A new species of stilt-legged mite of the genus Neophyllobius Berlese (Acari: Camerobiidae) from Brazil
Escobar-Garcia, Hector Alonso  1
; de Andrade, Daniel Júnior
1
; de Andrade, Daniel Júnior  2
; Mohammad-Doustaresharaf, Mojtaba
2
; Mohammad-Doustaresharaf, Mojtaba  3
and Ueckermann, Edward A.
3
and Ueckermann, Edward A.  4
4
1✉ São Paulo State University (UNESP), School of Agricultural and Veterinary Sciences, Jaboticabal, Brazil & Facultad de Agronomía, Universidad Nacional de Piura (UNP), Piura, Peru.
2São Paulo State University (UNESP), School of Agricultural and Veterinary Sciences, Jaboticabal, Brazil.
3Department of Plant Protection, Faculty of Agriculture, Azarbaijan Shahid Madani University, Tabriz, Iran.
4Unit for Environmental Sciences and Management, Potchefstroom Campus, North-West University, Private Bag X6001, Potchefstroom, 2520, South Africa.
2024 - Volume: 64 Issue: 2 pages: 592-601
https://doi.org/10.24349/x6a3-jlvyZooBank LSID: FBAA69ED-20E6-4532-B979-F081E0169CEE
Original research
Keywords
Abstract
Introduction
The family Camerobiidae Southcott (Acari: Trombidiformes: Prostigmata) is the second largest family in the superfamily Raphignathoidea; it consists of almost 171 species in seven genera; with the genus Neophyllobius, the largest of the family with 133 species reported (Beron 2020; Mirza et al. 2022; Escobar-Garcia et al. 2023). This stilt–legged mites, are widely distributed in both, temperate and tropical zones, and are free-living predators feeding on different phytophagous mites and crawlers of scale insects (Homoptera: Diaspididae, Margarodidae); however, in their habitats, they are usually in very low in numbers, as reported in the literature to date (Bolland 1986; Du Toit et al. 1998; Fan & Zhang 2005; Beron 2020; Mirza et al. 2022; Escobar-Garcia et al. 2023). Two stilt–legged mites' species have been recorded in South America, namely Tycherobius edaphon Flechtmann (Flechtmann 2001) described from Brazil, and Neophyllobius (Neophyllobius) unpensis Escobar-Garcia, Welbourn & Ueckermann (Escobar-Garcia et al. 2023) described from Peru.
Herein a new species of Neophyllobius is described and illustrated, on Couroupita guianensis Aubl. (Lecythidaceae), a native tree from the Amazon rainforest in South America recognized for its great economic, social, and environmental benefits, ethnomedicine, and its ability to control important pests and pathogens, such as larvicidal and ovicidal activities against the pest insects (Baskar et al. 2015; Esposito et al. 2023, Escobar-Garcia et al. 2024; CABI 2024). The purpose of this paper is to describe a new species of Neophyllobius.
Material and methods
Stilt–legged mites were collected from raceme-type inflorescences of Couroupita guianensis on the campus of the São Paulo State University (UNESP), Jaboticabal, Brazil (21°14′50.28″ S, 48°17′51.85″ W), in the spring of 2022 and 2023. Sampling involved collecting two raceme-type inflorescences from each of the four trees, every two weeks. Samples were placed in paper bags, and transferred to the Acarology laboratory (AcaroLab), UNESP, Jaboticabal, Brazil. The red mites with a dorsocentral opisthosomal white spot and long legs were collected with a fine-tipped brush under the stereomicroscope (Olympus SZ61), and then mounted dorso-ventrally on microscope slides in 7 μl Hoyer's medium and put in an oven at 50 °C for 7 days (Walter & Krantz 2009), the slides were then sealed with nail polish. Mite morphology was studied using a phase-contrast compound microscope (Nikon Eclipse E200). Specimens were measured with an ocular micrometre coupled to the microscope and presented in micrometres (μm); the holotype highlighted in bold. Distances between setae were calculated as the distance between the centres of two setal bases; the apparent length of a seta was measured from the setal base to the tip of the seta. Body length was measured between the posterior border of the idiosoma and the tip of the infracapitulum. Notations of gnathosomal follows those of Grandjean (1946) and for the idiosomal setae and leg setation those of Kethley (1990). The photos were taken using a camera (AixoCam MRc5) mounted on a phase-contrast compound microscope (Zeiss Axioscope AX10 Lab.A1). The type material is deposited in ESALQ— Department of Entomology and Acarology, Luiz de Queiroz College of Agriculture, University of São Paulo (USP), Brazil.
Taxonomy
Family Camerobiidae Southcott, 1957
Genus Neophyllobius Berlese, 1886
Type species: Neophyllobius elegans Berlese, 1886:19 [1].
Neophyllobius (Neophyllobius) unespensis Escobar-Garcia & Ueckermann n. sp.
ZOOBANK: 5B0ABC3F-B771-4DD5-B7D8-D9228858EB59 ![]()
(Figures 1–6)
Type materials
Holotype female, Brazil, collected on Couroupita guianensis, campus of the São Paulo State University (UNESP), Jaboticabal, Brazil (21°14′50.28″ S, 48°17′51.85″ W, 588 m a.s.l.), November 13, 2023; coll. H.A. Escobar-Garcia. Paratypes: a female (collected November 27, 2022), a protonymph (collected October 30, 2022), and a larva (collected December 11, 2022), same place of the holotype, coll. H.A. Escobar-Garcia, separate microscope slides and deposited in ESALQ.
General diagnosis. Female
Tarsi I–IV each with two mid–ventral setae; coxae II with one seta; femura I–IV with 4–3–3–2 setae; setae f1 ≥ e1, and are the longest of dorsocentral setae; most distal seta on femura I–IV not reaching articulation facet between genu and femur; genual setae I–III reaching slightly beyond the base of basal tibial seta; genual setae I–IV lengths as follows: I (70–74), II (65–66), III (70–74), IV (108–118).
Description. Female (n = 2)
(Figures 1–3)
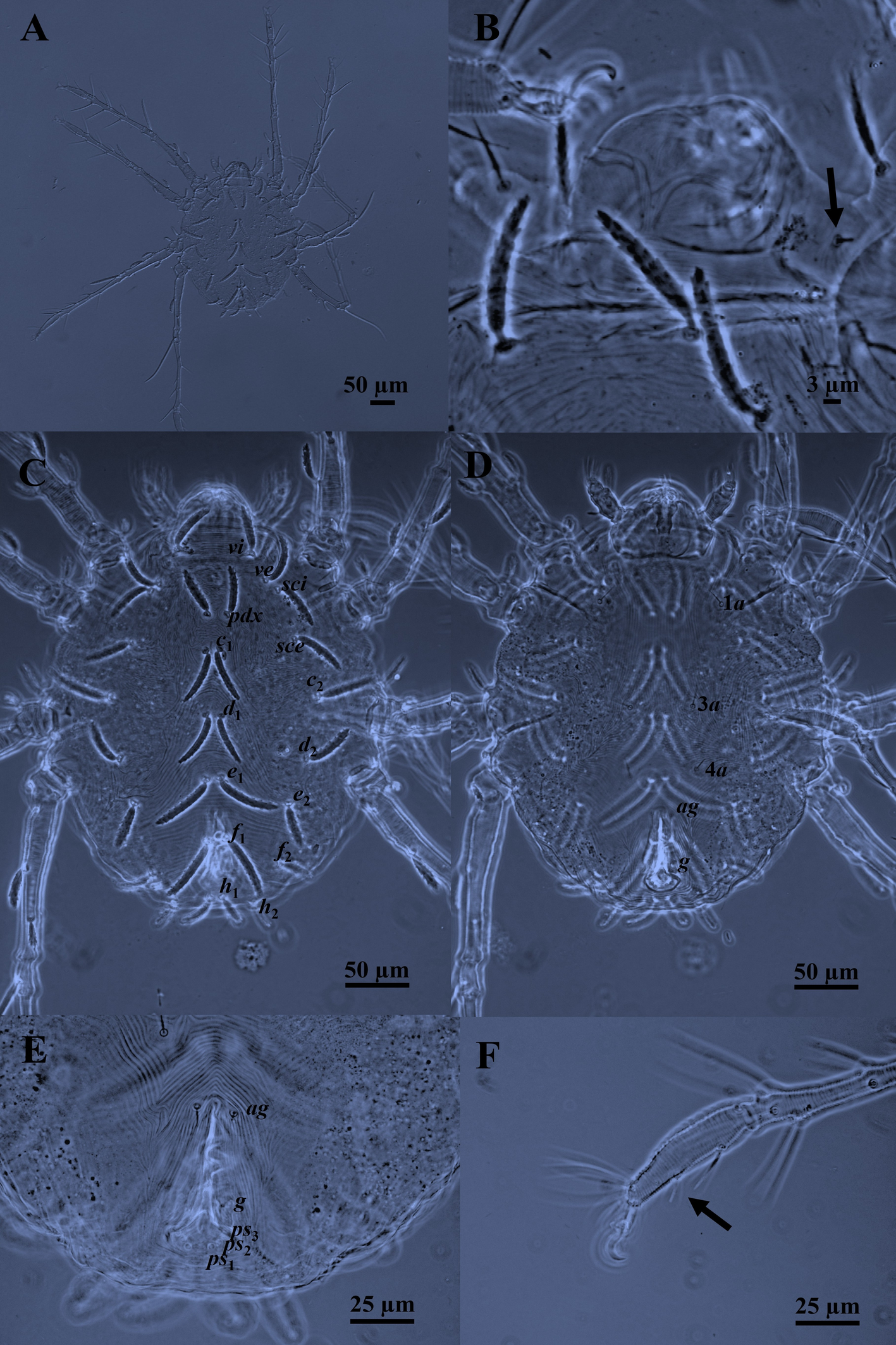






Body —In life, the color is red with a white dorsocentral opisthosomal spot. Dorsoventrally compressed, ovoid to nearly circular body with two lateral eyes on either side of the prodorsum. Body length 325–338. (Figs 1A and 2C).
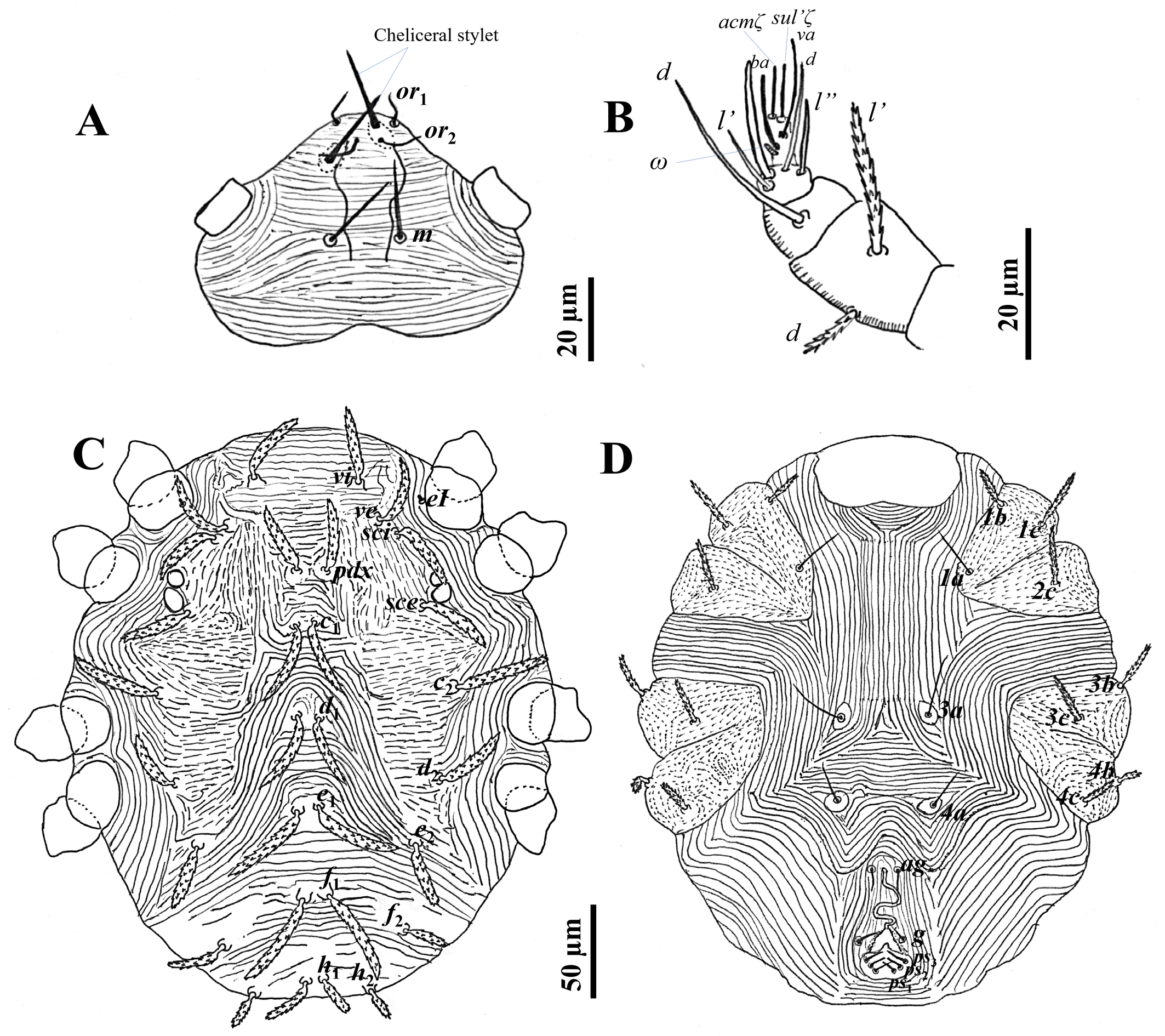
Idiosomal dorsum — Idiosoma broadly oval; two pairs of eyes anterolateral to sce, anterior pair smaller than posterior pair; cuticular striae transverse, oblique, and arched; cuticular striae transverse between vi–vi, striae arching between c1–d1–e2 (but diagonal behind e2); transverse between e1–f1–h1; longitudinal (and often oblique) thin striae laterally between c1–c2, d1–d2; shield-like areas carrying setae c2, d2 and e2; with 15 pairs of distinctly serrated setae, slightly curved; five pairs of prodorsal setae (vi, ve, sci, pdx, sce) and 10 pairs of dorsal opisthosomal setae (c1-2, d1-2, e1-2, f1-2, h1-2) all inserted on small tubercles (Figs 1C and 2C); some dorso–central setae shorter than interval to setae next behind; setae f1 ≥ e1 are the longest and h2 the shortest (f1 ≥ e1 ≥ c2 ≥ d1 ≥ c1 ≥ sci = pdx ≥ vi ≥ sce ≥ ve = d2 ≥ e2 > f2 ≥ h1 > h2, based on mean). Length of dorsal setae: vi 40–42, ve 38–40, sci 40–45, pdx 40–45, sce 38–43, c1 44–45, c2 50, d1 42–50, d2 38–40, e1 51–57, e2 36–39, f1 54–55, f2 30, h1 25–30, h2 23–24. Distance between setae: vi–vi 50–52, ve–ve 75–80, sci–sci 94–100, pdx–pdx 10–15, sce–sce 118–128, c1–c1 10–16, c2–c2 150–165, d1–d1 12–14, d2–d2 134–140, e1–e1 10–15, e2–e2 112–114, f1–f1 13–17, f2–f2 92–98, h1–h1 10, h2–h2 60–62.
Gnathosoma — Stylophore with one pair of peritremes forming a complete loop. Dorsal infracapitulum with pair of supracoxal setae ep (Figs 1B and 2A). The ventral infracapitulum with three pairs slender and simple setae: the m setae 20 long, two pairs of adoral setae or1 6 and or2 6. Distance between the m setae (m–m) 16–17, or1–or1 15, or2–or2 8–10. Cheliceral stylet 20–22 long (Fig. 2A). Palps, five-segmented (Fig. 2B): trochanter without setae; femur with two serrated setae (d 10, l′ 23), genu with one tactile seta d, tibia with three tactile setae (l′, l″ and d) and one sword‐like seta, tarsus with two eupathidia (acmζ, sulζ), two tactile setae (ba, va), and a small solenidion (ω) 3.
Idiosomal venter — Longitudinal striae between 1a–1a and 3a–3a; transverse between 4a–4a, striae arching between 4a–ag. The striations between 1a–3a setae are longitudinal. Setae 3a and 4a set on individual platelets. Striae form a diamond-shaped pattern between setae 3a–4a (Figs 1D and 2D). Striae on coxal fields I–II and III–IV stippled. One pair of aggenital setae (ag) present, genito-anal valves with a pair of genital setae (g) and three pairs of pseudanal setae ps1-3 (Figs 1E and 2D). Length of ventral setae: 1a 25–26, 3a 30, 4a 18–20, ag 13–14, g 10–12, ps1-3 9–10. Distance between setae: 1a–1a 72–92, 3a–3a 45–47, 4a–4a 49–50, ag–ag 15–17, g–g 18–25.
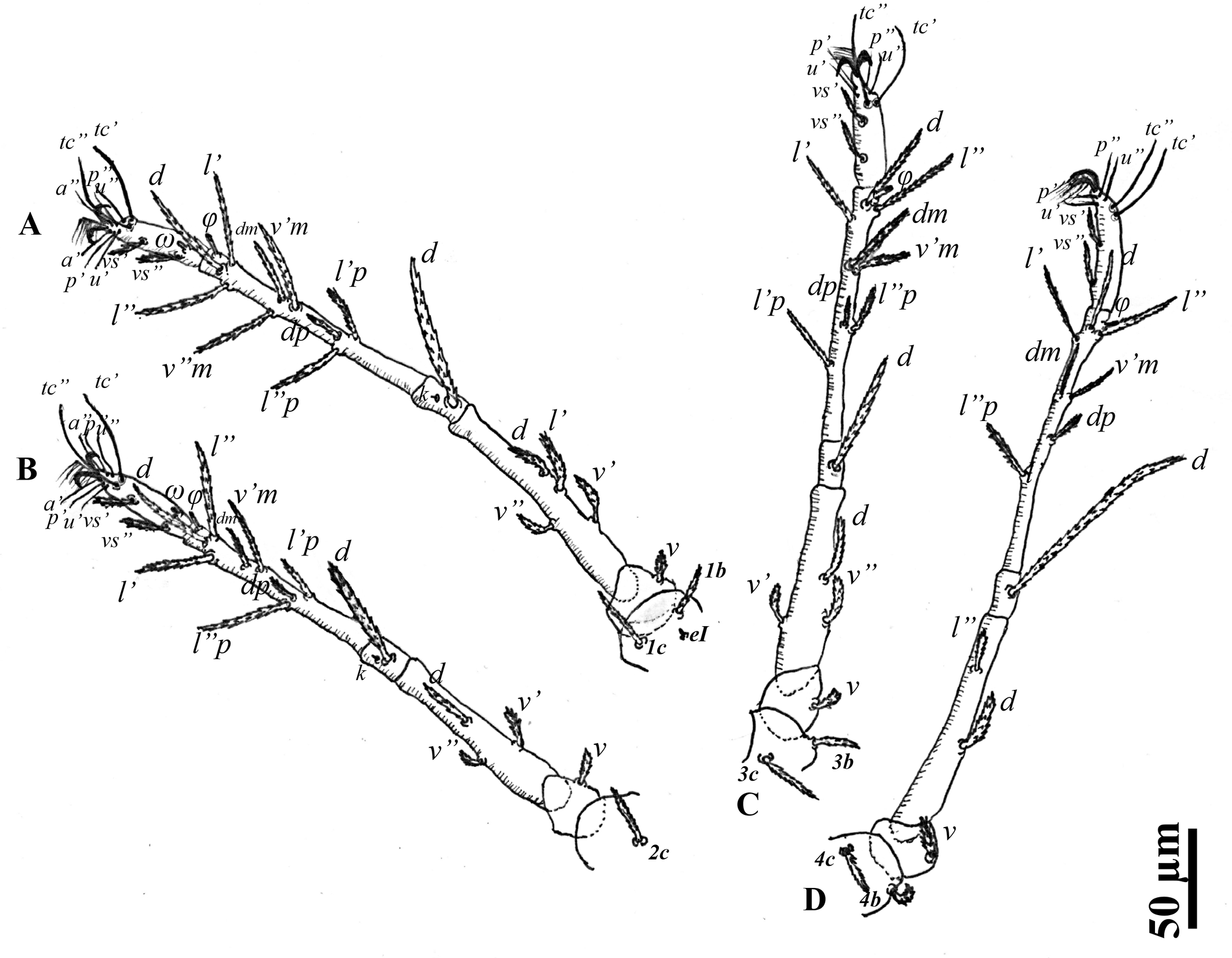
Leg chaetotaxy — Coxae I–II and coxae III–IV well separated; legs I–IV slender and long; all tarsi with ambulacrum bearing a pair of claws and an empodium with two rows of rayed hairs. Tarsi I–IV with two midventral setae (Figs 1F and 3). Genua I–IV each with one serrated seta; genu IV with a whip–like seta (d), IV (108–118) > III (70–74) = I (70–74) > II (65–66). Genua I and II each with a seta κ. Genual setae I–III reaching slightly beyond the base of basal tibial seta. Genual setae IV reaching beyond half the length of the tibia. Number of setae on leg segments (Fig. 3), with solenidia (on tibiae and tarsus), supracoxal setae (on coxae), and specialized setae (on genua) given in parentheses and not included in setal counts: coxae 3(+1eI)–1–2–2, trochanters 1–1–1–1, femora 4–3–3–2, genua 1(+1κ)–1(+1κ)–1–1, tibiae 9(+1φ)–8(+1φ)–8(+1φ)–7(+1φ), tarsi 10(+1ω)–10(+1ω)–8–8. Length of solenidia on tibiae (φ): Iφ (10–11); IIφ (7–8); IIIφ (7–8); IVφ (6). Length of solenidia on tarsi (ω): Iω (5); IIω (4).
Description. Protonymph (n = 1)
(Figure 4)
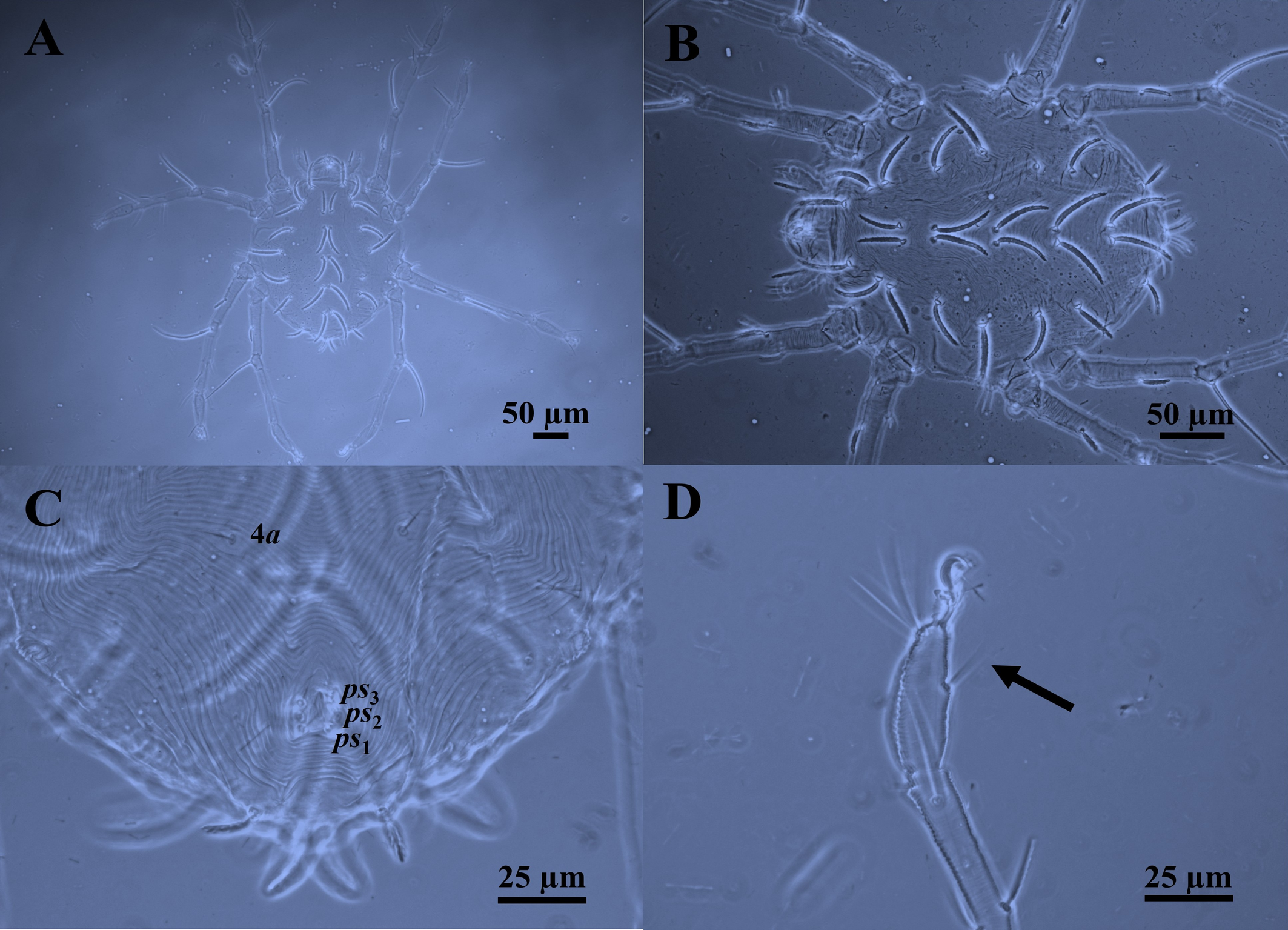


Body — Protonymph is also red, with two lateral prodorsal eyes and a dorsocentral opisthosomal white patch. Protonymph is dorsoventrally compressed, ovoid, or almost circular, and smaller than females. Body length 260 (Fig. 4A).
Idiosomal dorsum — As in female (Fig. 4B). Lengths of prodorsal setae: vi 35, ve 33, sci 38, pdx 33, sce 35 (sci > vi = sce > ve = pdx); lengths of dorsal opisthosomal setae: c1 40, c2 44, d1 40, d2 35, e1 45, e2 34, f1 45, f2 21, h1 22, h2 16, (e1 = f1 > c2 > c1 = d1 > d2 > e2 > h1 > f2 > h2); distances between dorsal idiosomal setae: vi–vi 43, ve–ve 65, sci–sci 74, pdx–pdx 12, sce–sce 95, c1–c1 10, c2–c2 125, d1–d1 10, d2–d2 105, e1–e1 10, e2–e2 95, f1–f1 10, f2–f2 70, h1–h1 7, h2–h2 35.
Gnathosoma — As in female. Infracapitulum with three pairs simple and slender setae: one pair infracapitular setae m 17, and two pairs of adoral setae or1 5 and or2 5, m–m 17, or1–or1 10, or2–or2 8. Dorsal infracapitulum with pair of supracoxal setae (ep). Cheliceral stylet 16 long. Palps five-segmented: trochanter without setae; femur with two serrated setae (d, l′), genu with one long dorsal tactile seta (d), tibia with three tactile setae (l′, l″ and d) and one sword‐like seta, tarsus with two eupathidia (acmζ, sulζ), two tactile setae (ba, va), and a small solenidion (ω) 2.
Idiosomal Venter — Similar to the female in having three pairs of pseudanal setae ps1-3, but without ag and g setae (Fig. 4C). Lengths of ventral setae: 1a 15, 3a 20, 4a 14, ps1-3 6. Distances between ventral idiosomal setae: 1a–1a 85, 3a–3a 37, 4a–4a 46.
Leg chaetotaxy — Legs like that of female. Tarsus I–IV each with one mid–ventral seta (Fig. 4D). Genua I–IV each with one serrated seta; genu IV with whip–like seta (d), IV 90 > III 85 > I 75 > II 70. Genua I and II each with a κ seta. Genual setae I–III reaching beyond half the length of the tibia. Genual setae IV reaching the base of the solenidion on tibia. Number of setae on leg segments, solenidia (on tibiae and tarsus), supracoxal setae (on coxae), and specialized setae (on genua) are given in parentheses and not included in setal counts: coxae 3(+1eI) –1–2–0, trochanters 1–1–1–0, femora 3–2–1–1, genua 1(+1κ) –1(+1κ) –1–1, tibiae 5(+1φ) –5(+1φ) –5(+1φ) –3(+1φ), and tarsi 9(+1ω) –9(+1ω) –7–5. Length of solenidia on tibiae (φ): Iφ 8; IIφ 5; IIIφ 5; IVφ 5. Length of solenidia on tarsi (ω): Iω 4; IIω 3.
Description. Larva (n = 1)
(Figure 5)
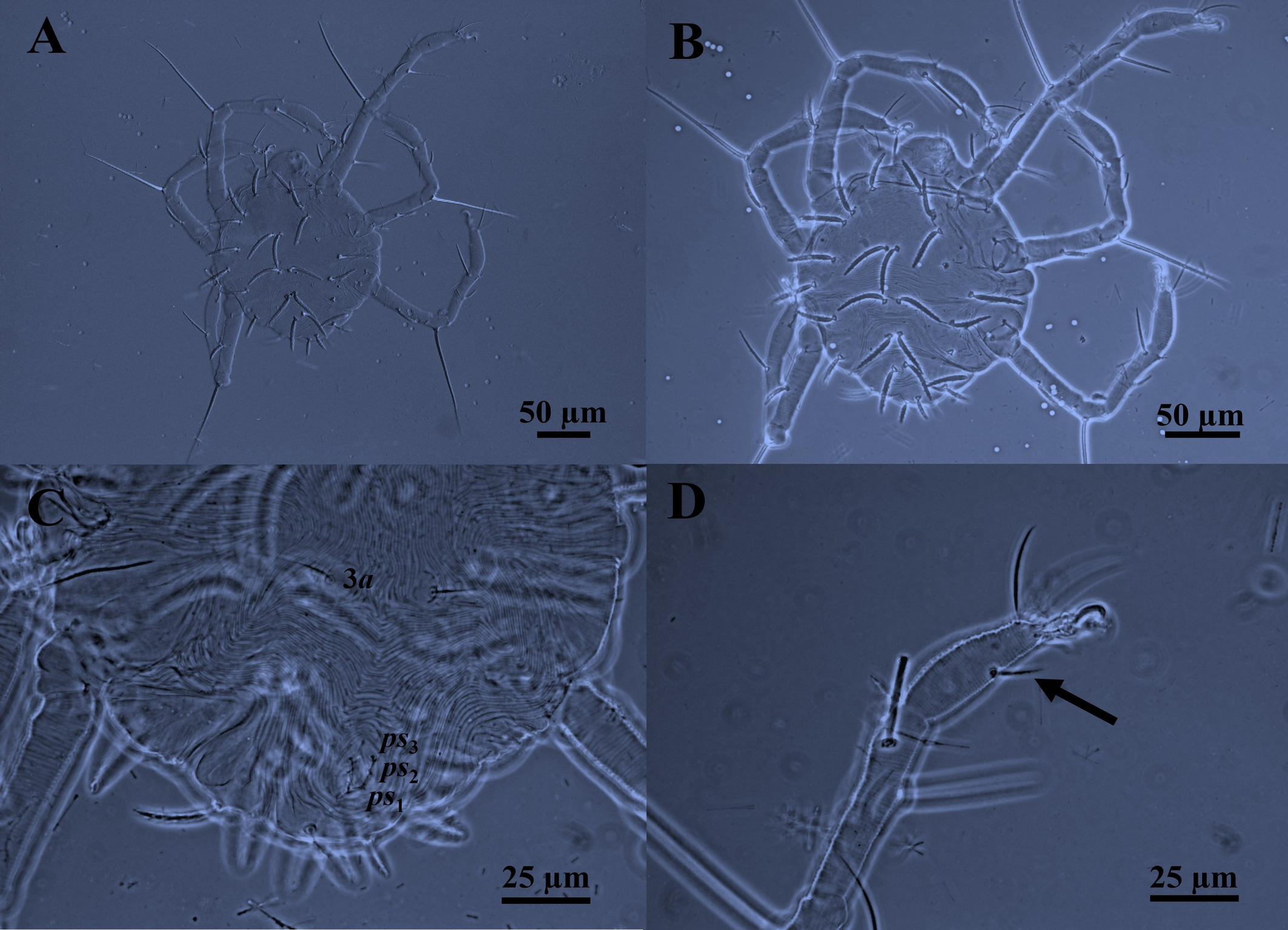


Body — Larva is likewise reddish yellow, with two lateral prodorsal eyes and a dorsocentral opisthosomal white patch. Larva are dorsoventrally compressed, ovoid, or almost circular, and smaller than protonymph. Body length 190 (Fig. 5A).
Idiosomal dorsum — In general, like that of protonymph. Idiosoma dorsally with 14 pairs of setae (pdx absent) situated on small tubercles (Fig. 5B). Shape of setae similar. Lengths of prodorsal setae: vi 28, ve 30, sci 33, sce 34 (sce > sci > ve > vi); lengths of dorsal opisthosomal setae: c1 36, c2 38, d1 35, d2 28, e1 34, e2 25, f1 34, f2 24, h1 16, h2 10, (c2 > c1 > d1 > e1 = f1 = d2 > e2 > f2 > h1 > h2); distances between dorsal idiosomal setae: vi–vi 33, ve–ve 55, sci–sci 65, sce–sce 77, c1–c1 8, c2–c2 110, d1–d1 10, d2–d2 80, e1–e1 8, e2–e2 75, f1–f1 7, f2–f2 50, h1–h1 8, h2–h2 36.
Gnathosoma – As in protonymph. Infracapitulum with three pairs simple and slender setae: one pair infracapitular setae m 6, and two pairs of adoral setae or1 6 and or2 6, m--m 20, or1–or1 8, or2–or2 6. Dorsal infracapitulum with pair of supracoxal setae (ep). Cheliceral stylet 16 long. Palps five-segmented: trochanter without setae; femur with a serrated setae d, genu with one long dorsal tactile seta d, tibia with three tactile setae (l′, l″ and d) and one sword‐like seta, tarsus with two eupathidia (acmζ, sulζ), two tactile setae (ba, va), and a small solenidion (ω) 2.
Idiosomal Venter — Like the protonymph in having three pairs of pseudanal setae ps1-3, but without 4a, ag, and g setae (Fig 5C). Lengths of ventral setae: 1a 12, 3a 20, ps1-3 5. Distances between ventral idiosomal setae: 1a–1a 55, 3a–3a 28.
Leg chaetotaxy — Legs like that of protonymph, but without leg IV. Tarsus I–III each with one mid–ventral seta (Fig. 5D). Genua I–III each with one whip–like serrated setae, III 112 > I 100 > II 87. Genua I and II each with a κ seta. Genual setae I–III reaching beyond half the length of the tarsus. Number of setae on leg segments, solenidia (on tibiae and tarsus), supracoxal setae (on coxae), and specialized setae (on genua) are given in parentheses and not included in setal counts: coxae 2(+1eI)–0–0, trochanters 0–0–0, femora 2–2–1, genua 1(+1κ)–1(+1κ)–1, tibiae 3(+1φ) –3(+1φ)–3(+1φ), and tarsi 7(+1ω) –7(+1ω)–5. Length of solenidia on tibiae (φ): Iφ 5; IIφ 5; IIIφ 5. Length of solenidia on tarsi (ω): Iω 2; IIω 2.
Etymology
The specific epithet ''unespensis'' refers to the name of the São Paulo State University (UNESP), Jaboticabal, São Paulo, Brazil, where this species was collected.
Remarks
The new species resembles Neophyllobius mkuzensis du Toit, Theron & Ueckermann 1998; and Neophyllobius gigantorum Bolland 1991; nevertheless Neophyllobius (N.) unespensis n. sp. can be distinguished from N. mkuzensis by a) serrated setae, slightly curved inserted on small tubercles (vs. serrated setae, slightly curved inserted on strong tubercles); b) cuticular striae arching between c1–d1–e2, (vs. transverse between c1–d1–e2); c) lengths of prodorsal setae vi (40–42), ve (38–40), sci (40–45), pdx (40–45), and sce (38–43) are shorter, [vs. (60–61), (63–72), 54, (47–54), and 54, respectively]; d) lengths of hysterosomal setae c2 50, d1 (42–50), d2 (38–40), e1 (51–57), e2 (36–39), f1 (54–55), f2 30, h1 (25–30), and h2 (23–24), are shorter, [vs. (88–95), (54–60), 57, (88–95), (50–54), 76, 44, (38–44), and (35–38) respectively]; e) seta on each of genua I and II exceeding the first row of tibial setae, (vs. seta on each of genua I and II not reaching base of basal tibial setae); f) setae f1 ≥ e1 are the longest of idiosomal setae, (vs. setae c2 ≥ e1 are the longest of idiosomal setae).
Neophyllobius (N.) unespensis n. sp. can be distinguished from N. gigantorum by a) all serrated setae, slightly curved inserted on small tubercles (vs. c2–e1–f1 serrated setae, slightly curved inserted on strong tubercles); b) lengths of prodorsal setae vi (40–42), ve (38–40), sci (40–45), and sce (38–43) are shorter, [vs. (45–50), (60–70), (55–60), and (45–55), respectively]; c) lengths of hysterosomal setae d1 (42–50), d2 (38–40), e1 (51–57), e2 (36–39), f2 30, h1 (25–30), and h2 (23–24), are shorter, [vs. (60–65), (70–75),(65–70), 55, (55–60), 35, and 30 respectively]; d) seta on each of genua I–III exceeding the first row of tibial setae, (vs. seta on each of genua I–III not reaching base of basal tibial setae); e) genual IV reaching beyond half the length of the tibia, (vs. genual IV reaching base of the first tibial seta); f) setae f1 ≥ e1 are the longest of idiosomal setae, (vs. setae d2 ≥ e1 are the longest of idiosomal setae).
Ecological observations
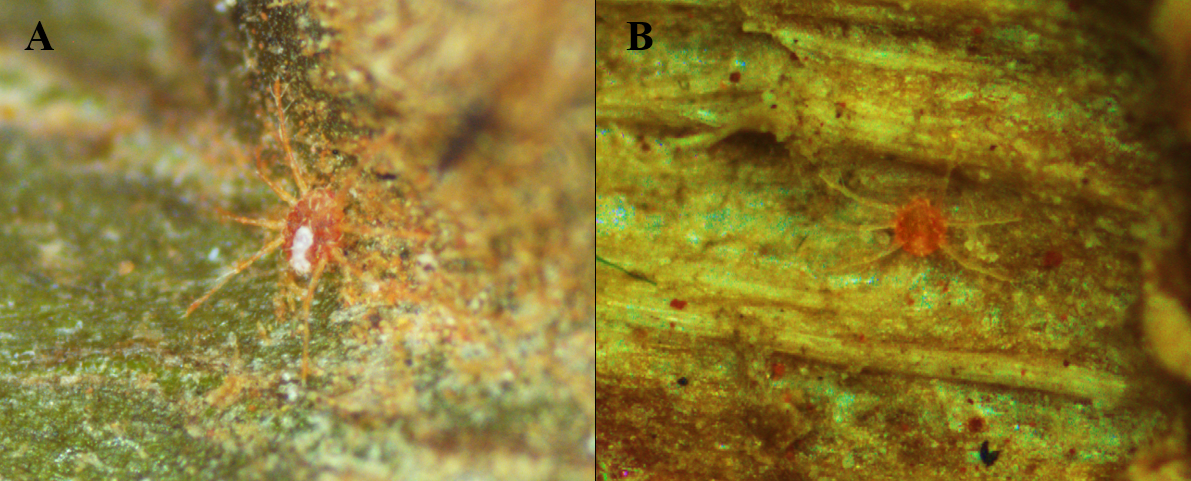


In this study Neophyllobius (N.) unespensis n. sp. was found in very low numbers on raceme-type inflorescences of Couroupita guianensis (Fig. 6). The orange eggs were observed to be placed individually and covered with webbing. Most mites were collected from October to December corresponding to the spring season in Jaboticabal, São Paulo, Brazil. Environmental conditions in the spring ranged from 11.8 to 21.7 °C to a maximum of 21.6 to 35.3 °C with a relative humidity ranging from 39.8 to 91.6%.
Acknowledgments
This study was financed in part by the Coordination of Superior Level Staff Improvement (CAPES), Brazil. We thank LCMAP Laboratory FCAV/UNESP—Brazil for the help with the figures. The meteorological elements used in this work were extracted from a set of data belonging to the collection of the Agroclimatological Station–Department of Exact Sciences, FCAV/UNESP–Jaboticabal, São Paulo, Brazil; 21°14′05″ S, 48°17′09″ W, 615 m a.s.l.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Baskar K., Ignacimuthu S., Jayakumar M. 2015. Toxic Effects of Couroupita guianensis Against Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Neotrop. Entomol., 44(1): 84-91. https://doi.org/10.1007/s13744-014-0260-7
- Berlese A. 1886. Acari dannosi alle piante coltivate. Premiata tipografia editrice. Padova: F. Sacchetto; p. 31.
- Beron P. 2020. Acarorum Catalogus VII. Trombidiformes, Prostigmata, Raphignathoidea (Fam. Barbutiidae, Caligonellidae, Camerobiidae, Cryptognathidae, Dasythyreidae, Dytiscacaridae, Eupalopsellidae, Homocaligidae, Mecognathidae, Raphignathidae, Stigmaeidae, Xenocaligonellididae). Pensoft & National Museum of Natural History, Sofia, p. 39-68. https://doi.org/10.3897/ab.e55087
- Bolland H.R. 1986. Review of the systematics of the family Camerobiidae (Acari, Raphignathoidea). I. The genera Camerobia, Decaphyllobius, Tillandsobius, and Tycherobius. Tijdschrift voor Entomologie, 129: 191-215.
- CABI Compendium. 2019. (accessed on February 2024). https://doi.org/10.1079/cabicompendium.15578
- Du Toit B.J., Theron P.D., Ueckermann, E.A. 1998. A new genus and four new species of the family Camerobiidae (Acari: Raphignathoidea) from South Africa. Int. J. Acarol., 24: 3-19. https://doi.org/10.1080/01647959808684122
- Escobar-Garcia H.A, de Andrade D.J, Welbourn C., Ueckermann E.A. 2023. Description of a new species of stilt-legged mite of the genus Neophyllobius Berlese (Acari: Camerobiidae) from Peru, Int. J. Acarol., 49: 214-221. https://doi.org/10.1080/01647954.2023.2236109
- Escobar-Garcia H.A., Nascimento V.F., De Melo M.A., Ramalho D.G., De Bortoli S.A. 2024. Aqueous botanical extracts, via different extraction methods, for control of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). J. Plant Dis. Prot., 131: 255-263. https://doi.org/10.1007/s41348-023-00809-6
- Esposito T., Pisanti S., Martinelli R., Celano R., Mencherini T., Re T., Aquino R.P. 2023. Couroupita guianensis bark decoction: From Amazonian medicine to the UHPLC-HRMS chemical profile and its role in inflammation processes and re-epithelialization. J. Ethnopharmacol., 313: 116579. https://doi.org/10.1016/j.jep.2023.116579
- Fan Q.-H., Zhang Z.-Q. 2005. Raphignathoidea (Acari: Prostigmata). Fauna of New Zealand. Manaaki Whenua Press, 52, p. 1-25.
- Flechtmann C.H.W. 2001. A new stilt-legged mite from the Brazilian Neotropics (Acari: Camerobiidae). Int. J. Acarol., 27(1): 41-44. https://doi.org/10.1080/01647950108684222
- Grandjean F. 1946. Au sujet de l'organe de Claparède, des eupathidies multiples et des taenidies mandibulaires chez les Acariens actinochitineux. Archives des Sciences Physiques et Naturelles. 28: 63-87.
- Kethley J. 1990. Acariformes, Prostigmata (Actinedida). In: Dindal, D.L. (Ed.), Soil Biology Guide. New York: John Wiley & Sons, p. 667-756.
- Mirza J.H., Kamran M., Alatawi F.J. 2022. New genus and new subgenera of camerobiid mites (Acari: Prostigmata: Camerobiidae) with a key to world species of the genus Neophyllobius. Insects, 13(344): 1-34. https://doi.org/10.3390/insects13040344
- Southcott R.V. 1957. Description of a new Australian Raphignathoid mite, with remarks on the classification of the Trombidiformes (Acarina). Proceedings of the Linnean Society of New South Wales, 81: 306-312.
- Walter D.E., Krantz G.W. 2009. Collecting, rearing, and preparing specimens. In: Krantz G.W., Walter D.E. (Eds), A manual of Acarology. 3rd ed. Texas Tech University Press, p. 83-96.



2024-03-08
Date accepted:
2024-04-22
Date published:
2024-04-30
Edited by:
Faraji, Farid

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Escobar-Garcia, Hector Alonso; de Andrade, Daniel Júnior; Mohammad-Doustaresharaf, Mojtaba and Ueckermann, Edward A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



