So, the situation regarding the `californicus' species group is currently unclear and requires further investigation (Ripka et al. 2022). It seems that the Turkish specimens in the current study have a close resemblance to the redescription of T. spathulatus (Andre 2005) in all aspects. This species was first described in Italy (Andre 2005). It was also reported from Hungary (Tempfli et al. 2015) and Russia (Khaustov 2023). In Türkiye, the species was found on M. domestica in Balıkesir (Kasap et al. 2013), and on S. dulcamara in Ankara (Kumral and Çobanoğlu 2015).
Order: Mesostigmata
Family: Phytoseiidae Berlese 1916
Amblyseius andersoni (Chant 1957)
Material examined — 4 ♀♀. 1 ♀ (Fatsa, N40°59′0.25″ E37°25′54.74″, 70 m, 06.VIII.2018), 1 ♀ (Perşembe, N41°4′51.30″ E37°37′50.47″, 28 m, 26.VII.2018), 1 ♀ (Perşembe, N41°4′29.02″ E37°43′52.77″, 455 m, 26.VII.2018), 1 ♀ (Perşembe, N41°0′26.05″ E37°49′22.80″, 6 m, 26.VII.2018)
Remarks — Amblyseius andersoni was first described from prunes in Canada by Chant (1957a). This predatory mite is found in over 30 countries worldwide (Demite et al. 2023). It also has been recorded in different habitats in Türkiye multiple times (Faraji et al. 2011; Çobanoğlu et al. 2020; Döker et al. 2020; Miroğlu and Çıkman 2022). In the current research area, it was previously discovered on persimmon (D. kaki) (Akyazı et al. 2017), vegetables (S. melongena, C. annuum, C. sativus) (Soysal and Akyazı 2018), stone fruits (P. avium, P. domestica, P. persica (Altunç and Akyazı 2019)) and P. laurocerasus (Akyazı et al. 2022b). Amblyseius. andersoni was classified as a Type 3 lifestyle (Subtype III-b) generalist predator living on glabrous leaves (McMurtry et al. 2013). According to Camporese and Duso (1995) and Duso et al. (2003), it was observed to be more abundant and effective as a predator of spider mites on grape varieties with more glabrous leaves (McMurtry et al. 2013). Polyphagotarsonemus latus, found in the current study, was given as a targeted species by A. andersoni (BFG, 2024; Terralink, 2024). Additionally, it has been reported that this predator can feed on fungi (Pozzebon and Duso 2008; McMurtry et al. 2013).
Amblyseius bryophilus Karg 1970
Material examined — 1 ♀ (İkizce, N41° 0′38.45″ E37°1′36.16″, 527 m, 31.VII.2019)
Remarks — Amblyseius bryophilus was first described on moss and humus in Germany by Karg (1970). It has been found in various countries worldwide including France, Hungary, Netherlands, Poland, Serbia, and Türkiye (Demite et al. 2023). In Türkiye, this species was collected from P. vulgaris in Rize province (Döker et al. 2014a, 2020), M. domestica (Akyol and Akyazı 2022) and P. laurocerasus trees (Akyazı et al. 2022b) in Ordu. According to McMurtry et al. (2013), Amblyseius spp. are classified as generalist predators with a Type III lifestyle. Additionally, it was found that most Amblyseius species live on glabrous leaves (subtype III-b), with some species found in soil/litter habitats (subtype III-e). It has also been observed that species with smooth bodies (mostly minute setae and no ornamentation of dorsal shield), such as Amblyseius spp., inhabit smooth leaves. However, there is no information directly on A. bryophilus's feeding habits and habitat preferences.
Amblyseius herbicolus (Chant 1959)
Material examined — 6 ♀♀. 1 ♀ (Altınordu, N40°57′8.13″ E37°56′18.50″, 4 m, 02.IX.2019), 1 ♀ (Altınordu, N40°56′50.42″ E37°56′20.31″, 9 m, 02.IX.2019), 1 ♀ (Fatsa, N40°58′7.31″ E37°34′15.96″, 273 m, 06.VIII.2018), 1 ♀ (Fatsa, N40°58′39.92″ E37°36′41.00″, 178 m, 06.VIII.2018), 1 ♀ (Gülyalı, N40°57′17.05″ E38°0′10.76″, 10 m, 02.IX.2019), 1 ♀ (Gülyalı, N40°58′37.75″ E37°59′56.64″, 4 m, 02.IX.2019)
Remarks — Amblyseius herbicolus was originally described from Portugal, intercepted at Boston, Massachusetts, USA on Bromeliaceae (Chant, 1959). This predatory mite is known in more than 50 countries around the world (Demite et al. 2023). In Türkiye, it was first reported on D. kaki and D. lotus trees in Ordu by Akyazı et al. (2016a). Later, this phytoseiid species was also found on stone (Altunç and Akyazı 2019) and pome fruit trees (Akyol and Akyazı 2022) in Ordu, citrus in Artvin, Gresun, Rize (Döker et al. 2020). This mite is classified as Subtype II-c-Generalist predators, which live in confined spaces on dicotyledonous plants. It has been observed to occur in domatia (McMurtry et al. 2013). It is known to be one of the most abundant and frequent phytoseiids associated with the pest mite species Brevipalpus phoenicis (Geijskes) (Trombidiformes: Tenuipalpidae) and Oligonychus ilicis (McGregor) (Trombidiformes: Tetranychidae) in Brazilian coffee crops (Reis et al. 2007). Moreover, the species has the potential to control P. latus (Rodriguez-Cruz et al. 2013).
Proprioseiopsis okanagensis (Chant 1957b)
Material examined — 1 ♀ (Kabadüz, N40°49′36.35″ E37°52′40.IX″, 703 m, 14.VI.2018)
Remarks — Proprioseiopsis okanagensis was originally reported from peaches in Canada by Chant (1957b). Later, it was recorded in Austria, Azerbaijan, Canada, China, Czech Republic, Finland, France, Germany, Georgia, Greece, Greenland, Hungary, Iceland, Iran, Kazakhstan, Latvia, Moldova, Netherlands, Norway, Poland, Russia, Slovakia, Slovenia, Sweeden, Türkiye, Ukraine, USA (Kazemi et al. 2022; Demite et al. 2023; Farazmand et al. 2023). Proprioseiopsis species are typically found in soil or litter habitats. Before this study, it was collected from Malus communis L. (Rosaceae) in Erzurum (Çobanoğlu 1989a), Rosa canina L., Rosa damascena (Mill.) (Rosaceae) in Ankara (Çobanoğlu and Bayram 1999), and Cucurbita sp. (Soysal and Akyazı 2018) in Ordu Türkiye. Although this group of mites is common in different parts of the world, very little is known about their biology. The few papers dealing with the biology of these mites refer to their ability to consume mites found on the aerial plant parts as prey. Some species of Proprioseiopsis are classified as Type I lifestyle- Specialized mite predators/subtype I-c, specialized predators of tydeiods (Momen 2011), On the other hand, most Proprioseiopsis have Subtype III-e lifestyle, Generalist predators from soil/litter habitats, (Salmane and Petrova 2002; Mcmurtry et al. 2013).
Euseius finlandicus (Oudemans 1915)
Material examined — 1 ♀ (Gülyalı, N40°56′54.57″ E38° 2′6.78″, 380 m, 18.IX.2019)
Remarks — Euseius finlandicus is a globally widespread species, with a presence in 57 countries based on Demite et al. (2023). This predatory mite was described by Oudemans (1915) based on the material collected from Salix caprea L. (Salicaceae) in Finland. The species was very common in different habitats around the world. It commonly occurs on various plants in Türkiye (Faraji et al. 2011). In previous studies in Ordu, which is the field of this study, E. finlandicus was collected from vegetables (Soysal and Akyazı 2018), stone (Altunç and Akyazı 2019), and pome (Akyazı et al. 2017; Akyol and Akyazı 2022) fruits. It was classified as Type 4 lifestyle, pollen-feeding generalist predator (McMurtry et al. 2013). Many members of this genus prefer glabrous leaves (Moraes et al. 1986; McMurtry et al. 2013). This species was collected from pubescent kiwi leaves in the current study, although it has also been reported mainly on glabrous substrates (Kabicek 2005, 2008; McMurtry et al. 2013). Kabicek (2008) has reported the common occurrence of this species in the glabrous region of the leaves of Corylus avellana L. (Betulaceae) moving to pubescent patches when disturbed. Additionally, type IV lifestyle phytoseiids contain species for which pollen constitutes an important part of the diet. It is known to feed on species including tetranychid, eriophyid, tyroglyphid, and tarsonemid mites, as well as pollen, fungal hyphae and spores, insect eggs and larvae, honeydew, and plant fluids. (Schausberger 1992; Kostainen and Hoy 1994; Abdallah et al. 2001). Additionally, Akyol and Akyazı (2022) found that the pair of E. finlandicus and B. rubrioculus had a very strong positive relationship. Schausberger (1997) reported that E. finlandicus tended to prey on motile immatures of T. pyri and Kampimodromus aberrans (Oudemans) (Mesostigmata: Phytoseiidae). Similarly, Akyol and Akyazı (2022) discovered that the pair of E. finlandicus and T. rapidus had a very highly positive association.
Euseius amissibilis Meshkov, 1991 [= E. gallicus Kreiter & Tixier 2010 (Döker et al., 2024)]
Material examined — 4 ♀♀. 1 ♀ (Altınordu, N40°56′50.42″ E37°56′20.31″, 9 m, 02.IX.2019), 1 ♀ (Gülyalı, N40°56′54.57″ E38° 2′6.78″, 380 m, 18.IX.2019), 1 ♀ (Gülyalı, N40°57′7.30″ E38°2′33.06″, 369 m, 18.IX.2019), 1 ♀ (Ünye, N41°6′18.31″ E37°19′39.58″, 9 m, 18.VII.2018)
Remarks — Euseius gallicus was first reported on Tilia platphyllos Scopoli (Tiliaceae), P. cerasus, Aesculus hippocastanum L. (Sapindaceae), and Viburnum tinus L. (Adoxaceae) in France by Tixier et al. (2009). It was also collected in Belgium (Döker et al. 2014b), Germany (Döker et al. 2014b), Italy (Tsolakis and Ragusa 2017), Slovenia (Chatti et al. 2017), Netherlands (Siepel et al. 2018), France (Tixier et al. 2020), Mauritius (Kreiter and Abo-Shnaf 2020) and Türkiye (Döker et al. 2014b; Soysal and Akyazı 2018; Çakır et al. 2020; Akyazı et al. 2022b). In Türkiye, the species was recorded on Ipomoea sp. (Convolvulaceae) in Trabzon (Döker et al. 2014b), vegetables (Soysal and Akyazı 2018), and P. laurocerasus (Akyazı et al. 2022b) in Ordu as well as walnut leaves in Samsun (Çakır et al. 2020). This mite is a Type IV generalist predator that feeds on pollen (Kreiter et al. 2020). However, a recent study, Döker et al. (2024), noted that E. gallicus is a junior synonym of E. amissibilis
Euseius stipulatus (Athias & Henriot 1960)
Material examined — 5 ♀♀. 4 ♀♀ (Ünye, N41°6′59.85″ E37°15′16.46″, 76 m, 18.VII.2018), 1 ♀ (Ünye, N41°7′1.20″ E37°15′15.81″, 74 m, 18.VII.2018)
Remarks — Euseius stipulatus has a worldwide distribution and has been detected in 21 countries across the globe according to Demite et al. (2023). This species was originally described from Algeria by Athias-Henriot (1960). While E. stipulatus is a common species found on many plants, it is particularly abundant in citrus orchards as reported by Sahraoui et al. (2012). Before this study, the species was known to be present in Türkiye and was collected from Citrus spp. and C. sativus (Faraji et al. 2011), as well as apple leaves (Akyol and Akyazı 2022). E. stipulatus is a Type IV lifestyle phytoseiid mite (McMurtry et al. 2013). Cruz-Miralles et al. (2021) reported that it is a zoophytophagous mite, which can engage in direct plant feeding on sour orange (Citrus aurantiıum L. (Rutaceae)) and Cleopatra mandarin (Citrus reshni hort. ex Tan (Rutaceae)). Additionally, it was found that E. stipulatus adversely affected the control of Tetrancychus urticae Koch (Trombidiformes: Tetranychidae) in Clementine orchards in Spain by negatively impacting the establishment of Neoseiulus californicus (McGregor) and P. persimilis (Abad-Moyano et al. 2010; McMurtry et al. 2013). Even if no clear correlation, E. stipulatus and Tetranychus sp. were also observed in citrus orchards in Tunisia (Sahraoui et al. 2014).
Neoseiulus umbraticus (Chant 1956)
Material examined — 2 ♀♀. 1 ♀ (İkizce, N41° 3′9.03″ E36°59′1.39″, 510 m, 31.VII.2019), 1 ♀ (Perşembe, N41°4′29.02″ E37°43′52.77″, 455 m, 26.VII.2018)
Remarks — Neoseiulus umbraticus was first described in England on Rubus fructicosus L. (Rosaceae) by Chant (1956) and then recorded mainly in 31 countries worldwide (Demite et al. 2023). In Türkiye, it was recorded on cucumber (Çobanoğlu 1989b), hazelnut (Çobanoğlu and Özman 2002) and persimmon (Akyazı et al. 2016a, 2017). There are only a few studies on the biology of this species. Sengonca and Dresher (2001) investigated the impact of feeding on Thrips tabaci Lindeman (Thysanoptera: Thripidae) on the biological parameters of this species, compared to T. urticae. Kazak et al. (2002) demonstrated that this species can feed on T. urticae under laboratory conditions. It was also discovered that N. umbraticus can develop and reproduce on P. ulmi, Calvolia lordi (Nesbitt) (Sarcoptiformes: Winterschmidtiidae), Aculus schlectendali (Nalepa) (Trombidiformes: Eriophyidae), and adults of Quadraspidiotus perniciosus (Comstock) (Hemiptera: Diaspididae), as well as on apple and cherry pollens. Eggs, larvae and protonymphs of T. urticae were the preferred prey for all stages of N. umbraticus (Knisley and Swift 1971). In addition, Wainstein and Vartapetov (1973) reported that it feeds on Panonychus citri (McGregor) (Trombidiformes: Tetranychidae) and T. urticae.
Typhlodromips sessor (De Leon 1962)
Material examined — 2 ♀♀. 1 ♀ (Altınordu, N40°54′36.26″ E37°50′22.97″, 140 m, 02.IX.2019), 1 ♀ (Fatsa, N40°58′39.37″ E37°24′59.45″, 96 m, 06.VIII.2018)
Remarks — De Leon (1962) first described Typhlodromips sessor from material collected from Vernonia sp. (Asteraceae) and apple leaves in Tennessee, USA. Later, the species were collected from Canada (Sciarappa et al. 1977), USA (Denmark and Evans 2011), Japan (Toyasima et al. 2014) and Türkiye (Samsun) (Baş et al. 2022). In Türkiye, Baş et al. (2022) reported it from Populus deltoides Bartr. Ex Marsh (Salicaceae) leaves in Samsun.
Phytoseius finitimus Ribaga 1904
Material examined — 9 ♀♀. 1 ♀ (Altınordu, N40°56′40.11″ E37°47′7.66″, 366 m, 02.IX.2019), 1 ♀ (Fatsa, N40°57′29.03″ E37°37′33.74″, 163 m, 06.VIII.2018), 1 ♀ (Gülyalı, N40°58′18.10″ E38° 2′11.96″, 0 m, 18.IX.2019), 1 ♀ (Gülyalı, N40°58′37.75″ E37°59′56.64″, 4 m, 02.IX.2019), 1 ♀ (İkizce, N41°4′29.84″ E37°0′40.80″, 490 m, 31.VII.2019), 1 ♀ (Perşembe, N40°59′39.76″ E37°48′54.06″, 15 m, 26.VII.2018), 1 ♀ (Perşembe, N40°59′32.68″ E37°48′47.75″, 16 m, 26.VII.2018), 2 ♀♀ (Ünye, N41°5′56.20″ E37°22′27.90″, 37 m, 18.VII.2018)
Remarks — Phytoseius finitimus was first discovered on Buddleja madagascariensis Lamarck (Scrophulariaceae) in Italy by Ribaga (1904). It is a widely distributed predatory species found in 18 countries worldwide (Demite et al. 2023). It is also a very common predatory species in Türkiye (İncekulak and Ecevit 2002; Akyazı and Ecevit 2003; Faraji et al. 2011; Gençer Gökçe et al. 2022; Miroğlu and Çıkman 2022). In the current study area, it was previously collected from various habitats including persimmon trees (Akyazı et al. 2017), vegetables (Soysal and Akyazı 2018), stone (Altunç and Akyazı 2019), and pome (Akyol and Akyazı 2022) fruits. It belongs to subtype III-a, which are generalist predators that live on pubescent leaves (leaves with trichomes) (McMurtry et al. 2013). The species' small, compressed idiosoma aids in moving between trichomes (Kreiter et al. 2003; Tixier et al. 2007). It was found that the species is commonly found on hairy plants (Pappas et al. 2013). Phytoseius finitimus has stout, usually serrate setae on its dorsal shield. It can colonize microhabitats that larger phytoseiids cannot, avoiding competition and escaping predation (Seelman et al. 2007). It takes advantage of the presence of prey that also prefer the same microhabitat. In addition, Duso and Vettorazzo (1999) indicated that P. finitimus could be potentially effective in controlling P. ulmi on grape plants. Pappas et al. (2013) also declared that the species is a natural enemy of both tetranychid and eriophyid mites. It can feed on pollen.
Conclusions
According to the study results, kiwi vines in Ordu have a rich fauna of beneficial mites due to the non-usage of pesticides. It is crucial to protect these mites in their environment, as they may hold the potential for biological control of pests in important fruit crops. On the other hand, various harmful mite species have also been identified. It is necessary to monitor the populations of these species and keep them under control in case they become potentially harmful to kiwi in the future. In future studies, it would also be possible to establish the relationships between these two mite groups.
References
- Abad-Moyano R., Urbaneja A., Hoffmann D., Schausberger P. 2010. Effects of Euseius stipulatus on establishment and efficacy in spider mite suppression of Neoseiulus californicus and Phytoseiulus persimilis in Clementine. Exp. Appl. Acarol., 50(4): 329-341. https://doi.org/10.1007/s10493-009-9320-9
- Abdallah A.A., Zhang Z.Q., Masters G.J., Mcneill S. 2001. Euseius finlandicus (Acari: Phytoseiidae) as a Potential Biocontrol Agent against Tetranychus urticae (Acari: Tetranychidae): Life History and Feeding Habits on Three Different Types of Food. Exp. Appl. Acarol., 25(10-11): 833-847. https://doi.org/10.1023/A:1020431531446
- Acuña-Soto J., Isiordia-Aquino N., Robles-Bermúdez A., Flores-Canales R. 2017. Mites associated to the cultivation of soursop (Annona muricata L.) in the municipality of Compostela, Nayarit, Mexico. GARJAS, 6(10): 306-309.
- Akyazı F., Ecevit O. 2003. Determination of mite species in hazelnut orchards in Samsun, Ordu and Giresun provinces. Ondokuz Mayıs Üniversitesi Ziraat Fakültesi Dergisi, 18(3): 39-45.
- Akyazı R., Soysal M., Altunç Y.E., Akyol D. 2022a. Efficacy of Nicotiana tabacum L. (Solanaceae), Allium sativum L. (Amaryllidaceae) and soft soap for controlling Polyphagotarsonemus latus (Banks, 1904) (Acari: Tarsonemidae). Turk. J. Entomol., 46(2): 211-226. https://doi.org/10.16970/entoted.1079195
- Akyazı R., Soysal M., Altunç Y.E. 2022b. Species complexes of leaf-inhabiting mites on Prunus laurocerasus L. (Rosaceae) trees in Ordu, Turkey. Acarol. Stud., 4(1): 9-20. https://doi.org/10.47121/acarolstud.996567
- Akyazı R., Ueckermann E.A., Soysal M., Akyol D. 2016a. Population dynamics of Mites (Acari) on Diospyros kaki Thunb. and Diospyros lotus L. (Ebenaceae) trees in Ordu, Turkey. Syst. Appl. Acarol., 21(10): 1334-1345. https://doi.org/10.11158/saa.21.10.4
- Akyazı R., Ueckermann E.A., Soysal M. 2016b. The new distribution of Amblyseius herbicolus in Turkey (Parasitiformes, Phytoseiidae) with a key of Amblyseius species found in Turkey. Acarologia, 56(2): 237-244. https://doi.org/10.1051/acarologia/20162241
- Akyazı R., Ueckermann E.A., Akyol D., Soysal M. 2017. Distribution of mite species (Acari) on persimmon trees in Turkey (Ordu), with one newly recorded mite species and one re-described species. Int. J. Acarol., 43(8): 563-581. https://doi.org/10.1080/01647954.2017.1373149
- Akyazı R., Welbourn C., Liburd O.E. 2021. Mite species (Acari) on blackberry cultivars in organic and conventional farms in Florida and Georgia, USA. Acarologia, 61(1): 31-45. https://doi.org/10.24349/acarologia/20214414
- Akyol D., Akyazı R. 2022. Comparative faunistic analysis of mite species on neglected and conventional pome fruit trees in Turkey. Acarologia, 62(4): 941-955. https://doi.org/10.24349/vt6l-svza
- Alp T. 2017. "Kivi″, Ordu Ticaret Borsası Yayını, pp. 25, Ordu.
- Altunç Y.E., Akyazı R. 2019. Ordu ilinde sert çekirdekli meyve ağaçlarında bulunan akar türleri. Anadolu Tarım Bilim. Derg., 34(1): 18-34. https://doi.org/10.7161/omuanajas.441274
- Altunç Y.E., Akyazı R. 2020. Two new records for spider mite fauna of Turkey, Tetranychus kanzawai Kishida and Eotetranychus rubiphilus Reck (Trombidiformes: Tetranychidae). Turkish Journal of Agriculture-Food Science and Technology, 8(8): 1598-1602. https://doi.org/10.24925/turjaf.v8i8.1598-1602.2387
- Andre H.M. 2005. In search of the true Tydeus (Acari, Tydeidae). J. Nat. Hist., 39(13): 975-1001. https://doi.org/10.1080/00222930400002838
- Andrade-Bertolo F., Moura R.B., Matioli A.L., Ott A.P. 2013. New records of mites (Acari: Prostigmata) on Vitis sp. (L.) in Brazil. Comun. Sci., 4(4): 414-418.
- Atak A. 2015. Kiwifruit research and production in Turkey. Acta Hortic., 1096: 63-67. https://doi.org/10.17660/ActaHortic.2015.1096.3
- Atak A. 2018. Modern approaches in new kiwifruit plantations in Turkey. Acta Hortic., 1218: 451-458. https://doi.org/10.17660/ActaHortic.2018.1218.62
- Athias-Henriot C. 1960. Nouveaux Amblyseius d' Algerie (Parasitiformes, Phytoseiidae). Acarologia, 2(3): 288-299.
- Aysan E., Kumral N.A. 2018. The tritrophic relationships among tomato varieties, tomato rust mite and its predators. Acarologia, 58(Suppl): 5-17. https://doi.org/10.24349/acarologia/20184283
- Baker E.W. 1970. The genus Tydeus: subgenera and species groups with descriptions of new species (Acarina: Tydeidae). Ann. Entomol. Soc. Am., 63(1): 163-177. https://doi.org/10.1093/aesa/63.1.163
- Baker E.W., Wharton G.W. 1952. An Introduction to Acarology. USA: The Macmillan Company New York, pp.465.
- Banks N. 1904. Class III, Arachnida, Order 1, Acarina, four new species of injurious mites. J. N. Y. Entomol. Soc., 12(1): 53-56.
- Baradaran P., Arbabi M. 2009. Population abundance of Pronematus ubiquitus (McGregor, 1932) (Acari: Tydeidae) on different fig varieties. IAU Entomol. Res. J., 1(2): 177-183.
- Baş H., Döker I., Özman-Sullivan S.K. 2022. New records and complementary descriptions of three Phytoseiidae (Acari: Mesostigmata) species from Turkey. Int. J. Acarol., 48(4-5): 393-400. https://doi.org/10.1080/01647954.2022.2082527
- Bayram Ş., Çobanoğlu S. 2007. Mite fauna (Acari: Prostigmata, Mesostigmata, Astigmata) of coniferous plants in Turkey. Turk. J. Entomol., 31(4): 279-290.
- Beard J.J., Ochoa R., Bauchan G.R., Trice M.D., Redford A.J., Walters T.W., Mitter C. 2015. Flat mites of the world [Internet]. [21 Mar 2022]. Available from: https://idtools.org/tools/1074/index.cfm
- BFG 2024. Predatory mite, Amblyseius andersoni. [Internet]. [07 Aug 2024]. Available from: https://bugsforgrowers.com/collections/products/products/predatory-mite-amblyseius-andersoni
- Bozkurt V. 1994. Brevipalpus obovatus Donnadieu (Phytoptipalpidae: Acarina)'un biyolojisi ve konukçuları üzerine araştırmalar [Ms Thesis]. Ankara Üniversitesi Fen Bilimleri Enstitüsü, pp. 42.
- Bulut E., Göçmen H. 2000. Pests and their natural enemies on greenhouse vegetables in Antalya. In: IOBCWPRS working group "Integrated control in protected crops, Mediterranean climate. Proceedings of the Meeting; Bulletin OILB-SROP., 23(1): 33-37.
- CABI 2004. CABI International, Cheletomimus berlesei [Internet]. [21 Apr 2023]. https://doi.org/10.1079/cabicompendium.12958
- CABI 2019. CABI International, Brevipalpus lewisi (citrus flat mite) [Internet]. [07 Aug 2024]. https://doi.org/10.1079/cabicompendium.10175
- CABI 2021. CABI International, Polyphagotarsonemus latus (broad mite) [Internet]. [21 Apr 2023] https://doi.org/10.1079/cabicompendium.26876
- Camporese P., Duso C. 1995. Life history and life table parameters of the predatory mite Typhlodromus talbii. Entomol. Exp. Appl., 77(2): 149-157. https://doi.org/10.1111/j.1570-7458.1995.tb01995.x
- Can M., Çobanoğlu S. 2010. Studies on the determination of mite (Acari) species and their hosts of greenhouse vegetables in Kumluca, Antalya. Akdeniz University Journal of the Faculty of Agriculture, 23(2): 87-92.
- Castagnoli M. 1984. Contribution to the knowledge of the tydeid mites (Acarina: Tydeidae) associated with cultivated plants in Italy. Redia, 47: 307-322.
- Castro E.B., Mesa N.C., Feres R.J.F., Moraes G.J.de, Ochoa R., Beard J.J., Demite P.R. 2024. Tenuipalpidae Database [Internet]. [10 Mar 2024]. Available from: http://www.tenuipalpidae.ibilce.unesp.br
- Chandurkar P.S. 2003. Integrated Pest Management For Kiwi. Government of India, Ministry of Agriculture, Department of Agriculture & Cooperation, Directorate of Plant Protection, Qurantine & Storagae, N. H. IV, Faridabad - 121 001 pp. 18.
- Chant D.A. 1956. Some mites of the subfamily Phytoseiinae (Acarina: Laelaptidae) from southeastern England, with descriptions of new species. Can. Entomol., 88(1): 37-26. https://doi.org/10.4039/Ent8826-1
- Chant D.A. 1957a. Descriptions of two new phytoseiid genera (Acarina: Phytoseiidae), with a note on Phytoseius Ribaga, 1902. Can. Entomol., 89(8): 357-363. https://doi.org/10.4039/Ent89357-8
- Chant D.A. 1957b. Descriptions of some phytoseiid mites (Acarina, Phytoseiidae). Part I. Nine new species from British Columbia with keys to the species of British Columbia. Part II. Redescriptions of eight species described by Berlese. Canada, Can. Entomol., 89(7): 289-308. https://doi.org/10.4039/Ent89289-7
- Chant D.A. 1959, Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. Can. Entomol., 61(12): 1-166. https://doi.org/10.4039/entm9112fv
- Chatti A., Kreiter S., Lebdi-Grissa K., Ksantini M. 2017. Phytophagous and predatory mites on olive trees in Tunisia. Catalogue, description of one new species and key for identification (Acari, Eriophyidae, Tetranychidae, Tenuipalpidae and Phytoseiidae). Acarologia, 57(2): 233-254. https://doi.org/10.1051/acarologia/20164152
- Childers C.C., French J.V., Rodrigues J.C.V. 2003. Brevipalpus californicus, B. obovatus, B. phoenicis, and B. lewisi (Acari: Tenuipalpidae): a Review of their Biology, Feeding Injury and Economic Importance. Exp. Appl. Acarol. 30: 5-28. https://doi.org/10.1023/B:APPA.0000006543.34042.b4
- Childers C.C., Rodrigues J.C.V, Grafton-Cardwell E.E., Morse J.G. 2013. Part II - Brevipalpus mites in California Citrus. Citrograph, 4(5): 52-58.
- CKC/CMCC 2003. A Pest Management Strategic Plan (PMSP) for Kiwifruit Production in California. California Kiwifruit Commission (CKC); California Minor Crops Council (CMCC) [Internet]. [10 Mar 2024]. Available from: https://ipmdata.ipmcenters.org/documents/pmsps/CAKIWIFRUIT.PDF
- Clements D.R., Harmsen R. 1993. Prey preferences of adult and immature Zetzellia mali Ewing (Acari: Stigmaeidae) and Typhlodromus caudiglans Schuster (Acari: Phytoseiidae). Can. Entomol., 125(5): 967-969. https://doi.org/10.4039/Ent125967-5
- Cortez-Mondaca E., Gutierrez-Soto G., Santillan-Galicia T., Valenzuela-Escoboza F.A., Lopez M.A., Osuna A.O. 2022. Natural Enemies Associated with Citrus Flat Mite1 in a Commercial Orchard of Persian Lime at Sinaloa, Mexico. Southwest. Entomol., 47(1): 107-112. https://doi.org/10.3958/059.047.0109
- Croft B.A. 1994. Biological control of apple mites by a phytoseiid mite complex and Zetzellia mali (Acari: Stigmaeidae) (long-term effects and impact of azinphosmethyl on colonization by Amblyseius andersoni (Acari: Phytoseiidae)). Environ. Entomol., 23(5): 1317-1325. https://doi.org/10.1093/ee/23.5.1317
- Cruz-Miralles J., Cabedo-López M., Guzzo M., Ibáñez-Gual V., Flors V., Jaques J.A. 2021. Plant-feeding may explain why the generalist predator Euseius stipulatus does better on less defended citrus plants but Tetranychus-specialists Neoseiulus californicus and Phytoseiulus persimilis do not. Exp. Appl. Acarol., 83(2): 167-182. https://doi.org/10.1007/s10493-020-00588-x
- Çakır S., Tixier M.S., Özman-Sullivan S. 2020. Phytoseiid species (Acari: Phytoseiidae) on walnut trees in Samsun Province, Turkey. Acarol. Stud., 2(1): 24-33.
- Çobanoğlu S. 1989a. Türkiye için üç yeni faydalı akar (Acari: Phytoseiidae) türü. Türk. Entomol. Derg., 13(4): 229-238.
- Çobanoğlu S. 1989b. Antalya ili sebze alanlarinda tespit edilen Phytoseiidae Berlese, 1915 (Acarina: Mesostigmata) türleri. Türk. Bit. Kor. Bül., 29(1-2): 47-64.
- Çobanoğlu S. 1995. Some new tarsonemidae (Acarina, Prostigmata) species for Turkish acarofauna. Türk. Entomol. Derg., 19(2): 87-94.
- Çobanoğlu S., Akçakoyunluoğlu K., Çalmaşur Ö. 2020. Mite Diversity (Acari) from Ornamental Plants in Erzurum in Turkey. J. Agric. Sci., 26(2): 236-245. https://doi.org/10.15832/ankutbd.518260
- Çobanoğlu S., Bayram S. 1999. Mite (Acari) species associated with cultivated and wild rose plants in Camlidere, Turkey. Entomol. Mon. Mag., 135: 245-248.
- Çobanoğlu S., Kazmierski A. 1999. Tydeidae and Stigmeidae (Acari, Prostigmata) from orchards, trees and shurbs in Turkey. Biol. Bull. Poznań, 36(1): 71-82.
- Çobanoğlu S., Kumral N.A. 2014. The biodiversity and population fluctuation of plant parasitic and benificial mite species (Acari) in tomato fields of Ankara, Bursa and Yalova provinces. Turk. J. Entomol., 38(2): 197-214.
- Çobanoğlu S., Özman S.K. 2002. Beneficial mite species of hazelnut orchard ecosystems from the Black Sea Region of Turkey. Proceedings of the 2nd Meeting of WG 4: Bio-control of Arthropod Pests in the Stored Products 30-31st May 2002, Prague, pp. 91-99.
- Çobanoğlu S., Ueckermann E.A., Sağlam H.D. 2016. The Tenuipalpidae of Turkey, with a key to species (Acari: Trombidiformes). Zootaxa, 4097(2): 151-186. https://doi.org/10.11646/zootaxa.4097.2.1
- Çobanoğlu S., Uysal C., Ökten E. 2003. The complex of the beneficial mite fauna of ornamental trees and shrubs in Ankara, Turkey. Entomol. Mon. Mag., 139(1664/1666): 7-12.
- Da Silva G.L., Metzelthin M.H., Da Silva O.S., Ferla N.J. 2016. Catalogue of the mite family Tydeidae (Acari: prostigmata) with the world key to the species. Zootaxa, 4135(1): 1-68. https://doi.org/10.11646/zootaxa.4135.1.1
- Dayoub A.M., Boubou A. 2023 New records of phytoseiid mites (Acari: Phytoseiidae) on solanaceous plants in the Syrian coastal region. Acarologia, 63(3), 744-750. https://doi.org/10.24349/f56z-s6rx
- De Leon D. 1962. Twenty-three new phytoseiids, mostly from southeastern United States (Acarina: Phytoseiidae). Fla. Entomol. 45(1): 11-27. https://doi.org/10.2307/3492899
- Demite P.R., Cavalcante A.C.C., Lofego A.C., Rodrigues R.R., Moraes G.J. de. 2022. Tarsonemid mites (Acari: Tarsonemidae) on myrtaceous plants of the Atlantic Forest, Brazil, with description of a new species of Tarsonemus Canestrini & Fanzago. Zootaxa, 5094(1): 153-168. https://doi.org/10.11646/zootaxa.5094.1.6
- Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2023. Phytoseiidae database [Internet]. [5 Mar 2023]. Available from: \textless www.lea.esalq.usp.br/phytoseiidae>
- Denizhan E., Çobanoğlu S. 2008. Aculus schlechtendali (Nalepa) (Acarina: Eriophyidae)'nin Ankara'da Malus floribunda L. (Rosaceae) üzerinde popülasyon değişimi ve predatörleri. Tarım Bilimleri Dergisi, 14(3): 288-296. https://doi.org/10.1501/Tarimbil_0000001043
- Denizhan E., Çobanoğlu S. 2009. Ankara ili ceviz (Juglans regia L.) ağaçlarinda bulunan eriophyid akarlar ve predatörleri. YYÜ. Tar. Bil. Der., 19(1): 33-37.
- Denmark H.A. 1980. Broad mite, Polyphagotarsonemus latus (Banks). FDACS-DPI Bureau of Entomology Circular No. 213, pp. 2.
- Denmark H.A., Evans G.A. 2011. Phytoseiidae of North America and Hawaii (Acari: Mesostigmata). West Bloomfield (MI): Indira Publishing House. pp. 451.
- Denmark H.A., Evans G.A. 2019. Additions to the world fauna of the family Phytoseiidae (Acari: Mesostigmata) with an illustrated key to the subfamilies, tribes, subtribes and genera of Phytoseiidae of the World. Indira Publishing House, West Bloomfield, Michigan, USA, 1-315.
- Diler H., Yazıcı G., Saçtı Z., Yücel C., Barış A. 2022. Survey of mite species of tea plantations in Rize. Plant Prot. Bull., 62(3): 37-49. https://doi.org/10.16955/bitkorb.1112756
- Doğan S. 2007. Checklist of Raphignathoid mites (Acari: raphignathoidea) of Turkey. Zootaxa, 1454(1): 1-26. https://doi.org/10.11646/zootaxa.1454.1.1
- Doğan S. 2022. An overview of research on the family Cheyletidae (Acariformes) in Turkey, with a checklist of the Turkish cheyletid mites. Syst. Appl. Acarol., 27(6): 1132-1151. https://doi.org/10.11158/saa.27.6.12
- Döker I, Kazak C, Karut K. 2016. Contributions to the Phytoseiidae (Acari: Mesostigmata) fauna of Turkey: morphological variations, twelve new records, re-description of some species and a revised key to the Turkish species. Syst. Appl. Acarol., 21(4): 505-527. https://doi.org/10.11158/saa.21.4.10
- Döker I., Kazak C., Karut K. 2020. The genus Amblyseius Berlese (Acari: Phytoseiidae) in Turkey with discussion on the identity of Amblyseius meridionalis. Syst. Appl. Acarol., 25(8): 1395-1420. https://doi.org/10.11158/saa.25.8.4
- Döker I., Stathakis T.I., Kazak C. 2014a. First record of Amblyseius bryophilus Karg (Acari: Phytoseiidae) for the Turkish fauna. Turk. J. Zool., 38(3): 375-377. https://doi.org/10.3906/zoo-1305-15
- Döker I., Witters J., Pijnakker J., Kazak C., Tixier M.S., Kreiter S. 2014b. Euseius gallicus Kreiter and Tixier (Acari: Phytoseiidae) is present in four more countries in Europe: Belgium, Germany, The Netherlands and Turkey. Acarologia, 54(3): 245-248. https://doi.org/10.1051/acarologia/20142132
- Döker I., Khaustov V.A., Joharchi O., Khaustov A.A., Kazakov D.V., Meshkov Y.I. 2024. Integrative taxonomy demonstrates synonymy between Euseius amissibilis Meshkov and Euseius gallicus Kreiter & Tixier (Acari: Phytoseiidae). Syst. Appl. Acarol., 29(1): 60-77. https://doi.org/10.11158/saa.29.1.5
- Dönel G., Doğan S. 2013. Predatör bir akar olan Zetzellia mali (Ewing) (Acari: Stigmaeidae)'nin Kelkit Vadisi'nden ilk kaydı. Erzincan Üniversitesi Fen Bilimleri Enstitüsü Dergisi, 6(2): 157-163.
- Duarte M.V.A., Vangansbeke D., Pijnakker J., Moerkens R., Benavente A., Arijs Y., Saucedo A.L.F., Wäckers F. 2021. Evaluation of Natural and factitious food sources for Pronematus ubiquitus on tomato plants. Insects, 12(12): 1111. https://doi.org/10.3390/insects12121111
- Duso C., Pasini M., Pellegrini M. 2003. Distribution of the predatory mite Typhlodromus pyri (Acari: Phytoseiidae) on different apple cultivars. Biocontrol Sci. Techn., 13(7): 671-681. https://doi.org/10.1080/09583150310001606264
- Duso C., Vettorazzo E. 1999. Mite population dynamics on different grape varieties with or without phytoseiids relased (Acari: Phytoseiidae). Exp. Appl. Acarol., 23(9): 741-763. https://doi.org/10.1023/A:1006297225577
- Düzgüneş Z. 1952. Türkiye'de turunçgil akarları. Bit. Kor. Bül., 1: 6-11.
- Düzgüneş Z. 1963. Türkiye de yeni bulunan akarlar. Bit. Kor. Bül., 3: 237-246.
- Düzgüneş Z. 1965. Türkiye'de bitkilerde zarar veren Tenuipalpidae Sayed familyası türleri üzerine incelemeler. Ankara Üniversitesi Ziraat Fakültesi Yıllığı, Fasikül 3, 120-148.
- Düzgüneş Z. 1977. The phytophagus mites on different economic plants and their control in Çukurova. Journal of Agricultural Faculty of Çukurova University, Public Lecture, 91: 1-25.
- Ewing H.E. 1921. New nearctic spider mites. Proc. U.S. Natl. Mus., 59(2394): 633-666. https://doi.org/10.5479/si.00963801.59-2394.659
- Ewing H.E. 1939. A revision of the mites of the subfamily Tarsoneminae of North America, the West Indies and the Hawaiian Islands. U.S. Dep. Agr. Tech. Bull., 653: 1-63.
- Fan Q.H., Flechtmann C.H.W., Moraes G.J. de. 2016. Annotated catalogue of Stigmaeidae (Acari: Prostigmata), with a pictorial key to genera. Zootaxa, 4176(1): 1-199. doi:10.11646/zootaxa.4176.1.1 https://doi.org/10.11646/zootaxa.4176.1.1
- FAO 2022. Food and agriculture data. [Internet]. [22 March 2024]. Available from: https://www.fao.org
- Faraji F., Çobanoğlu S., Çakmak İ. 2011. A checklist and a key for the Phytoseiidae species of Turkey with two new species records (Acari: Mesostigmata). Int. J. Acarol., 37(suppl.1): 221-243. https://doi.org/10.1080/01647954.2011.558851
- Farazmand A., Jalaeian M., Kamali H., Saboori A., Tixier M.S., Kreiter S. 2023. Phytoseiidae (Acari: Mesostigmata) in four provinces of Iran. Persian J. Acarol., 12(1): 21-58.
- Fasulo T.R. 2004. Broad Mite, Polyphagotarsonemus latus (Banks) (Arachnida: Acari: Tarsonemidae): EENY-183/IN340, Rev. 9/2005. EDIS 2004 (3). Gainesville, FL. https://doi.org/10.32473/edis-in340-2005
- Garga N., Proctor H., Belczewski R. 1997. Leg size affects mating success in Tarsonemus confuscus Ewing (Prostigmata: Tarsonemidae). Acarologia, 38(4): 369-375.
- Gençer Gökçe P., Kılıç N., Çobanoğlu S. 2022. Tekirdağ ili park ve süs bitkilerinde akar (Acari) türleri ve konukçularinin belirlenmesi. Tekirdağ Ziraat Fakültesi Dergisi, 19(3): 697-711. https://doi.org/10.33462/jotaf.1124376
- Gerson U., Smiley R.L., Ochoa R. 2003. Mites (Acari) for pest control. Oxford, UK: Blackwell Science Publishing Ltd. pp. 558. https://doi.org/10.1002/9780470750995
- Gonzalez-Rodriguez R.H. 1965. A taxonomic study of the genera Mediolata, Zetzellia and Agistemus (Acarina: Stigmaeidae). Univ. Calif. Publ. Entomol., 41: 1-64.
- Gökdoğan O. 2022. Energy and economic efficiency of kiwi fruit production in Turkey: A case study from Mersin province. Erwerbs-Obstbau, 64(1): 55-60. https://doi.org/10.1007/s10341-021-00610-5
- Göven M.A., Çobanoğlu S., Güven B. 2009. Predatory mite fauna in Aegean vineyards. Plant Prot. Bull., 49(1): 1-10.
- Grinberg M., Perl-Treves R., Palevsky E. Shome I., Soroker V. 2005. Interaction between cucumber plants and the broad mite, Polyphagotarsonemus latus from damage to defense gene expression. Entomol. Exp. Appl., 115(1): 135-144. https://doi.org/10.1111/j.1570-7458.2005.00275.x
- Han X., Wang Y., Liu K.C., Ai J., Chen R.Z. 2020. A new Leipothrix (Trombidiformes: Eriophyoidea) infesting Actinidia fruit trees in Jilin province, Northeastern China. Int. J. Acarol., 46(7): 479-488. https://doi.org/10.1080/01647954.2020.1808059
- Hao B., Yu L., Xu C. 2007. Tarsonemus confusus, a new pest for bagged apples. J. Fruit. Sci. 24(2): 180-184.
- Hao B., Yu L., Xu C., He L., Jiao R. 2010. Tarsonemus confusus causes black-dot disease of bagged fruits on apple trees and its control. J. Fruit. Sci. 27(6): 956-960.
- Hernandes F.A., Feres R.J.F. 2006. Review about mites (Acari) of rubber trees (Hevea spp., Euphorbiaceae) in Brazil. Biota Neotrop., 6(1): 1-24. https://doi.org/10.1590/S1676-06032006000100005
- İnak E., Çobanoğlu S. 2018. Determination of mite species on vineyards of Ankara, Turkey. Fresenius Environ. Bull., 27(2): 1232-1239.
- İncekulak R., Ecevit O. 2002. Amasya Elma bahçelerinde bulunan akar türleri ve popülasyon dinamiklerinin belirlenmesi. Türkiye 5. Biyolojik Mücadele Kongresi Bildirileri, p. 297-314.
- Jalilirad M., Hajizadeh J., Noei J. 2013. Fauna of Prostigmatic mites (Acari: Prostigmata) associated with citrus orchards in Guilan Province. J. Plant Pest Res., 2(4): 1-13.
- Kabicek J. 2005. Intra-leaf distribution of the phytoseiid mites (Acari, Phytoseiidae) on several species of wild broad leaf trees. Biologia, 60(5): 523-528.
- Kabicek J. 2008. Cohabitation and intra-leaf distribution of phytoseiid mites (Acari: Phytoseiidae) on leaves of Corylus avellana. Plant Prot. Sci., 44(1): 32-36. https://doi.org/10.17221/3/2008-PPS
- Kain D.P., Nyrop J.P. 1995. Predatory mites. Insect Identification, Sheet No. 23. Cornell Cooperative Extension, Cornell University, Ithaca and New York State IPM Program, New York, USA.
- Karg W. 1970. Neue arten der raubmilbenfamilie Phytoseiidae Berlese, 1916 (Acarina: Parasitiformes). Dtsc. Entomol. Z., 17(4-5): 289-301. https://doi.org/10.1002/mmnd.19700170402
- Kasap İ., Atlihan R., Özgökçe M.S., Kaydan M.B., Polat E., Yarimbatman A. 2007. Vangölü havzası ceviz bahçelerinde önemli zararlı ve yararlı akarların popülasyon gelişmesi. Türkiye II. Bitki Koruma Kongresi Bildirileri; 27-29 Ağustos 2007; Isparta, p. 68.
- Kasap İ., Çobanoğlu S. 2007. Mite (Acari) fauna in apple orchards of around the Lake Van basin of Turkey. Turk. J. Entomol., 31(2): 97-109.
- Kasap İ., Çobanoğlu S., Pehlivan S. 2013. Predatory mite species on pome fruit trees and weeds in the province of Çanakkale and Balıkesir. Turk. J. Biol. Control., 4(2): 109-124.
- Kazak C., Yildiz S., Şekeroglu E. 2002. Biological characteristics and life tables of Neoseiulus umbraticus Chant (Acari, Phytoseiidae) at three constant temperatures. Anzeiger für Schädlingskunde / J. Pest. Science, 75(5): 118-121. https://doi.org/10.1046/j.1472-8206.2002.02034.x
- Kazemi S., Mohammad-Doustaresharaf M., Döker I. 2022. An annotated checklist of the Iranian Phytoseiidae (Acari: Mesostigmata), with an updated key to the species. Syst. Appl. Acarol., 27(4): 697-748. https://doi.org/10.11158/saa.27.4.6
- Khanjani M., Ueckermann E.A. 2002. The stigmaeid mites of Iran (Acari: Stigmaeidae). Int. J. Acarol., 28(4): 317-339. https://doi.org/10.1080/01647950208684309
- Khaustov A.A. 2023. New data to the fauna of Tydeidae (Acari: Prostigmata) of Western Siberia, Russia with discovery of secondary sexual dimorphism Syst. Appl. Acarol., 28(8): 1335-1343. https://doi.org/10.11158/saa.28.8.5
- Knisley C.B., Swift F.C. 1971. Biological studies of Amblyseius umbraticus (Acarina: Phytoseiidae). Ann. Entomol. Soc. Am., 64(4): 813-822. https://doi.org/10.1093/aesa/64.4.813
- Kostiainen T., Hoy M.A. 1994. Egg-harvesting allows large scale rearing of Amblyseius finlandicus (Acari: Phytoseiidae) in the laboratory. Exp. Appl. Acarol., 18(3): 155-165. https://doi.org/10.1007/BF02353683
- Krantz G.W., Walter D.E. 2009. A Manual of Acarology, 3rd edition. Texas Tech University Press, pp. 816.
- Kreiter S., Abo-Shnaf R.I.A. 2020. New records of phytoseiid mites from Mauritius Island (Acari: Mesostigmata). Acarologia, 60(3): 520-545. https://doi.org/10.24349/acarologia/20204382
- Kreiter S., Amiri K., Douin M., Bohinc T., Trdan S., Tixier M.S. 2020. Phytoseiid mites of Slovenia (Acari: Mesostigmata): new records and first description of the male of Amblyseius microorientalis. Acarologia, 60(2): 203-242. https://doi.org/10.24349/acarologia/20204364
- Kreiter S., Tixier M.S., Bourgeois T. 2003. Do generalist phytoseiid mites (Gamasida: Phytoseiidae) have interactions with their host plants? Int. J. Trop. Insect. Sci., 23(1): 35-50. https://doi.org/10.1017/S1742758400012236
- Kumral N.A. 2005. Bursa ilinde ılıman iklim meyvelerinde bulunan zararlı ve doğal düşman akarların saptanması ve Panonychus ulmi (Koch)'nin bazı pestsitlere karşı duyarlılığı üzerinde araştırmalar [Phd Thesis]. Uludağ Üniversitesi. Bursa: Fen Bilimleri Enstitüsü, Bitki Koruma Anabilim Dalı, pp. 157.
- Kumral N.A., Çobanoğlu S. 2015. The potential of the nightshade plants (Solanaceae) as reservoir plants for pest and predatory mites. Turk. J. Entomol., 39(1): 91-108. https://doi.org/10.16970/ted.55042
- Laniecki R., Kaźmierski A., Mąkol J., Laniecka I., Magowski W. 2021. Know your campus: salient research potential of prostigmatic soil mite fauna (Acariformes: Prostigmata, Endeostigmata) within university campus area. Acarologia, 61(3): 650-663. https://doi.org/10.24349/TTpf-5Q3l
- Lay-Yee M., Whiting D.C. 1996. Response of `Hayward' kiwifruit to high-temperature controlled atmosphere treatments for control of two-spotted spider mite (Tetranychus urticae). Postharvest Biol. Technol., 7(1-2): 73-81. https://doi.org/10.1016/0925-5214(95)00035-6
- Li L., Jiao R., Yu L., He X.Z., He L., Xu C., Zhang L., Liu J. 2018. Functional response and prey stage preference of Neoseiulus barkeri on Tarsonemus confusus. Syst. Appl. Acarol., 23(11): 2244-2258. https://doi.org/10.11158/saa.23.11.16
- Li L., Yu L., He L. H, XZ., Jiao R., Xuy C. 2022. Temperature-dependent development and reproduction of Tarsonemus confusus (Acari: Tarsonemidae): an important pest mite of horticulture. Exp. Appl. Acarol., 88(3-4): 301-316. https://doi.org/10.1007/s10493-022-00761-4
- Lindquist E.E. 1986. The world genera of Tarsonemidae (Acari: Heterostigmata): a morphological, phylogenetic and systematic revision, with classification of family-group taxa in the Heterostigmata. The Entomological Society of Canada, Ottawa, pp. 517. https://doi.org/10.4039/entm118136fv
- Livshits I.Z., Kuznetsov N.N., Zapletina V.P. 1972. New species of the family Tydeidae (Acariformes) from Crimea and Azerbaijan. Zool. Zhurnal, 51(10): 1578-1580.
- Luypaert G. 2015. The broad mite, Polyphagotarsonemus latus (Acari: Tarsonemidae), and its interactions with pot azalea, Rhododendron simsii hybrid. [Phd Thesis]. Belgium, Ghent: Ghent University, pp. 209.
- Madanlar N. 1991. İzmir ilinde turunçgillerde bulunan Acarina türleri ve populasyon yoğunluklarının saptanması üzerinde araştırmalar [Phd Thesis]. İzmir: Ege Üniversitesi Fen Bilimleri Enstitüsü, Bitki Koruma Anabilim Dalı, pp. 258.
- Maughan T., Black B. 2015. "Hardy Kiwi in the Garden″, Vegetables, Fruits and Herbs Book [Internet]. [15 June 2023]. Utah State University Press, p. 95-98. Available from: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=1684&context=extension_curall
- Maynard G.V., Lepschi B.J., Malfroy S.F. 2018. Norfolk Island Quarantine Survey 2012-2014-a Comprehensive Assessment of an Isolated Subtropical Island. Proc. Linn. Soc. N.S.W., 140: 7-243.
- McGregor E.A. 1932. The ubiquitous mite, a new species on citrus. Proceedings of the Entomological Society of Washington, 34(4): 60-63.
- McMurtry J.A., de Moraes G.J., Famah-Sourassou N. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18(4): 297-320. https://doi.org/10.11158/saa.18.4.1
- Mesa N.C., Ochoa R., Welbourn W.C., Evans G.A. Moraes G.J. de. 2009. A catalog of the Tenuipalpidae (Acari) of the world with a key to genera. Zootaxa, 2098(1): 1-185. https://doi.org/10.11646/zootaxa.2098.1.1
- Michailides T.J., Morgan D.P., Mitcham E., Crisosto C.H. 1994. Occurrence of moldy core and core rot of Fuji apple in California. KAC. Plant Prot., 3(1): 4-6.
- Migeon A., Dorkeld F. 2024. Spider Mites Web: a comprehensive database for the Tetranychidae. [Internet]. [20 March 2024]. Available from: https://www1.montpellier.inrae.fr/CBGP/spmweb
- Miroğlu M., Çıkman E. 2022. Beneficial mite fauna of Hevsel Gardens-Diyarbakır. Bit. Kor. Bül., 62(1): 34-45. https://doi.org/10.16955/bitkorb.985322
- Moerkens R., Vangansbeke D., Duarte M.V.A., Bellinkx S., De Roo E., Pijnakker J., Wäckers F. 2023. Modelling the interaction between a pest (Aculops lycopersici), two predators (Pronematus ubiquitus and Macrolophus pygmaeus) and climate variables: a 3-year greenhouse study in a tomato crop. Pest Manag. Sci., 79(12): 5362-5373. https://doi.org/10.1002/ps.7747
- Momen F.M. 2011. Life tables and feeding habits of Proprioseiopsis cabonus, a specific predator of tydeid mites (Acari: Phytoseiidae and Tydeidae). Acarina, 19(1): 103-109.
- Momen F.M., Lundqvist L. 1996. Taxonomy of non-Tydeus genera of the mite family Tydeidae (Acari: Prostigmata) from moss, lichens and trees in Southern Sweden. Acarologia, 37(4): 281-297.
- Moraes G.J., McMurtry J.A., Denmark H.A. 1986. A catolog of the mite family Phytoseiidae. References to taxonomy, synonymies, distribution and habitat. Brasilia: EMBRAPA. pp. 353.
- Naves P., Nóbrega F., Auger P. 2021. Updated and annotated review of Tetranychidae occurring in mainland Portugal, the Azores, and Madeira Archipelagos. Acarologia, 61(2): 380-393. https://doi.org/10.24349/acarologia/20214437
- Oudemans A.C. 1915. Acarologische Aanteekeningen 56. Entomol. Ber., 4: 180-188. https://doi.org/10.5962/bhl.part.1128
- Oudemans A.C. 1928. Acarologische Aanteekeningen 94. Entomol. Ber., 7: 374-382.
- Oudemans A.C. 1929. Acarologische Aanteekeningen 95. Entomol. Ber., 7: 393-399.
- Ovando-Garay V., Gonzalez-Gomez R., Zarza E., Castillo-Vera A., de Coss-Flores M.E. 2022. Morphological and genetic characterization of the broad mite Polyphagotarsonemus latus Banks (Acari: Tarsonemidae) from two Mexican populations. PLoS ONE, 17(4): e0266335. https://doi.org/10.1371/journal.pone.0266335
- Önuçar A., Ulu O. 1988. Notes on the mite species present on chestnut trees. Turk. J. Entomol., 12: 33-38.
- Özman-Sullivan S.K., Kazmierski A., Cobanoglu S. 2005. Alycina and Eupodina mites in hazelnut orchards in Turkey. Acta Hortic. 686: 401-406. https://doi.org/10.17660/ActaHortic.2005.686.55
- Özman-Sullivan S.K., Öcal H., Mıcık M. 2006. Mites of tea plantations in Turkey. Izmir: VIII. European Congress of Entomology; Sept 2006. p. 45.
- Özman-Sullivan S.K., Öcal H., Mıcık M. 2007.Occurence of mite species in tea plantations in Turkey. Glasgow, Scotland: XVI International Plant Protection Congress; Oct 2007. 2: 764-765.
- Panou H.N., Emmanouel N.G. 1996. Two new species of Lorryia (Acari: Prostigmata) from Greece. Ent. Mitt. Zool. Mus. Hamburg, 12(154): 91-103.
- Pappas M.L., Xanthis C., Samaras K., Koveos D.S., Broufas G.D. 2013. Potential of the predatory mite Phytoseius finitimus (Acari: Phytoseiidae) to feed and reproduce on greenhouse pests. Exp. Appl. Acarol., 61(4): 387-401. https://doi.org/10.1007/s10493-013-9711-9
- Pijnakker J., Moerkens R. Vangansbeke D., Duarte M., Bellinkx S., Benavente A., Merckx J., Stevens I., Wäckers F. 2022a. Dual protection: A tydeoid mite effectively controls both a problem pest and a key pathogen in tomato. Pest Manag. Sci., 78(1): 355-361. https://doi.org/10.1002/ps.6647
- Pijnakker J., Hürriyet A., Petit C., Vangansbeke D., Duarte M.V.A., Arijs Y., Moerkens R., Sutter L., Maret D., Wäckers F. 2022b. Evaluation of Phytoseiid and Iolinid mites for biological control of the tomato russet mite Aculops lycopersici (Acari: Eriophyidae). Insects, 13(12): 1146. https://doi.org/10.3390/insects13121146
- Pozzebon A., Duso C. 2008. Grape downy mildew Plasmopora viticola, an alternative food for generalist predatory mites occurring in vineyards. Biol. Control., 45(3): 441-449. https://doi.org/10.1016/j.biocontrol.2008.02.001
- Rasmy A.H. 1971. Relation between predaceous and phytophagous mites on Citrus. Z. Angew. Entomol., / J. Appl. Entomol., 67(1-4): 6-9. https://doi.org/10.1111/j.1439-0418.1971.tb02088.x
- Reck G.F. 1948. Descriptions of species of the genus Schizotetranychus (Träg.) from Georgia. Soobshcheniya Akademii Nauk Gruzinskoi SSR, 9: 445-452.
- Reis P.R., Teodoro A.V., Pedro Neto M., Da Silva E.A. 2007. Life history of Amblyseius herbicolus (Chant) (Acari: Phytoseiidae) on coffee plants. Neotrop. Entomol., 36(2): 282-287. https://doi.org/10.1590/S1519-566X2007000200016
- Ribaga C. 1904. Gamasidi planticoli. Riv. Patol. Veg., 10: 175-178.
- Ripka G., Fain A., Kaźmierski A., Kreiter S., Magowski W.Ł. 2002. Recent data to the knowledge of the arboreal mite fauna in Hungary (Acari: Mesostigmata, Prostigmata, and Astigmata). Acarologia, 42(3): 271-281.
- Ripka G., Király G., Szabó A., Kaźmierski A. 2022. Recent additions to the plant-inhabiting mite fauna of three European countries (Acari: Acariformes: Tydeoidea, Stigmaeidae). Syst. Appl. Acarol., 27(5): 865-887. https://doi.org/10.11158/saa.27.5.4
- Rodríguez-Cruz F.A., Venzon M., Pinto C.M. 2013. Performance of Amblyseius herbicolus on broad mites and on castor bean and sunn hemp pollen. Exp. Appl. Acarol., 60(4): 497-507. https://doi.org/10.1007/s10493-013-9665-y
- Saccaggi D.L., Ueckermann E.A. 2024. The problem of taxonomic uncertainty in biosecurity: South African mite interceptions as an example. Acarologia, 64(2): 363-369. https://doi.org/10.24349/top1-r59v
- Sadeghi H., Łaniecka I., Kaźmierski A. 2012. Tydeoid mites (Acari: Triophtydeidae, Iolinidae, Tydeidae) of Razavi Khorasan Province, Iran, with description of three new species. Ann. Zool., 62(1): 99-114. https://doi.org/10.3161/000345412X633685
- Sağlam H.D., Çobanoğlu S. 2010. Determination of Tenuipalpidae (Acari: prostigmata) species in parks and ornamental plants of Ankara, Turkey. Turk. J. Entomol., 34(1): 37-52.
- Sahraoui H., Grissa K.L., Kreiter S., Douin M., Tixier M.S. 2012. Phytoseiid mites (Acari: Mesostigmata) of Tunisian citrus orchards: Catalogue, biogeography and key for identification. Acarologia, 52(4): 433-452. https://doi.org/10.1051/acarologia/20122072
- Salarzehi S., Hajizadeh J., Hakimitabar M., Ueckermann E.A. 2018. A contribution to the knowledge of cheyletid mites of Iran with redescription of Eucheyletia flabellifera (Michael, 1878) (Prostigmata: Cheyletidae). Acarologia, 58(2): 457-470. https://doi.org/10.24349/acarologia/20184253
- Salmane I., Petrova V. 2002. Overview on Phytoseiidae mites (Acari: Mesostigmata, Gamasina) of Latvia. Latvijas Entomologs, 39: 48-54.
- Santos M.A. 1976. Evaluation of Zetzellia mali as a predator of Panonychus ulmi and Aculus schlechtendali. Environ. Entomol., 5(1): 187-191. https://doi.org/10.1093/ee/5.1.187
- Santos P.T.M. 2011. Acarofauna da vinha e infestantes em zonas edafoclimáticas diferentes na região de Setúbal [Ms Thesis] - Lisboa, Portugal: Instituto Superior de Agronomia, Universidade Técnica de Lisboa, pp. 111.
- Satyagopal K., Sushil S.N., Jeyakumar P., Shankar G., Sharma O.P., Sain S.K., Boina D.R., Srinivasa Rao N., Sunanda B.S., Asre S.R., Murali R., Arya S., Kumar S., Mahendiran G., Kalra V.K., Panda S.K., Sahu K.C., Mohapatra S.N., Ganguli J., Lakpale N. 2015. AESA based IPM package for Kiwi. National Institute of Plant Health Management, Rajendranagar, Hyderabad - 500 030 pp. 35.
- Schausberger P. 1992. Comparative investigations on the effect of different foods on development and reproduction of Amblyseius aberrans Oud. and A. finlandicus Oud. (Acarina, Phytoseiidae). J. Appl. Entomol., 113(1-5): 476-486. https://doi.org/10.1111/j.1439-0418.1992.tb00692.x
- Schausberger P. 1997. Inter- and intraspecific predation on immatures by adult females in Euseius finlandicus, Typhlodromus pyri and Kampimodromus aberrans (Acari: Phytoseiidae). Exp. Appl. Acarol., 21(3): 131-150. https://doi.org/10.1023/A:1018478418010
- Sciarappa W.J., Swift F., Knisley C.B. 1977. Distribution, abundance, and inter- specific associations of Typhlodromips sessor (Acarina: Phytoseiidae). Ann. Entomol. Soc. Am., 70(6): 955-959. https://doi.org/10.1093/aesa/70.6.955
- Seelmann L., Auer A., Hoffmann D., Schausberger P. 2007. Leaf pubescence mediates intraguild predation between predatory mites. Oikos, 116(5): 807-817. https://doi.org/10.1111/j.0030-1299.2007.15895.x
- Sengonca C., Drescher K. 2001. Laboratory studies on the suitability of Thrips tabaci Lindeman (Thysanoptera, Thripidae) as prey for the development, longevity, reproduction and predation of four predatory mite species of the genus Amblyseius (Acari, Phytoseiidae). Z. PflKrankh. PflSchutz., 108(1): 66-76. http://www.jstor.org/stable/43215384
- Siepel H., Cremers H., Dimmers W.J., Loomans A., Vierbergen B. 2018. Checklist of the mesostigmatic mites of the Netherlands. Nederlandse Faunistische Mededelingen, 51: 115-188.
- Sousa J.M., Gondim Jr. M.G.C., Lofego A.C., Moraes G.J. 2015. Mites on Annonaceae species in northeast Brazil and in the state of Pará. Acarologia, 55(1): 5-18. https://doi.org/10.1051/acarologia/20152147
- Soysal M., Akyazı R. 2018. Mite species of the vegetable crops in Ordu province with first report of Amblyseius rademacheri Dosse, 1958 (Mesostigmata: Phytoseiidae) in Turkey. Turk. J. Entomol., 42(4): 265-286. https://doi.org/10.16970/entoted.447218
- Steven D., Valenzuela L., Gonzalez R.H. 1997. Kiwifruit pests in Chile. Acta Hortic., 444: 773-777. https://doi.org/10.17660/ActaHortic.1997.444.118
- Summers F.M. 1960. Eupalopsis and Eupalopsellid mites (Acarina: Stigmaeidae, Eupalopsellidae). Fla. Entomol., 43(3): 119-138. https://doi.org/10.2307/3492677
- Summers F.M., Price D.W. 1970. Review of the mite family Cheyletidae. Univ. Calif. Publ. Entomol., 61: 1-153.
- Tempfli B., Pénzes B., Fail J., Szabó Á. 2015. The occurrence of tydeoid mites (Acari: tydeoidea) in Hungarian vineyards. Syst. Appl. Acarol., 20(8): 937-954. https://doi.org/10.11158/saa.20.8.9
- Terralink 2024. Bio Anso-Mite. [Internet]. [07 Aug 2024]. Available from: https://tlhort.com/products/bio-andersoni?_pos=1&_sid=d795a6afa&_ss=r
- Tixier M.S., Douin M., Kreiter S. 2020. Phytoseiidae (Acari: Mesostigmata) on plants of the family Solanaceae: Results of a survey in the south of France and a review of world biodiversity. Exp. Appl. Acarol., 81(3): 357-388. https://doi.org/10.1007/s10493-020-00507-0
- Tixier M.S., Kreiter S., Bourgeois T., Cheval B. 2007. Factors affecting density and diversity of phytoseiid mite communities in two arboreta in the South of France. J. Egypt. Soc. Parasitol., 37(2): 493-510.
- Tixier M.S., Kreiter S., Okassa M., Cheval B. 2009. A new species of the genus Euseius Wainstein (Acari: Phytoseiidae) from France. J. Nat. Hist., 44(3-4): 241-254. https://doi.org/10.1080/00222930903383529
- Tokkamış F.N. 2011. Tokat İlinde Yetiştirilen Bazı Sebze Türlerinde Faydalı ve Zararlı Akar (Acari) Türlerinin Belirlenmesi [Ms Thesis]. Tokat: Gaziosmanpaşa Üniversitesi, Fen Bilimleri Enstitüsü, Bitki Koruma Anabilim Dalı. pp. 69.
- Toyoshima S., Kishimoto H., Morii H., Amano H. 2014. Occurrence of Typhlodromips sessor (De Leon) (Acari: Phytoseiidae) on Mexican sunflower Tithonia rotundifolia (Miller) (Asteridae: Asteraceae) planted around a tea plantation in Japan. J. Acarol. Soc. Japan., 23(1): 29-33. https://doi.org/10.2300/acari.23.29
- TRG 2021. Leading Countries in Kiwi Exports in The World. Turkish Goods [Internet]. [10 March 2024]. Available from: https://www.turkishgoods.com/post/blog/leading-countries-in-kiwi-exports-in-the-world
- Tseng Y.H. 1982. Mites of the family Stigmaeidae of Taiwan with key to genera of the world (Acarina: Prostigmata). Phytopathologist and Entomologist of the National Taiwan University, 9: 1-52.
- Tseng Y.H. 1985. A taxonomic study of the Tydeidae of Taiwan (Acarina: Trombidiformes). J. Natl. Taiwan. Mus., 38(1): 129-168. https://doi.org/10.6532/JTM.198506_38(1).0007
- Tshikhudo P.P., Nnzeru L.R., Saccaggi D.L., Makhado R.A., Munyai T.C. 2021. Risk analysis of Brevipalpus species (Acari: Tenuipalpidae) introduction via kiwifruit (Actinidia spp.) imported to South Africa. Afr. Entomol., 29(2): 463-470. https://doi.org/10.4001/003.029.0463
- Tsolakis H., Ragusa E. 2017. Phytoseiid mites from the Basilicata region (Southern Italy): species diversity and redescription of Typhloseiulus arzakanicus (Arutunjan) with a key of the species of Typhloseiulus Chant and McMurtry 1994 (Parasitiformes: Phytoseiidae). Acarologia, 57(4): 805-821. https://doi.org/10.24349/acarologia/20174195
- TUIK 2023. Turkish Statistical Institute (TURKSTAT) Reports [Internet]. [10 March 2024]. Available from: https://www.tuik.gov.tr/
- Ueckermann E.A., Grout T.G. 2007. Tydeoid mites (Acari: Tydeidae, Edbakerellidae, Iolinidae) occurring on Citrus in southern Africa. J. Nat. Hist., 41(37-40): 2351-2378. https://doi.org/10.1080/00222930701589921
- Ueckermann E.A., Çobanoğlu S., Öğreten A. 2019. Re-description of two new tydeid records (Acari: Trombidiformes) with a key to Tydoidea species of Turkey. Syst. Appl. Acarol., 24(3): 497-507. https://doi.org/10.11158/saa.24.3.13
- Ueckermann E.A., de Vis R.M.J., Reybroeck E., Vervaet L., Van Lommel W., DeWitte J., Foque D., Gautam S., Ouyang Y., Vangansbeke D., De Clercq P., Van Leeuwen T. 2024. Redescription of Pronematus ubiquitus (McGregor, 1932) (Acari, Iolinidae) description of two new species and redescription of two additional species with a review of and key to all Pronematus species. Acarologia, 64(1): 277-311. https://doi.org/10.24349/tyki-9xlp
- Ueckermann E.A., Palevsky E., Gerson U., Recht E., Theron P.D. 2018. The Tenuipalpidae (Acari: Trombidiformes) of Israel. Acarologia, 58(2): 483-525. https://doi.org/10.24349/acarologia/20184255
- Uygun N., Ulusoy M.R., Karaca I. 1995. A citrus pest in the east Mediterranean region of Turkey, Polyphagotarsonemus latus (Banks) (Acarina: Tarsonemidae). Turk. J. Entomol., 19(1): 1-4.
- Van der Walt L., Spotts R.A., Ueckermann E.A., Smit F.J., Jensen T., McLeod A. 2011. The association of Tarsonemus mites (Acari: Heterostigmata) with different apple developmental stages and apple core rot diseases. Int. J. Acarol., 37(1): 71-84. https://doi.org/10.1080/01647954.2010.539981
- Vela J.M., Wong E., Jaques J.A., Ledesma C., Boyero J.R. 2017. Mite diversity (Acari: Tetranychidae, Tydeidae, Ioinidae, Phytoseiidae) and within-tree distribution in citrus orchards in southern Spain, with a special refernce to Eutetranychus orientalis. Exp. Appl. Acarol., 73(2): 191-207. https://doi.org/10.1007/s10493-017-0180-4
- Volgin V.I. 1987. Acarina of the family Cheyletidae of the world. New Delhi: Amerind Publishing Company. pp. 532.
- Wainstein B.A., Vartapetov S.G. 1973. Predatory mites of the family Phytoseiidae (Parasitiformes) of Adzharskaya ASSR. Akad. Nauk. Armenian. SSR. Biol. Zh. Arm., 26(2): 102-105.
- Walter D.E., Proctor H.C. 2013. Mites: Ecology, Evolution & Behaviour. Sydney: Springer Science+Business Media Dordrecht. pp. 494. https://doi.org/10.1007/978-94-007-7164-2
- Wang H., Jia J., Yu X., Han X., Yao Z. 1999. New pest mites of the peach fruits - Tarsonemus confusus and Tarsonemus bilobatus. Deciduous Fruit Tree, 2: 18-18
- Wrensch D.L., Ebbert M.A. 1993. Evolution and diversity of sex ratio in insects and mites. New York: Springer New York, NY, Routledge, Chapman & Hall. pp. 634. https://doi.org/10.1007/978-1-4684-1402-8
- Yaşarakıncı N., Hıncal P. 1997. The Research on determining the pests and beneficial species and their population densities on the tomato, cucumber, pepper and lettuce glasshouses in İzmir. Plant. Prot. Bull., 37(1-2): 79-89.
- Yeşilayer A., Çobanoğlu S. 2012. A new record of Cheletomimus berlesei (Oudemans) (Acari; Cheyletidae) for the Turkish fauna. Turk. Bull. Entomol., 2(3): 183-188.
- Yeşilayer A., Çobanoğlu S. 2013. Determination of Raphignathoid mites (Acari: Prostigmata: Raphignathoidea) ornamental plants of Istanbul (Turkey). Turk. J. Entomol., 37(1-2): 93-103.
- Zhang Z.Q. 2000. Key to Tarsonemidae of New Zealand. Final report to MAF Science Policy for Project FMA102, 1-35.
- Zhang Z.Q. 2003. Mites of Greenhouses. Identification, Biology and Control. Wallingford:



2024-03-27
Date accepted:
2024-09-21
Date published:
2024-10-10
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Akyazı, Rana; Soysal, Mete and Ueckermann, Edward A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



