Free-living mites (Acari) of the Shokalsky Island, off the northern Gyda Peninsula, Kara Sea, High Arctic
Bizin, Mikhail  1
and Makarova, Olga L.
1
and Makarova, Olga L.  2
2
1✉ Laboratory of Synecology, Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences, Moscow, Russia.
2Laboratory of Synecology, Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences, Moscow, Russia.
2024 - Volume: 64 Issue: 1 pages: 172-191
https://doi.org/10.24349/634f-jb4iOriginal research
Keywords
Abstract
Introduction
The soil mite fauna of arctic landscapes is quite species-rich and diverse, including representatives of all main acarine orders (Behan 1978; Danks 1981; Hodkinson et al., 2013).
In terms of species diversity, local polar acarofaunas are comparable to those of insects (Chernov 2004; Coulson et al. 2014; Böcher et al. 2015; Seniczak et al. 2020b), even exceeding them in the northernmost polar desert regions (Makarova 2002а, 2023).
However, the knowledge of free-living mites varies extremely throughout the Arctic depending on either acarine suborder or geographical region. There are only a few areas, such as Greenland, the Svalbard Archipelago, Severnaya Zemlya Archipelago, Franz Josef Land Archipelago, as well as several territories of the Canadian Arctic and Alaska where the mite faunas have been investigated relatively comprehensive (Behan 1978, 1997; Danks 1981; Makarova 2002a, 2015a, 2023; Coulson et al. 2014; Seniszak et al. 2020b). In the Russian Arctic, oribatid and mesostigmatan checklists have been published for many Eastern European (including Novaya Zemlya and Vaygach Island) and Siberian localities (Grishina 1985; Davydova and Nikolsky 1986; Grishina et al. 1998; Makarova 1999, 2002a, b, 2012, 2013; Krivolutsky et al. 2003; Malkova 2009; Zenkova et al. 2011; Melekhina 2011, 2020; Melekhina and Zinovyeva 2012; Marchenko 2012; Makarova and Rosenfeld 2014; Ryabinin et al. 2015; Melekhina et al. 2019; Makarova and Bizin 2020, etc.). At the same time, there are virtually no regional studies dealing with other acarine suborders, primarily Prostigmata and Endeostigmata. Both are often the most abundant (Prostigmata more diverse as well) taxa in the acarocenoses of tundra and polar deserts (McAlpine 1965; Douce and Crossley 1977; MacLean et al. 1978; Hodkinson et al. 2013; Makarova 2002а, 2015а, 2023).
Over the vast Siberian Far North, communities of soil mites still remain virtually unexplored (Makarova et al. 2015). Several publications have contributed to the knowledge of oribatid and mesostigmatic mite assemblages in some areas of Taymyr (Ananjieva et al. 1973, 1979; Bogdanov 1975; Davydova et al. 1980; Grishina and Mordkovich 1996; Grishina et al. 1998) or Kolyma River delta (MacLean et al. 1978, Tolstikov et al. 1996). Only a few comprehensive studies on the free-living mites of Severnaya Zemlya and Franz Josef Land include detailed ecological information on all mite taxa (Makarova 1999, 2002a, b, 2023).
In August 2016, we carried out a two-week long field survey on the small Shokalsky Island in the southern part of Kara Sea. This island is of interest because (1) it lies very closely to the Gyda Peninsula, adjoining from the north, and its fauna could be considered as the northernmost along the whole West Siberian transect. (2) The island is located within the realm of the arctic tundra subzone (Alexandrova 1980), a transitional belt between the tundra and the polar desert zone. So, the analysis of the Shokalsky Island mite fauna could be useful for delimitation of these two natural zones, since such a subdivision of High Arctic is still in debate (see references in Makarova 2002b; Ermokhina et al. 2023). (3) Finally, landscapes of Shokalsky Island and the entire Gyda Peninsula are similar to those of the neighboring Yamal Peninsula, while being much less modified by property development. In this regard, an inventory of the local biodiversity of an environmentally intact area in the West Siberian arctic tundra could be of use for environmental monitoring programs.
Material and methods
Study area
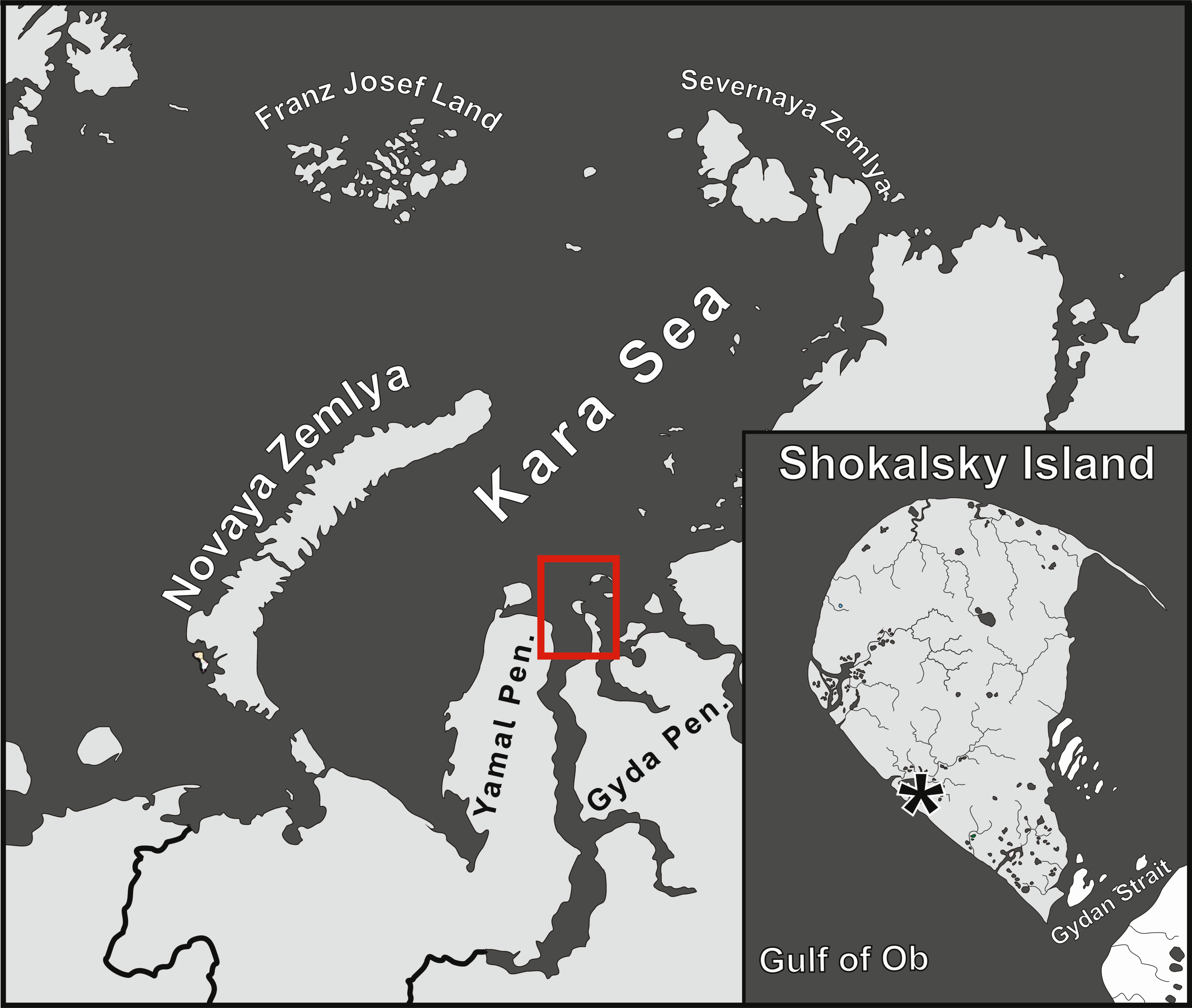
The Shokalsky Island is located in the Kara Sea (72°58′N 74°27′E), east of the Gulf of Ob mouth (Figure 1), 5 km north of the Gyda Peninsula, being separated from the latter by the narrow (5–9 km) and shallow (0.5–6 m) Gydan Strait with pronounced tides, about 0.8 m (Kalyakin et al. 1999). The island is small, 30 km × 20 km (428 km2), and rather flat with only low hills (up to 10 m above sea level; Figure 2). Its surface is mainly composed of sands and almost entirely covered with vegetation (Figure 2; Kalyakin et al. 1999). The seasonally thawing layer of the soil varies between 0.9–1.2 m depth.


Due to oceanic impact, the Shokalsky Island has a harsh humid Arctic climate. The frost-free period does not exceed two months per year. During the last decade, the mean annual temperature at the nearest observation point, the M.V. Popov Weather Station (Bely Island, northern Yamal Peninsula), was –8.7 °C, the mean temperature of July amounting to 5.8 °C, and that of January to about –20 °C. Annual precipitation is not more than 300 mm, with about 50% as rains. Relative air humidity is high, almost 90% on average (Weather Archive of M.V. Popov Weather Station 2023).
The dominant soil types are Reductaquic Turbic Cryosols and Histic Reductaquic Turbic Cryosols (Kalyakin et al. 1999; IUSS Working Group WRB 2015). Like the northern part of Gyda Peninsula, the Shokalsky Island is located within Yamal-Gyda Subprovince of the European-West Siberian Province of the Arctic Floristic Region (Yurtsev et al. 1978).



Sampling
The material was collected on 5–17 August 2016 in the west of the island (Figure 1). Soil samples were cut out using a knife and a plastic frame (5 x 5 cm) in the most typical habitats (Table 1). All cores included the aboveground parts of plants, litter (or turf) and topsoil fragments down to 5 cm. A total of 53 samples were processed.
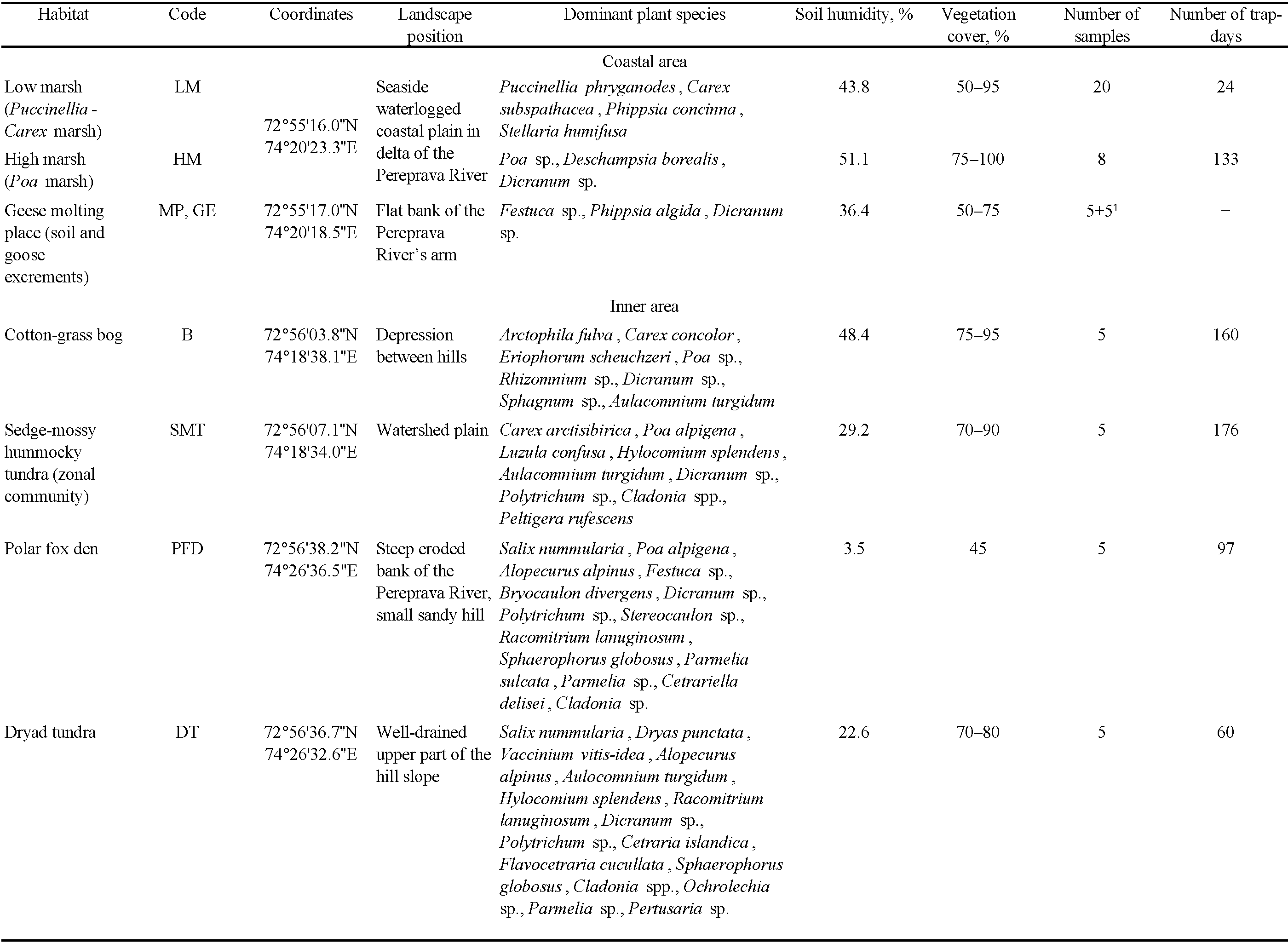


Four sets of samples were taken on the coastal floodplain in the delta of Pereprava River, the largest river of the island (Table 1). The vegetation cover of this area is the characteristic high-arctic salt marsh influenced by marine tidal inundation. The first series of samples was taken in the zone of low marsh, dominated by the halophilous grasses Puccinellia phryganodes (Trin.) Scribn. & Merr. and Carex subspathacea Wormsk. ex Hornem. The second series concerned the zone of high marsh with prevalence of Poa sp. and Deschampsia borealis (Trautv.) Roshev. The third series was a molting place of brants, Branta bernicla (Linnaeus, 1758), with Festuca sp. and Phippsia algida (Soland.) R. Br. In addition, samples of geese excrements (5 samples, each 125 cm³ in volume) were collected on the molting place (Table 1).
The other habitats studied were located in the inner part of the island. Plots of a cotton-grass bog, of sedge-mossy hummocky tundra, and of dryad tundra, as well as the meadow covering the polar fox den were explored (Table 1).
The samples taken on salt marshes were more numerous than others (Table 1) because of our special interest in the formation of coastal acarocenoses (Bizin et al. 2021).
Besides soil samples, mites were collected using pitfall trapping, hand sorting of sod patches and moss cushions, and through mowing herbs and dwarf-shrubs with an insect net (Makarov et al. 2018; Nekhaeva 2018).
Mite extraction and identification
The samples were kept in the cold, each sample being wrapped in paper and placed inside a plastic bag for no more than 5–7 days. Microarthropods were extracted into 96% alcohol from the cores in the lab (in Moscow) using Tullgren funnels by drying the samples until their complete desiccation (8–10 days) with neither additional heating nor light. Mites were slide-mounted in Hoyer's medium and identified. The material is currently kept in the collection of the Laboratory of Synecology, Severtsov Institute of Ecology and Evolution RAS, Moscow.
The following publications were mainly used for taxonomic identifications: Chant and Hansell (1971); Ghilarov et al. (1975, 1977); Balogh and Mahunka (1983); Behan-Pelletier (1985); Karg (1993); Makarova (2000a, 2015b, c); Chant and McMurtry (2003); Weigmann (2006); Khaustov (2008); Bayartogtokh (2010); Lindquist and Makarova (2011); Makarova and Behan-Pelletier (2015). Immature Oppiidae (impossible to identify) were collected in the dryad tundra only (142 juvenile specimens vs 350 adults). They were attributed to species proportionately to the adult ratio and combined with adults in each sample.
Data analysis
The mite density was calculated per m². Quantitative data were previously square-root-transformed prior to analysis.
To determine the efficiency of sampling efforts and to understand if further sampling was likely to reveal more taxa, we used cumulative curve and general sample rarefaction curve.
To examine the assemblage structure, we used the non-metric multidimensional scaling ordination (NMDS) based on Bray-Curtis dissimilarity of species abundance data in separate samples, as well as the principal coordinates analysis (PCoA) of qualitative data based on Jaccard index.
The following common diversity indicators were used: number of mite species per sample and per habitat, the Shannon index, and the Berger-Parker index (Magurran 1988). We tested the differences between total mite density and diversity indicators in various habitats using the Kruskal-Wallis test with pairwise comparisons through the Dunn's test via ''dunn.test'' package in R software (Dinno 2017; R Core Team 2021).
Rarefaction curve and both ordinations (NMDS, PCoA) were carried out in PAST software vers. 4.02 (Hammer et al. 2001).
Results
A total of 13,343 specimens were examined, including immature stages. In soil samples, we identified 77 species belonging to all major orders of mites. Additionally, four species, namely Melichares parvanalis (Thor, 1930) (Mesostigmata), Neomolgus sp., Eupodes cf. voxencollinus Thor, 1934, and Penthaleus cf. major (Dugès, 1834) (Prostigmata), were found in pitfall material. In total, 81 free-living mite species representing 36 families are presently known to occur in the Shokalsky Island (Table 2).



The suborder Prostigmata was especially diverse (43–68% of the species list in a separate habitat; Figure 3A), being represented by 38 species (31 genera, 15 families). Cyta latirostris (Hermann, 1804), Claveupodes delicatus Strandtmann & Prasse, 1977, Eupodes cf. voxencollinus, and Poecilophysis cf. saxonica (Willmann, 1934) were recorded from the Russian Arctic at first time. The species, Charadracarus hurdi Newell, 1960 (Johnstonianidae), identified by J. Mąkol (Wroclaw University of Environmental and Life Sciences), was found to occur in Russia at first time.
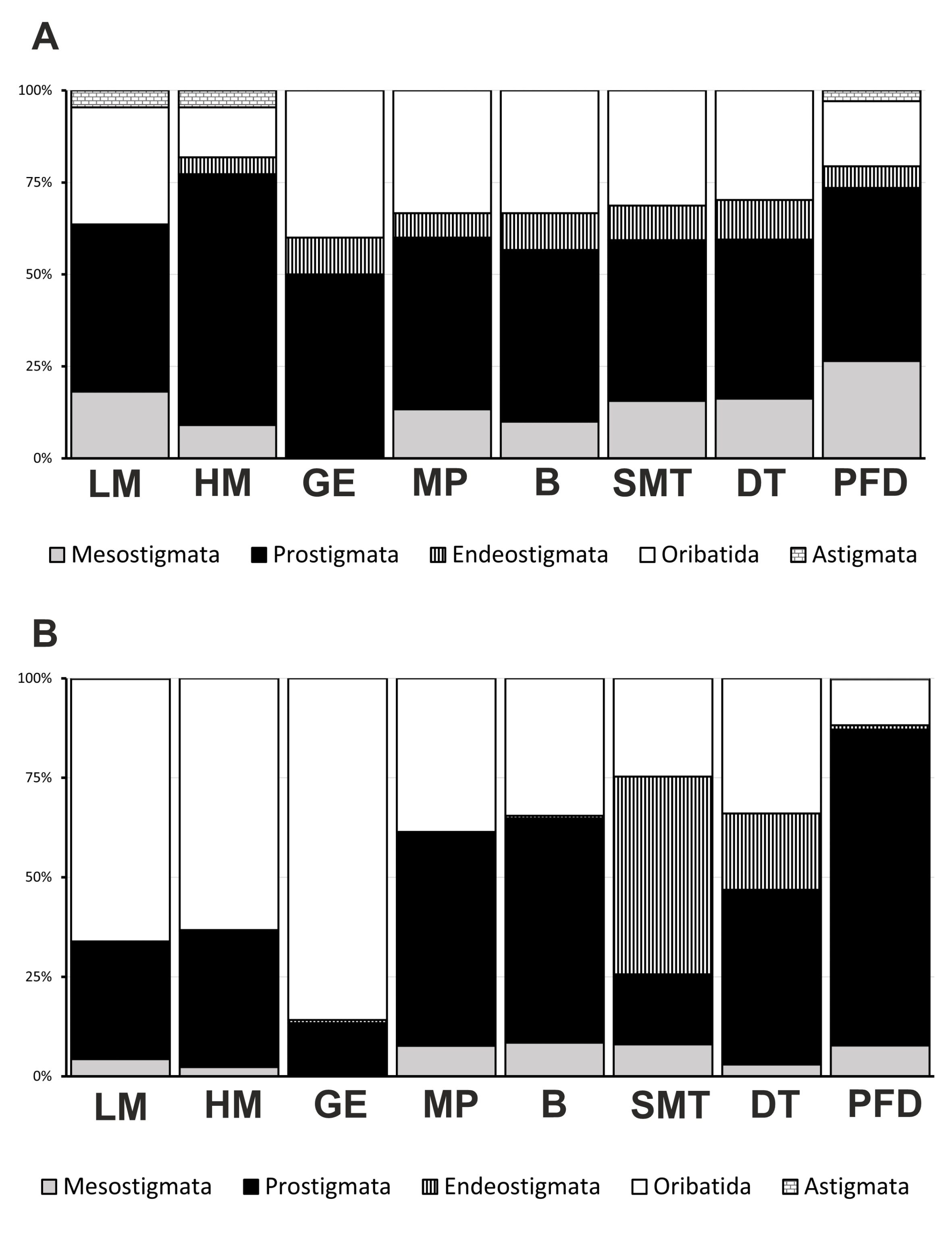


The suborder Oribatida amounted to 18 species (13 genera, 9 families), Mesostigmata included 17 species (13 genera, 7 families), while Endeostigmata (5 species, 3 genera, 3 families) and Astigmata (3 species, 2 genera, 2 families) were less diverse. The most species-rich families were Ascidae (9 species), Eupodidae (7), Tarsonemidae (4), and Ceratozetidae (4) comprising about a third of the total checklist. Most genera (77%) were represented by one species only, whereas Arctoseius, Zercon (Mesostigmata), Neoprotereunetes (Prostigmata), Alicorhagia (Endeostigmata), Liochthonius, and Moritzoppia (Oribatida) contained from 3 to 9 species each (Table 2).
Of the 58 securely identified species (Table 2), at least seven are distributed worldwide (cosmopolitan or subcosmopolitan), and 19 are polyzonal. At the same time, 24 identified species (41%) showed circumpolar or similar distribution patterns (Holarctic arctic or Holarctic arctic-montane). The proportion of these cryobiontic species varied between suborders from 30% (Prostigmata) to 58% (Mesostigmata).
The species, Liochthonius sellnicki (Thor, 1930), Hermannia scabra (L. Koch, 1879), Nanorchestes cf. gilli Strandtmann, 1982, Svalbardia lucens (L. Koch, 1879), as well as Cocceupodes cf. mollicellus (C.L. Koch, 1838), Eupodes cf. boerneri (Thor, 1934), Cheilostigmaeus longisetosus Willmann, 1951 occurred in six of the eight studied habitats (Table 2). Two other species, Steneotarsonemus arcticus Lindquist, 1986 and Paratriophtydeus sp., were recorded in five and four habitats, respectively. At the same time, 28 species were found only in one biotope.
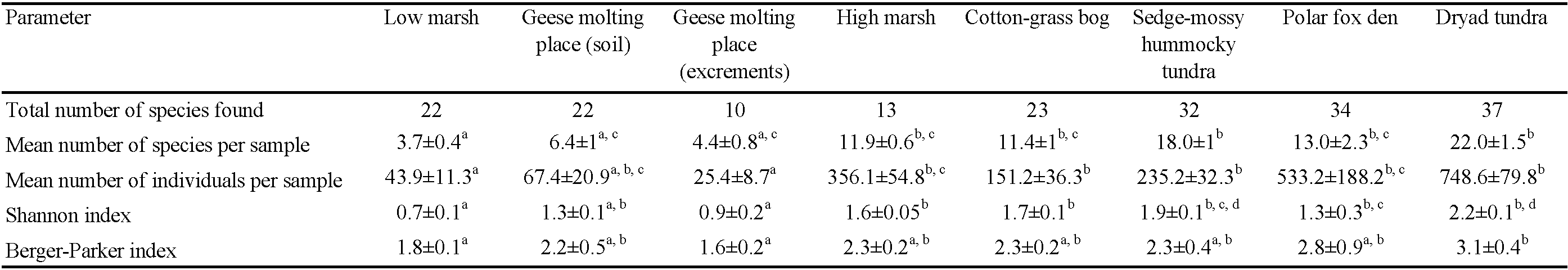
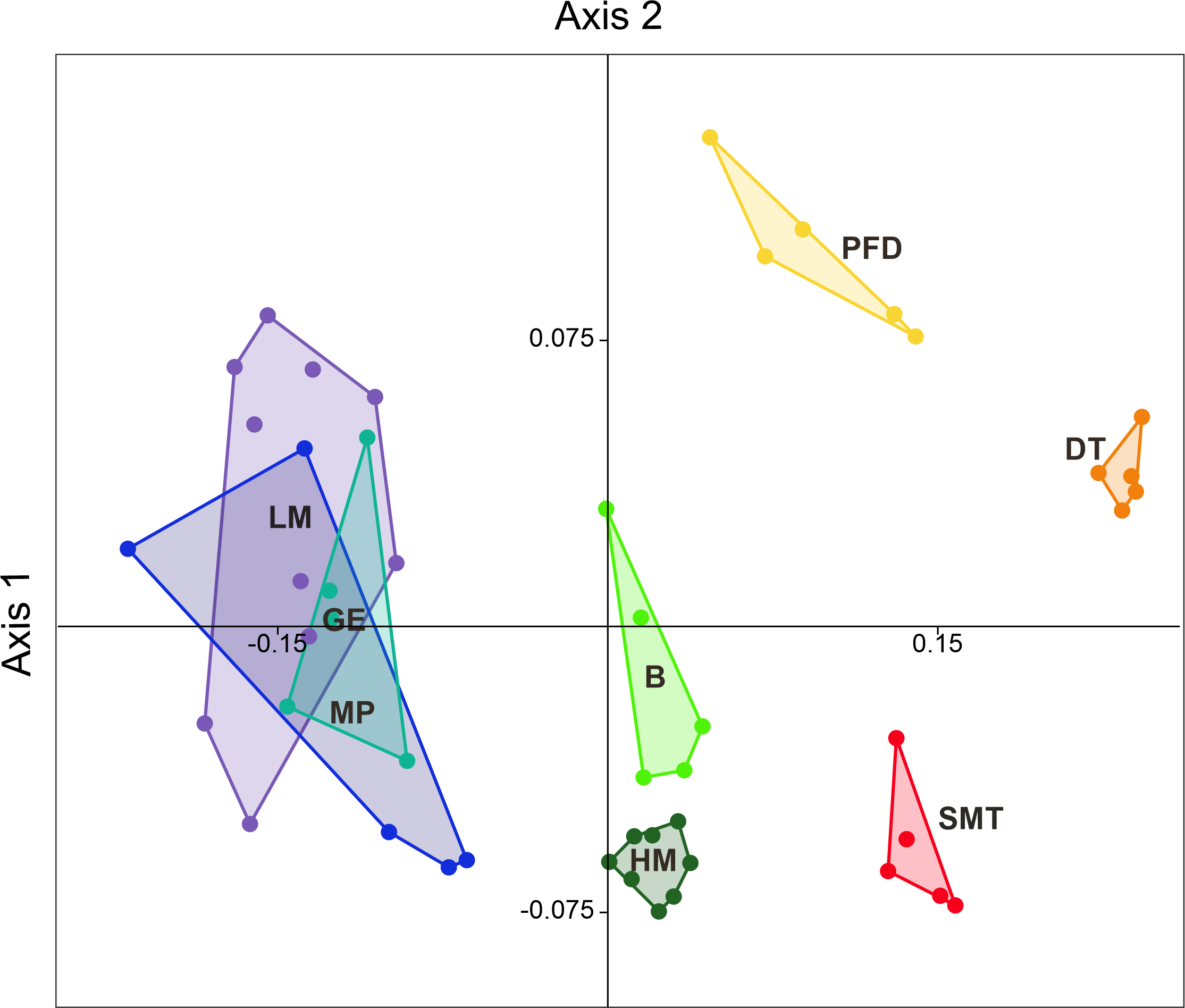
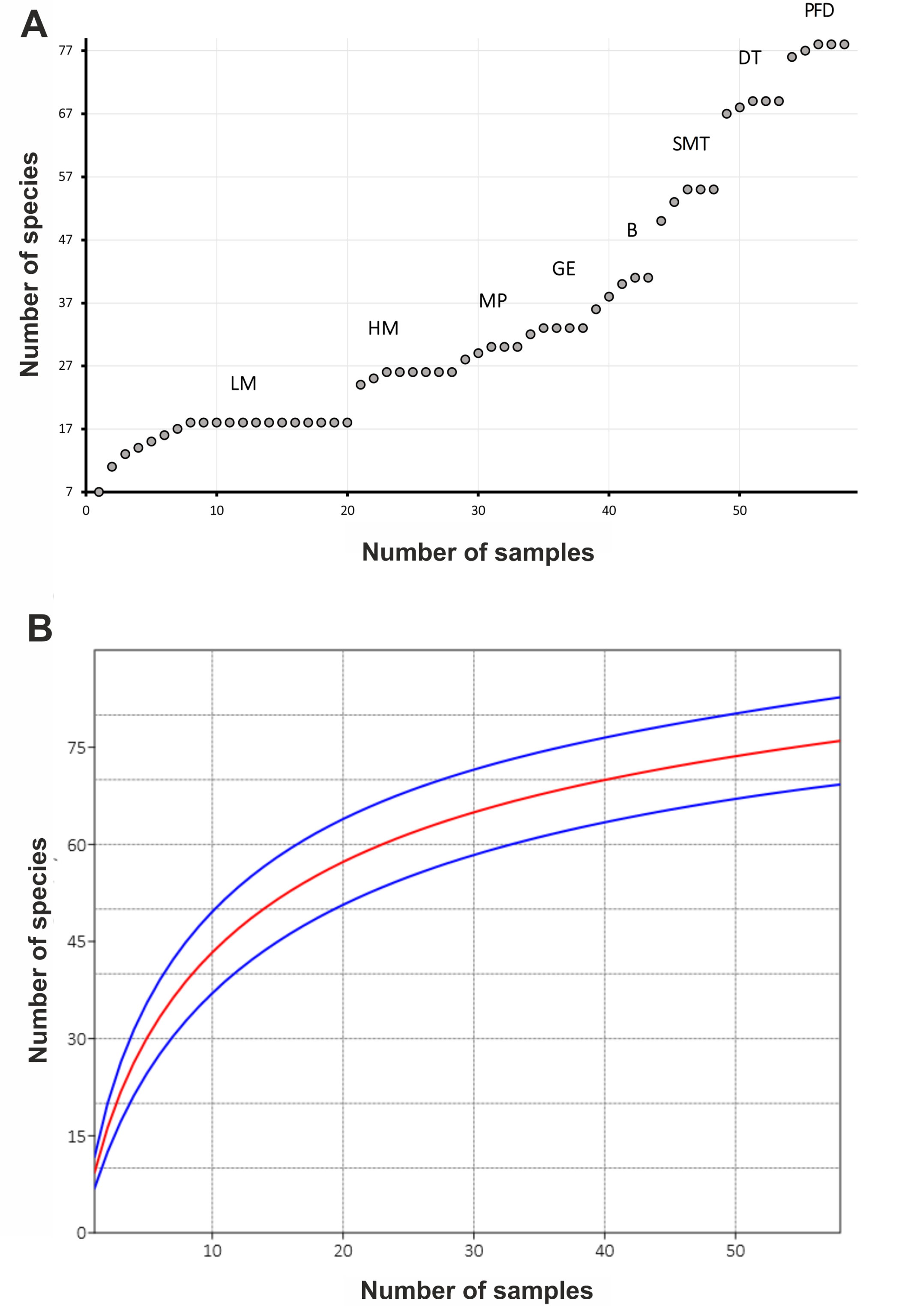
The NMDS plot showed that samples generally grouped into clusters in accordance with habitat attribution (Figure 4). Each separate habitat series of samples supported 10–37 species (mean = 24.1, Table 3), while the individual samples yielded 3–22 species (mean = 11.4) in different habitats. The cumulative curve (Figure 5A) grows regularly, where sets of samples from the coastal plain found their places, and shows a steeper trajectory in the right half, where data from the sedge-mossy tundra, the dryad tundra and the meadow of the polar fox den were accumulated. The overall sampling rarefaction curve failed to approach an asymptote (Figure 5B).






The total mite density of individual habitats varied significantly (χ² = 41.6; df = 7; p-value = 0.000), increasing along the hydrological gradient from low marsh and cotton-grass bog (10160–60480 ind./m²) to dryad tundra and polar fox den, reaching 213280–299440 ind./m² (Table 2).
In the material examined (including specimens collected by pitfalls and hand sorting), the most abundant species were found to be Steneotarsonemus arcticus (13% all individuals), Paratriophtydeus sp. (12%), Liochthonius sellnicki (10%), Nanorchestes cf. gilli (7%), Svalbardia lucens (7%), and Hermannia scabra (4%). Members of Prostigmata were dominant in a half of habitats (Table 2, Figure 3B). Only on salt marshes they were replaced by Oribatida.
The Shannon index of the assemblages varied from 0.7 (low marsh) to 2.2 (dryad tundra), the mean value being 1.3 (Table 3). The differences between all habitats were statistically significant (χ2 = 38.0; df = 7; p-value = 0.000003). On the studied landscape profile, the index changed in accordance with species number (both increased with elevation).
The dominance structure estimated based on the Berger–Parker index (used as 1/D) changed irregularly along the profile (Table 3). The highest values belonged to polar fox den (2.8) and dryad tundra (3.1), the lowest to low marsh (1.8) and geese molting place (for samples of excrements, 1.6). As a whole, the values of that index for all habitats were barely differed from one another (χ2 = 14.4; df = 7; p-value = 0.04).
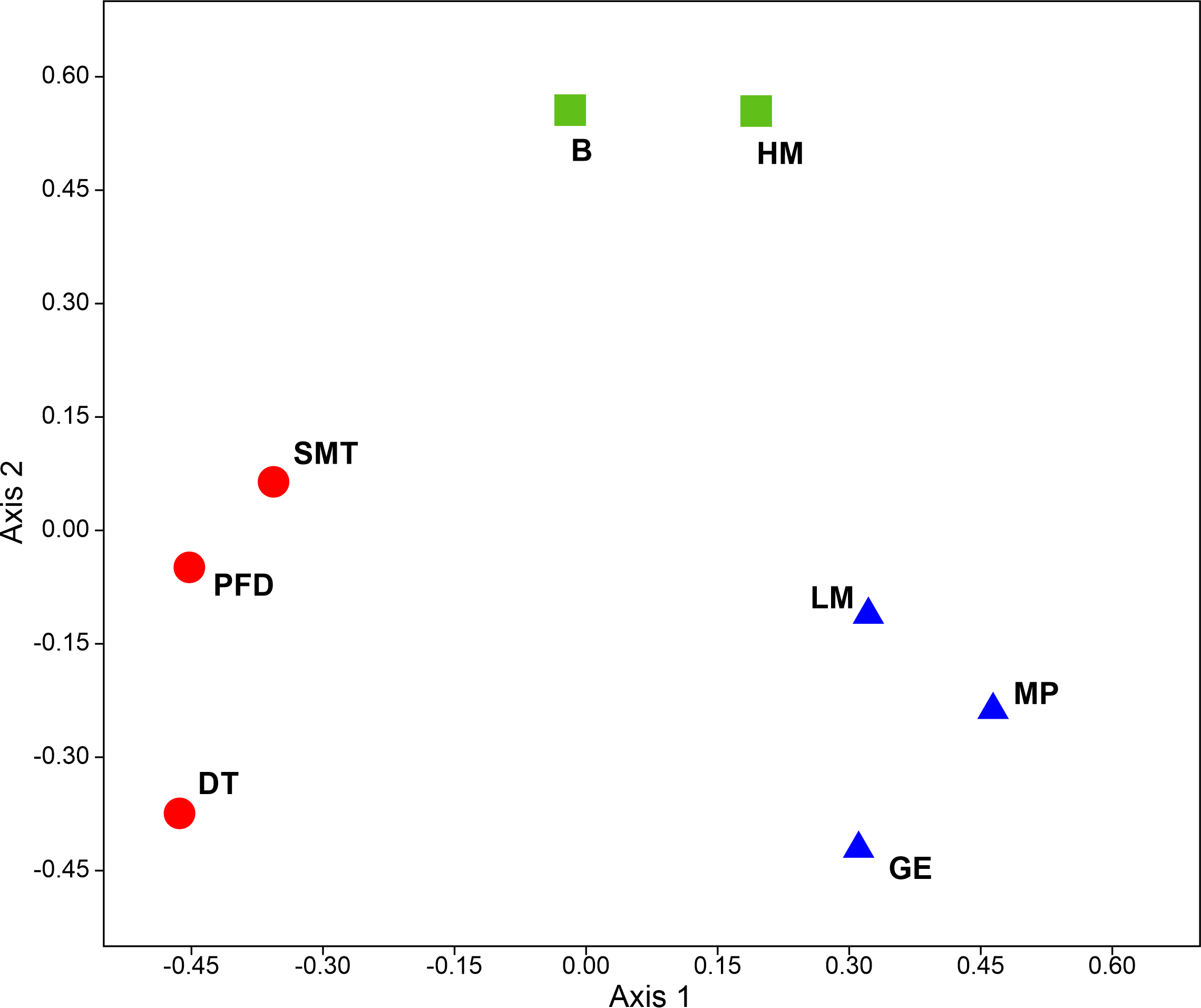
In general, mite diversity was higher in well-drained habitats (tundra sites, polar fox den). Species compositions clearly form three groups (Figure 6) encompassing the coastal, wet mossy, and well-drained habitats.


Discussion
Even though the above information on the mite communities of Shokalsky Island is based on relatively limited material collected in small areas, it is interesting to compare it to the polar desert acarocenoses of the Bolshevik Island (Severnaya Zemlya), and Franz Josef Land, the closest Arctic archipelagos where soil mites have been studied in detail (Makarova 1999, 2002a, b, 2023).
The acarocenoses of polar deserts show some peculiarities making them distinguishable from those in the tundra zone (Makarova, 2002a, b, 2023): (1) first, they support a relatively high taxonomic diversity of mites (and arachnids in general) compared to the insects. (2) Species of the suborder Prostigmata (usually members of Eupodoidea) in terms of both diversity and abundance prevail in most habitats, while the representation of the suborder Oribatida is considerably lesser. (3) Most acarine genera are represented by a single species only, while the diversity of Arctoseius remains as high as in the subarctic regions. (4) Mites appear to be unevenly distributed within individual habitats (due vegetation heterogeneity), as well as across a landscape: many species prefer the warmest substrates, often of zoogenic origin.
Species diversity and taxonomic structure of the fauna
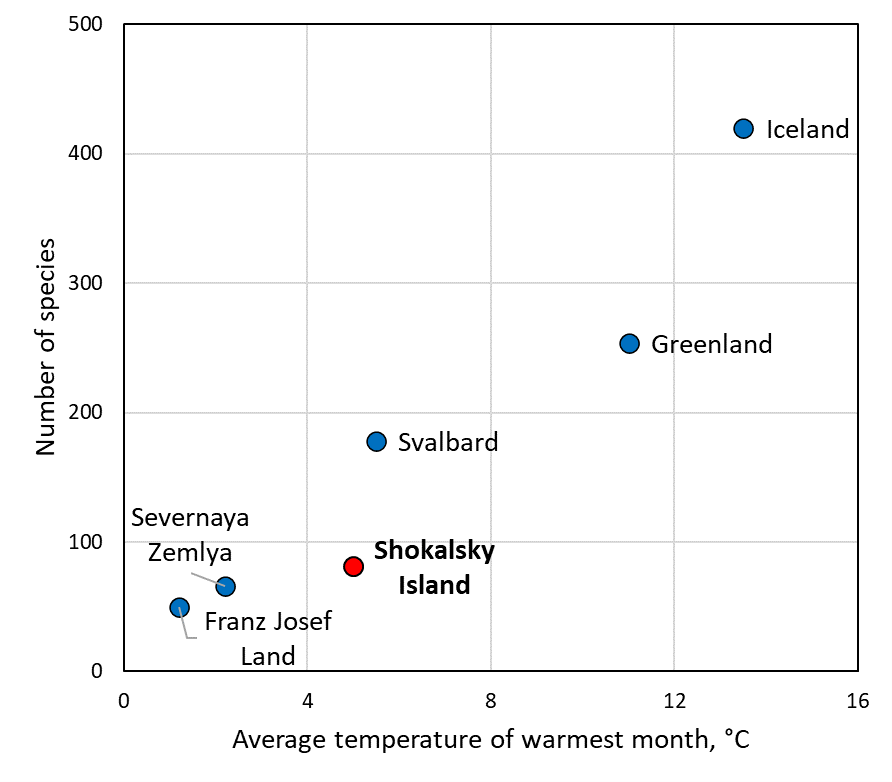
The number of mite species revealed on the Shokalsky Island (81 species; Table 2) makes up about 13% of all mite diversity across the Arctic (Hodkinson et al. 2013) and about 20–25% of the species richness known from such large arctic regions as Taymyr, Greenland, the Canadian Arctic, or the Bolshezemelskaya Tundra (Behan 1978; Makarova 2015a; Rozhnov et al. 2019; Makarova et al. 2022). This number of species (81) represents less than half of the mite species diversity of the mountainous Svalbard Archipelago located nearly at about the same latitude and where acarofauna had been studied for over a century (Seniczak et al. 2020b). However, in comparison with polar desert districts hosting 15–61 species, the acarofauna of Shokalsky Island is noticeably enriched (cf. McAlpine 1965; Makarova 2002а, 2023). As can be easily seen (Figure 7), the numbers of species revealed in relatively well studied local faunas differ in high-arctic and low-arctic regions. Thus, the widely adopted division of polar area into High and Low Arctic demarcated by the mean July isotherm of 5–6 °С (Alexandrova 1980; Böcher et al. 2015) appears to be supported by acarological evidence as well.


In comparison to vascular plants, mosses, and lichens, soil mites appeared to maintain similarly high levels of diversity (Table 4). Among the animal taxa studied in the island (Table 4), only springtails were represented by a similar number of species (68 species; A.B. Babenko, personal communication, March 15, 2023).
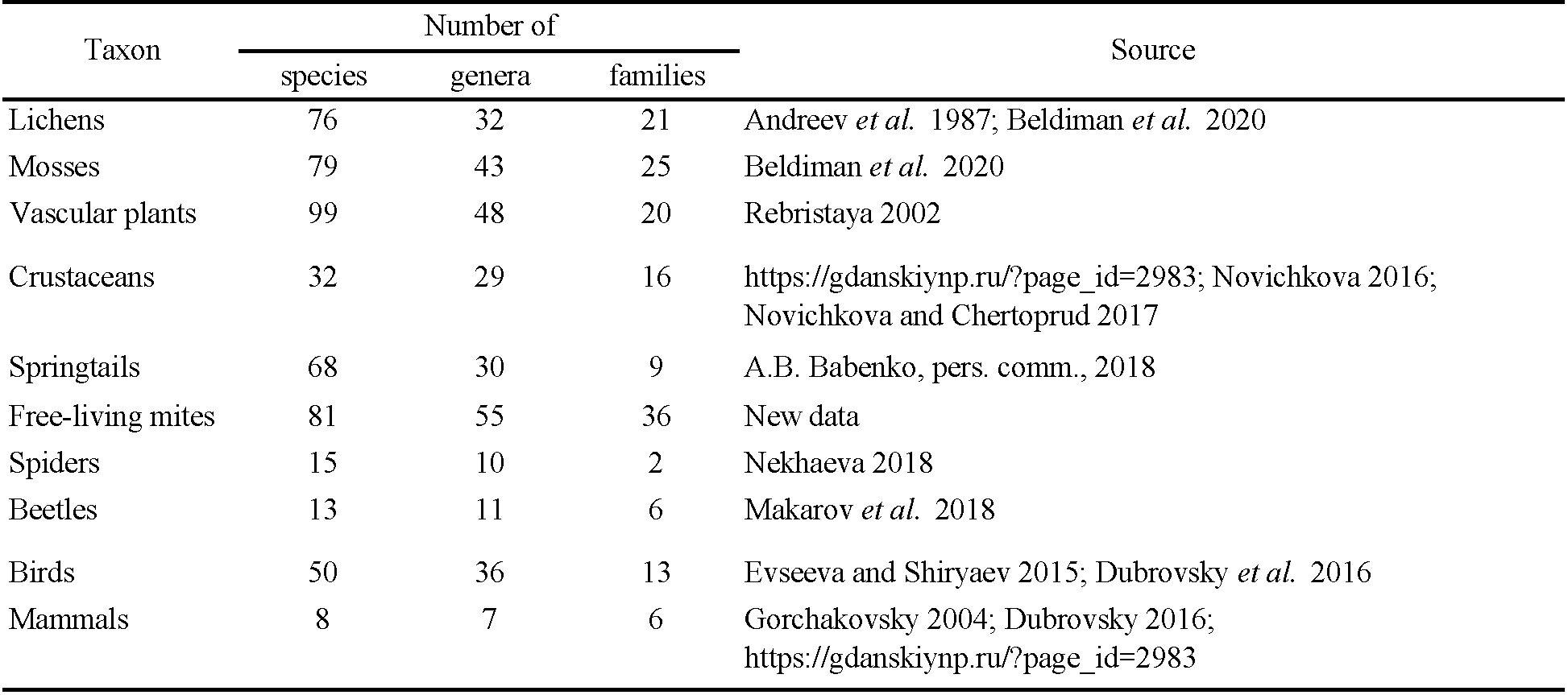


The macrotaxonomic structure of the acarofauna (the proportion of the number of species in different orders and suborders) was closer to that known for the arctic polar desert areas (McAlpine 1965; Makarova 2002a, 2023) than for ones of the tundra zone. So, more than half of the species found were members of Prostigmata (Table 2; Figure 3A). Comparably to tundrian study areas of Yamal, Taymyr, and the Vaygach Island, all located south of Shokalsky Island, the diversity of Oribatida (18 species) was about twice as low, and the diversity of Mesostigmata (17 species) appeared to be 2–4 times lower (Grishina 1985; Davydova and Nikolsky 1986; Grishina et al. 1998; Marchenko 2012; Makarova 2013; Melekhina et al. 2019). At the same time, the species richness of Oribatida and Mesostigmata in the Shokalsky Island was similar to that revealed in the mountainous tundras of the northern Palaearctic (Sidorchuk 2009; Marchenko 2010, 2011; Melekhina and Zinovyeva 2012; Makarova 2019; Leonov 2020; Leonov and Rakhleeva 2020). Despite the similarity of the macrotaxonomic structure of the tundrian Shokalsky Island's acarofauna to that in the true polar desert regions, a clear diagnostic feature of these natural zones appeared to be the diversity of Oribatida (Makarova 2002a). On the Shokalsky Island, this suborder contained twice as many species as on the Severnaya Zemlya Archipelago or Franz Josef Land (Makarova 2002a, 2023). The shorter mesostigmatic mite list (17 species vs 20 on Severnaya Zemlya) was obviously due to our undersampling of the mites from zoogenic substrates (from seabird and lemming colonies) that are usually inhabited by specialized species.
The most genera (76%) comprising the Shokalsky Island's acarofauna were represented by one species only (Table 2), thus showing a strongly ''fragmented'' taxonomic structure. This also held true for other animal taxa studied on the island (Table 4). The fragmented biota is typical of polar deserts (Korotkevich 1972; Chernov and Matveeva 1979; Makarova 2002а, 2023; Babenko 2018). On the Shokalsky Island, this reflects its position at the northernmost margin of the tundra zone.
At the same time the genus Arctoseius Thor, 1930 is unprecedentedly diverse in the entire Arctic (more than 30 species, up to 12–15 species per local fauna) (Lindquist 1961; Makarova 1999, 2000b, 2013, 2015a, 2023). The number of its species is believed to reflect the age of the fauna to some extent (Makarova et al. 2022). At least 9 species dwell on the Shokalsky Island (up to 5 species per habitat and up to 3 species per individual sample), this being a moderate value close to that of both Svalbard (Seniсzak et al. 2020b), Greenland (Makarova 2015a) and Franz Josef Land (Makarova 2023).
Distributions
In the north of Eastern Europe and West Siberia, representatives of the European (West Palaearctic) and Siberian faunogenetic complexes coexist (Eskov 1988; Babenko 2012; Babenko et al. 2017; Makarova et al. 2019). The proportion of these zoogeographic elements in the acarofauna of Shokalsky Island (totaling about 9%) was not as significant as that observed in the tundra areas lying more southerly in the same sector of the Arctic (27–36%; Marchenko 2012; Babenko et al. 2017; Makarova et al. 2019; Babenko and Antipova 2022). Only two species, Zercon forsslundi Sellnick, 1956 and Halolaelaps cf. gerlachi Hirschmann, 1966, are associated with the West Palaearctic (the latter species is also known from Greenland), while Arctoseius productus Makarova, 2000, Zercon aff. bajkalensis Blaszak, 1979, and Pyroppia arctica Krivolutsky, 1974 can be considered as ''Siberian'' species (see Ghilarov et al. 1975; Grishina 1985; Krivolutsky et al. 1995; Grishina et al. 1998; Marchenko 2012; Makarova 2013).
At the same time, 16 mite species found, which belong to different suborders, could be considered as true arctic elements (Table 2). In addition, nine species, namely Zercon michaeli Halašková, 1977; Arctoseius idiodactylus Lindquist, 1961; Melichares parvanalis; Coccorhagidia pittardi Strandtmann, 1971; Poecilophysis cf. saxonica; Camisia dictyna Colloff, 1993; Ceratozetes spitsbergensis Thor, 1934; Melanozetes interruptus Willmann, 1953; Svalbardia lucens, inhabit both Arctic and mountainous landscapes more to the south (Lindquist 1963; Ghilarov et al. 1975; Krivolutsky et al. 1995; Makarova 2000b, 2013; Kamali et al. 2001; Zacharda and Kučera 2006; Fischer et al. 2016; Behan-Pelletier and Lindo 2019). On the Shokalsky Island, the proportion of cryobiont species is the highest among Mesostigmata (58%), but much lower in both Oribatida (31%) and Prostigmata (30%). The high specialization of the arctic mesostigmatan fauna has repeatedly been discussed (Makarova and Böcher 2009; Hodkinson et al. 2013; Makarova 2015a, 2023; Makarova et al. 2019).
The pattern characterized, namely a reduced sectoral specificity and an increased proportion of Holarctic cryobiont species seem to be among the most distinctive features of high-arctic biota (Chernov 1978; Babenko 2005; Bayartogtokh et al. 2011).
Abundance and diversity indicators
The total mite density in the soil of the studied habitats ranged similar to other tundra regions across the Arctic (e.g., Bengtson et al. 1974; Douce and Crossley 1977; MacLean et al. 1978; Byzova et al. 1995) or tundra-like alpine heaths (e.g., Petrova and Grechanichenko 1987; Minor et al. 2016a). Similar values were revealed for the polar deserts of both Severnaya Zemlya (Makarova 2002a) and Franz Josef Land (Makarova 2023). Thus, as pointed out earlier (Makarova 2002a), the acarofaunas of the tundra zone and polar deserts cannot be distinguished based on this parameter alone.
The number of species in every habitat series on Shokalsky Island reached a plateau on the cumulative curve (Figure 5A) already following the 3rd to 7th sample, showing the sampling effort's sufficiency.
The main diversity indicators (number of species per sample and habitat, and the Shannon index; Table 3) increased along an environmental gradient from cold waterlogged sites of low marsh to the warmest and well-drained habitats (sedge-mossy tundra, dryad tundra and meadow of polar fox den). Such a pattern is considered to be quite typical of the soil mites of high-arctic landscapes (Ananjieva et al. 1979; Makarova 2002b).
Assemblage structure
Mite assemblages of all studied habitats showed a mono- or an oligodominant structure (one to four dominant species). Prostigmatans were most diverse and abundant virtually in all habitats (Figure 3B), being mainly represented by species of Eupodoidea. These are members of this superfamily that constitute the main body of acarocenoses everywhere in the polar regions (Strandtmann 1971; Usher and Booth 1984; Makarova 2002b, 2023) and in cold treeless nival heaths (Zacharda and Kučera 2006, 2010).
Ordination based on both abundance and diversity split all habitats in three separate groups, i.e. wet coastal habitats, wet mossy habitats, and drained habitats of the inner area (Figures 4, 6).
Wet coastal habitats
Both the geese molting place located in the river delta and the low marsh on the marine coast were regularly inundated by tides. The tidal regime supported the high density of such a characteristic littoral species as Ameronothrus nigrofemoratus (L. Koch, 1879), as well as some hygrophilous mites (Eustigmaeus cf. tjumeniensis Khaustov & Tolstikov, 2014, Svalbardia lucens, Cheylostigmaeus longisetosus, Arctoseius ornatus Lindquist, 1961 etc.). Remarkably, A. nigrofemoratus and Halolaelaps cf. gerlachi, another seashore-dweller [along with Ameronothrus lineatus (Thorell, 1871)], are probably the northernmost members of the specialized littoral species complex along the latitudinal transect (compare with Seniczak et al. 2020b). On Severnaya Zemlya, such species failed to occur at all (Makarova 2002a). The regular residence of Ameronothrus members on Franz Josef Land needs confirmation. Until now, this genus is known from the archipelago from a single deutonymph (Makarova 2023).
Wet mossy habitats
The vegetation cover of both the high marsh and cotton-grass bog is characterized by the presence of a thick moss cover incorporating sedge and graminoid tussocks. This type of vegetation was believed to ensure more stable environmental conditions and provided a variety of suitable microhabitats for mites showing different biotic preferences (Minor et al. 2016b; Seniczak et al. 2020a; Bizin et al. 2021). Most of the mass species in these habitats were hygrophilous or associated with grasses (Steneotarsonemus arcticus). On the Shokalsky Island, S. arcticus inhabited a wide range of biotopes like salt marshes and meadow of polar fox den where graminoids (Alopecurus, Poa, Calamagrostis, Festuca) prevailed, yet it was absent from both the dryad tundra where grasses were sparse, and bird-transformed sites with suppressed vegetation (Table 2).
The leading roles played by oribatid mites in waterlogged habitats (Figure 3B and Table 2) was a notable feature to distinguish the mite assemblages of the Shokalsky and Bolshevik islands. In areas where environmental conditions were intermediate between a tundra and polar deserts (like Shokalsky Island), some oribatid species, S. lucens and A. nigrofemoratus among them, showed their northernmost range limits (see Figure 2 in Ermilov et al. 2022; Artamonova et al. 2023) but they were virtually absent from polar deserts (Makarova 2002a, 2023).
Inner drained habitats
This cluster was formed by the warmest habitats characterized by the greatest taxonomic diversity and the highest densities (Tables 2 and 3). Only these habitats hosted relatively larger species (the idiosoma being more than 450 µm in length). Among them, were the arctic-boreal Diapterobates notatus (Thorell, 1871), Acugamasus montanus (Willmann, 1936), Zercon cf. bajkalensis, as well as the true arctic species Hermannia scabra, Melanozetes interruptus, and Dinychus micropunctatus Evans, 1955.
However, the dominant complex included only smaller species (the idiosoma not exceeding 300 µm in length). These were the prostigmatans Steneotarsonemus arcticus, Lorrya cf. obstinata (Livshitz, 1973), Paratriophtydeus sp., as well as Kerdabania sp. The genus Paratriophtydeus was only rarely to be recorded in polar regions of the Nearctic (Behan 1978; Makarova 2015). In the cosmopolitan genus Kerdabania, only one species, K. arctica (Thor, 1934), is presently known from the Arctic alone (Svalbard, as ''Pediculoides arcticus''; Khaustov 2009).
The endeostigmatan, Nanorchestes cf. gilli, was the most abundant species both in the sedge-mossy and dryad tundra (Table 2). This species is very common in polar deserts of the Northern Hemisphere where it occupies a wide range of habitats (Makarova 2002a, 2023). Members of the algivorous genus Nanorchestes are the most common components of the acarofauna in Antarctic polar deserts as well (Rounsewell 1977). These mites usually prefer bare and/or pioneer sites (see references in Makarova 2002a; Usher and Booth 1984; Russell and Alberti 2010) where soil algae are also abundant.
The vegetation cover of the sedge-mossy and dryad tundras of Shokalsky Island was characterized by an increased share of lichens (Beldiman et al. 2020). That was probably why the lichenophagous Ceratozetes spitsbergensis (Makarova 2002b; Fischer et al. 2016) was found just in those habitats (Table 2).
The mite assemblage in the sandy grounds of polar fox den demonstrated the most complicated structure of dominance (Table 2). About a quarter of the species recorded (9; 26%) were found only in pitfall material including the characteristic arctic-montane species, Melichares parvanalis, which was absent from soil cores of all habitats.
Conclusion
Our study dealing with all groups of mites presents the first review of a local acarofauna of the West Siberian tundra. Compared to the data from polar deserts, the acarofauna of Shokalsky Island is more diverse and some of its features are transitional between the ones characteristic of the polar-desert and tundra zones. So, the Prostigmata is the most species-rich suborder in almost all habitats, just like this is observed in polar deserts. Nevertheless, the list of dominant mite species of the Shokalsky Island includes member of Endeostigmata and larger species of Oribatida (even in waterlogged habitats). The latter feature is typical only of the tundra zone. As in polar deserts, the proportion of genera represented by one species only is high (76%) while the genus Arctoseius is the most diverse (9 species). Besides this, our data confirm high specialization (Makarova and Böcher 2009; Makarova 2023) of the arctic mesotigmatan fauna (58% species showing arctic or arctic-montane distribution patterns) as opposed to the Oribatida fauna which mainly comprises arctic-boreal or polyzonal species (70% in total).
Along the other recent publications (Makarova 2002a, 2015a, b, 2023; Seniczak et al. 2020b), our research highlights the sharp need in identifying the arctic Prostigmata, especially the superfamily Eupodoidea, the leading taxon in high-arctic acarocenoses.
Acknowledgements
The 2016 expedition to the Shokalsky Island was organized and partly funded by the ''Gydanskiy'' State Natural Park (through the kind mediation of its director, V.V. Berlinsky, and the staff members A.A. Gorchakovsky and V.L. Lapsuy). Lab work was supported by the Russian Foundation for Basic Research, project No. 20-54-56054 Iran_t.
We are grateful to our colleagues A.A. Nekhaeva and N.B. Korostelev (SIEE RAS) for their help in collecting material during the expedition; to A.A. Khaustov (University of Tjumen), J. Mąkol (Wroclaw University of Environmental and Life Sciences), and Ph.E. Chetverikov (Zoological Institute RAS) for taxonomic help; to K.A. Ermokhina and I.N. Pospelov (SIEE RAS) for the identification of mass plant species. We thankfully acknowledge the information support of E.S. Chertoprud, A.B. Babenko, N.B. Korostelev (SIEE RAS), V.Yu. Dubrovsky (Moscow Zoo), A.M. Evseeva and S.V. Goryachkin (Institute of Geography RAS).
S.I. Golovatch (SIEE RAS) kindly edited the English.
References
- Alexandrova V.D. 1980. The Arctic and Antarctic, their division into geobotanical areas. Cambridge: Cambridge University Press. pp. 247.
- Ananjieva S.I., Krivolutsky D.A., Chernov Yu. I. 1973. Oribatid mites (Oribatei) of the typical tundra subzone of the Western Taymyr. In: Tikhomirov B.A. Biogeocenoses of the Taymyr tundra and their productivity. Issue 2. Leningrad: Nauka. p. 148-151. (In Russian)
- Ananjieva S.I., Krivolutsky D.A., Chernov Yu. I. 1979. Oribatid mites (Oribatida) in artic tundras and polar deserts of the North-East of Taymyr. In: Alexandrova V.D., Matveeva N.V. (Eds). Arctic tundras and polar deserts of Taymyr. Leningrad: Nauka. p. 144-147. (In Russian)
- Andreev M.P., Drobysh A.A., Rebristaya O.V. 1987. Lichens of the Bely and Shokalsky Islands (Kara Sea). Nov. Sist. Nizs. Rast., 24: 126-131. (In Russian)
- Artamonova V.S., Bizin M.S., Efeykin B.D., Makarova O.L. 2023. Two lineages of oribatid mites morphologically correspond to the circumpolar species Ameronothrus nigrofemoratus (Acari, Oribatida) but differ genetically as distinct species are revealed on the Kolguev Island. Dokl. Biol. Sci., 512: 321-325. https://doi.org/10.1134/S0012496623700631
- Babenko A.B. 2005. Collembolas of the Arctic: structure of the fauna and chorological characteristics [DSc Thesis]. Moscow: Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences. pp. 382. (In Russian)
- Babenko A.B. 2012. Springtails (Hexapoda, Collembola) of tundra landscapes of the Kola Peninsula. Entomol. Rev., 92 (5): 497-515. https://doi.org/10.1134/S0013873812050028
- Babenko A.B. 2018. Springtails (Collembola) in the subpolar landscapes of the Northern Hemisphere. Entomol. Rev., 98 (4): 383-406. https://doi.org/10.1134/S0013873818040012
- Babenko A.B., Antipova M.D. 2022. Peculiarities of the collembolan fauna and assemblages (Hexapoda, Collembola) of Eastern Yamal. Entomol. Rev., 102 (5): 639-657. https://doi.org/10.1134/S0013873822050074
- Babenko A.B., Potapov M.B., Taskaeva A.A. 2017. The collembolan fauna of the East European tundra. Russ. Entomol. J., 26 (1): 1-30. https://doi.org/10.15298/rusentj.26.1.01
- Balogh J., Mahunka S. 1983. Primitive Oribatids of the Palaearctic region. The soil mites of the world. 1. Budapest: Akadémiai Kiadó. pp. 372.
- Bayartogtokh B. 2010. Oribatid mites of Mongolia (Acari: Oribatida). Moscow: KMK Scientific Press. pp. 371. (In Russian)
- Bayartogtokh B., Schatz H., Ekrem T. 2011. Distribution and diversity of the soil mites of Svalbard, with redescriptions of three known species (Acati: Oribatida). Int. J. Acarol., 37 (6): 467-484. https://doi.org/10.1080/01647954.2010.525525
- Behan V.M. 1978. Diversity, distribution, and feeding habitats of North American Arctic soil Acari [Phd Thesis]. Quebec: Macdonald College of McGill University. pp. 487.
- Behan-Pelletier V.M. 1985. Ceratozetidae of the Western North American Arctic. Can. Entomol., 117(11): 1287-1366. https://doi.org/10.4039/Ent1171287-11
- Behan-Pelletier V.M. 1997. Oribatid mites (Acari: Oribatida) of the Yukon. In: Danks H.V., Downes J.A. (Eds). Insects of Yukon. Ottawa: Biological Survey of Canada (Terrestrial Arthropods). p. 115-149.
- Behan-Pelletier V.M., Lindo Z. 2019. Checklist of oribatid mites (Acari: Oribatida) of Canada and Alaska. Zootaxa, 4666 (1): 001-180. https://doi.org/10.11646/zootaxa.4666.1.1
- Beldiman L.N., Urbanavichene I.N., Fedosov V.E., Kuzmina E.Yu. 2020. Mosses and lichens of Shokalsky Island (Kara Sea, Yamal-Nenets Autonomous Area). Nov. Sist. Nizs. Rast., 54(2): 497-613. (In Russian) https://doi.org/10.31111/nsnr/2020.54.2.497
- Bengston S.-A., Fjellberg A., Solhöy T. 1974. Abundance of tundra arthropods in Spitzbergen. Ent. scand., 5: 137-142. https://doi.org/10.1163/187631274X00164
- Bizin M.S., Borisenko G.V., Makarova O.L. 2021. Impact of environmental factors on the formation of soil-mite (Acari) assemblages on coastal marshes of Shokalsky Island, Kara Sea. Contemp. Probl. Ecol., 14 (2): 144-161. https://doi.org/10.1134/S1995425521020037
- Bogdanov I.I. 1975. Lemming mites of the Eastern Taymyr. Parasitologia, 9(6): 522-525. (In Russian)
- Böcher J., Kristensen N.P., Pape T., Vilhelmsen L (Eds). 2015. An identification manual of insects, spiders and their allies. Leiden - Boston: Brill. pp. 898.
- Byzova Ju.B., Uvarov A.V., Petrova A.D. 1995. Seasonal changes in communities of soil invertebrates in tundra ecosystems of Hornsund, Spitsbergen. Pol. Polar. Res. 16 (3-4): 245-266.
- Chant D.A., Hansell I.C. 1971. The genus Amblyseius (Acarina: Phytoseiidae) in Canada and Alaska. Can. J. Zool., 49 (5): 703-758. https://doi.org/10.1139/z71-110
- Chant D.A., McMurtry J.A. 2003. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part I. Neoseiulini new tribe. Int. J. Acarol., 29 (1): 3-46. https://doi.org/10.1080/01647950308684319
- Chernov Yu.I. 1978. Animal community of the Subarctic. Moscow: Nauka. pp. 176. (In Russian)
- Chernov Yu.I. 2004. The animal world of the polar desert on the Devon Island Plateau, Canadian Arctic Archipelago. Entomol. Rev., 84 (Suppl. 1): S15-S24.
- Chernov Yu.I., Matveeva N.V. 1979. Patterns of zonal distribution of communities on Taymyr. In: Alexandrova V.D., Matveeva N.V. (Eds). Arctic tundras and polar deserts of Taymyr. Leningrad: Nauka. p. 166-200. (In Russian)
- Coulson S.J., Convey P., Aakra K., Aarvik L., Ávila-Jiménez M. L., Babenko A., Biersma E.M., Boström S., Brittain J. E., Carlsson A.M., Christoffersen K., De Smet W.H., Ekrem T., Fjellberg A., Füreder L., Gustafsson D., Gwiazdowicz D.J., Hansen L.O., Holmstrup M., Hullé M., Kaczmarek Ł., Kolicka M., Kuklin V., Lakka H.-K., Lebedeva N., Makarova O., Maraldo K., Melekhina E., Ødegaard F., Pilskog H.E., Simon J.C., Sohlenius B., Solhøy T., Søli G., Stur E., Tanasevitch A., Taskaeva A., Vellea G., Zawierucha K., Zmudczyńska-Skarbek K. 2014. The terrestrial and freshwater invertebrate biodiversity of the archipelagoes of the Barents Sea; Svalbard, Franz Josef Land and Novaya Zemlya. Soil Biol. Biochem., 68: 440-70. https://doi.org/10.1016/j.soilbio.2013.10.006
- Danks H.V. 1981. Arctic Arthropods. Ottawa: Tyrell Press Limited. pp. 608.
- Davydova M.S., Nikolskij V.V. 1986. Gamasid mites of West Siberia. Novosiborsk: Nauka. pp. 125. (In Russian)
- Davydova M.S., Nikolskij V.V., Yudin B.S., Dudareva G.V., Belova O.S. 1980. Gamasid mites of middle Siberian tundra. Parasitic insects and mites of Siberia. Novosibirsk: Nauka. pp. 141-148. (In Russian)
- Dinno A. Package ''dunn.test'' [Internet]. [26 October 2017]. [27 June 2023]. Available from: https://CRAN.R-project.org/package=dunn.test
- Douce G.K., Crossley D.A. 1977. Acarina abundance and community structure in an arctic coastal tundra. Pedobiologia, 17 (1): 32-42. https://doi.org/10.1016/S0031-4056(23)00139-7
- Dubrovsky V.Yu. 2016. Population of small mammals on the Shokalsky Island (Kara Sea). Zool. Zh., 95 (2): 245-248. (In Russian) https://doi.org/10.7868/S0044513416010049
- Dubrovsky V.Yu., Shiruaev D.M., Korostelev N.B., Chertoprud E.M. 2016. Post-nesting avifauna of the Shokalsky Island. Zool. Zh., 95 (3): 344-347. (In Russian) https://doi.org/10.7868/S0044513416030053
- Ermilov S.G., Makarova O.L., Behan-Pelletier V.M. 2022. Taxonomy and ecology of the Arctic oribatid mite Svalbardia lucens comb. nov. (Acari, Oribatida, Ceratozetidae): resolving of a long-standing confusion. Syst. Appl. Acarol., 27(3): 497-510. https://doi.org/10.11158/saa.27.3.8
- Ermokhina K.A., Terskaia A.I., Ivleva T.Y., Dudov S.V., Zemlianskii V.А., Telyatnikov M.Y., Khitun O.V., Troeva E.I., Koroleva N.E., Abdulmanova S.Y. 2023. The High-Low Arctic boundary: How is it determined and where is it located? Ecol. Evol. 13(10): e10545. https://doi.org/10.1002/ece3.10545
- Eskov K.Yu. 1988. Spiders of Central Siberia. In: Contribution to the fauna of Central Siberia and adjacent regions of Mongolia. Moscow: Severtsov Institute of Evolutionary Morphology and Ecology of Animals of the Academy of Sciences of the USSR. pp. 101-155. (In Russian)
- Evseeva A.M., Shiryaev D.M. 2015. Avifauna of the Shokalsky Island, Kara Sea. Russ. J. Ornithol., 24 (1226): 4490-4494. (In Russian)
- Fischer B.M., Schatz H., Querner P., Pauli H. 2016. Ceratozetes spitsbergensis Thor, 1934: an Arctic mite new to continental Europe (Acari: Oribatida). Int. J. Acarol., 42 (2): 135-139. https://doi.org/10.1080/01647954.2015.1133702
- Ghilarov M.S., Krivolutsky D.A., Bulanova-Zakhvatkina E.M., Wainstein B.A., Wolgin W.I., Golosova L.D., Lange A.B., Sevastjanov W.D., Sitnikova L.G., Shaldybina E.S. 1975. Key to soil-inhabiting mites. Sarcoptiformes. Moscow: Nauka. pp. 491. (In Russian)
- Ghilarov M.S., Bregetova N.G., Wainstein B.A., Kadite B.A., Koroleva E.V., Petrova A.D., Tikhomirov S.I., Shcherbak G.I. 1977. Key to soil-inhabiting mites. Mesostigmata. Leningrad: Nauka. pp. 718. (in Russian)
- Gorchakovsky A.A. 2004. Vertebrates of the Gydansky State Nature Reserve. In: Current state and environmental monitoring of the Ob-Taz region. Saint-Petersburg: Hydrometeoizdat. p. 5-32. (In Russian)
- Grishina L.G. 1985. Oribatida of the North of Siberia. Arthropods of Siberia and Far East. Novosibirsk: Nauka. p. 14-23. (In Russian)
- Grishina L.G., Mordkovich V.G. 1996. Oribatid mites of Taymyr Nature Reserve. In: Minoransky V.A. (Ed). Problems of soil zoology. Abstract-book of the First All-Russian Meeting on Soil Zoology. Rostov-on-Don: Izdatelstvo OblIUU. p. 33-34. (In Russian)
- Grishina L.G., Babenko A.B., Chernov Yu.I. 1998. The Oribatid Mites (Sarcoptiformes, Oribatei) of Taimyr Peninsula Western Coast. Vestnik zoologii, 32 (1-2): 116-118. (In Russian)
- Hammer Ø., Harper D.A. T., Ryan P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron., 4 (1): 1-9. Available from: http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
- Hodkinson I.D., Babenko A., Behan-Pelletier V., Bocher J., Boxshall G., Brodo F., Coulson S.J., De Smet W., Dózsa-Farkas K., Elias S., Fjellberg A., Fochetti R., Foottit R., Hessen D., Hobaek A., Holmsstrup M., Koponen S., Liston A., Makarova O., Marusik Y.M., Michelsen V., Mikkola K., Pont A., Renaud A., Rueda L.M., Savage J., Smith H., Samchyshyna L., Velle G., Viehberg F., Wall D.H., Weider L.J., Wetterich S., Yu Q., A. Zinovjev A. 2013. Chapter 7, Terrestial and Freshwater Invertebrates. In: Meltofte H. (Ed.). Arctic Biodiversity Assessment. Reykjavik: The Arctic Council. p. 195-223.
- IUSS Working Group WRB. 2015. International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports. No.106. Rome: FAO. pp. 192.
- Kalyakin V.N., Romanenko F.A., Molochaev A.V., Rogacheva E.V., Syroechkovsky E.E. 1999. Gydanskyi Nature Reserve. In: Sokolov V.E., Pavlov D.S., Syroechkovsky E.E. (Eds). Zapovedniki Sibiri. T.1. Moscow: Logata. p .47-55. (In Russian)
- Kamali K., Ostovan H., Atamehr A. 2001. A catalog of mites and ticks (Acari) of Iran. Tehran: Islamic Azad University Scientific Publication Center. pp. 192.
- Karg W. 1993. Acari (Acarina), Milben. Parasitiformes (Anactinochaeta). Cohors Gamasina Leach. Raubmilben (2nd edn.). Die Tierwelt Deutschlands. 59. Jena-Stuttgart-New York: Gustav Fischer Verlag. pp. 523.
- Khaustov A.A. 2008. Mites of the family Scutacaridae of Eastern Palaearctic. Kiev: Akadem-periodyka. pp. 209.
- Khaustov A.A. 2009. A description of new genus, Kerdabania gen. n. with four new species (Acari: Heterostigmata: Neopygmephoridae). Acarina, 17(2):171-188.
- Korotkevich E.S. 1972. Polar deserts. Leningrad: Hydrometeoizdat. pp. 419. (In Russian)
- Krivolutsky D.A., Lebren Ph., Kunst M., Akimov I.A. et al. 1995. Oribatid mites: morphology, development, phylogeny, ecology, studying methods, and characteristics of the model species Nothrus palustris. Moscow: Nauka. pp. 224. (In Russian)
- Krivolutsky D.A., Drozdov N.N., Lebedeva N.V., Kaljakin V.N. 2003. Geography of soil microarthropods on the arctic islands. Vestnik Moskovskogo universiteta. Series. 5. Geography, 6: 33-40. (In Russian)
- Lindquist E.E. 1961. Taxonomic and biological studies of the mites of the genus Arctoseius Thor from Barrow, Alaska (Acarina: Aceosejidae). Hilgardia. 30 (11): 301-350. https://doi.org/10.3733/hilg.v30n11p301
- Lindquist E.E. 1963. A revision of mites of the subfamily Blattisocinae (Acarina: Blattisocidae) in America north of Mexico [Doctoral Dissertation in Biology]. Berkeley: University of California. pp. 402.
- Lindquist E.E., Makarova O.L. 2011. Two new circumpolar mite species of the genus Arctoseius Thor (Parasitiformes, Mesostigmata, Ascidae). Entomol. Rev., 91(8): 1054-1072 https://doi.org/10.1134/S0013873811080100
- Leonov V.D. 2020. The first report on the oribatid mites (Acari: Oribatida) in the tundra of the Chunatundra Mountains on the Kola Peninsula, Russia. Acarologia, 60 (4): 722-734. https://doi.org/10.24349/acarologia/20204398
- Leonov V.D., Rakhleeva A.A. 2020. The first report on oribatid mites in tundra belts of the Lovozersky Mountains on the Kola Peninsula, Russia. Acarologia, 60 (2): 301-316. https://doi.org/10.24349/acarologia/20204369
- MacLean S.F., Behan V., Fjellberg A. 1978. Soil Acari and Collembola from Chaun Bay, Northern Chukotka. Arct. Antarct. Alp. Res., 10 (3): 559-568. https://doi.org/10.2307/1550679
- Magurran A.E. 1988. Ecological diversity and its measurement. Princeton: Princeton University Press. pp. 192. https://doi.org/10.1007/978-94-015-7358-0
- Makarov K.V., Gusarov V.I., Makarova O.L., Bizin M.S., Nekhaeva A.A. 2018. The first data on beetles (Coleoptera) of the High Arctic Shokalsky Island (Kara Sea). Russian Entomol. J., 27 (4): 387-398. https://doi.org/10.15298/rusentj.27.4.06
- Makarova O.L. 1999. Mesostigmatic mites (Parasitiformes, Mesostigmata) of polar deserts in the Severnaya Zemlya. Entomol. Rev., 79 (8): 982-990.
- Makarova O.L. 2000a. To a study of mites of the genus Arctoseius (Parasitiformes, Ascidae) from the Far North: 2. Description of A. productus sp. n. and A. babankoi sp. n. and key to High Arctic species. Entomol. Rev., 80 (Suppl. 1): S131-S142.
- Makarova O.L. 2000b. To a study of mites of the genus Arctoseius (Parasitiformes, Ascidae) from the Far North: 3. Ranges and ecological preferences of species. Entomol. Rev., 80 (Suppl. 1): S143-S150.
- Makarova O.L. 2002a. Acarocenoses (Acariformes, Parasitiformes) in polar deserts. 1. Mite assemblages in the Severnaya Zemlya Archipelago. Structure of fauna and abundance. Entomol. Rev., 82(7): 839-856.
- Makarova O.L. 2002b. Acarocenoses (Acariformes, Parasitiformes) in polar deserts. 2. Cenotic relations. Structure of communities. Proportion of suborders. Entomol. Rev., 82(7): 857-875.
- Makarova O.L. 2012. Peculiarities of the gamasid mite fauna (Parasitiformes, Mesostigmata) of the Novaya Zemlya and Svalbard archipelagoes. In: Matyshov G.G., Tarasov G.A. (Eds). Comprehensive studies of the Spitzbergen's nature, Issue 11; Moscow: Geos. p. 164-173. (In Russian)
- Makarova O.L. 2013. Gamasid mites (Parasitiformes, Mesostigmata) of the European Arctic and their distribution patterns. Entomol. Rev., 93(1): 113-133. https://doi.org/10.1134/S0013873813010156
- Makarova O.L. 2015a. The fauna of free-living mites (Acari) of Greenland. Entomol. Rev., 95(1): 108-125. https://doi.org/10.1134/S0013873815010133
- Makarova O.L. 2015b. Mesostigmata (Gamasida, Gamasid mites). In: Böcher J., Kristensen N.P., Pape T., Vilhelmsen L (Eds). The Greenland Entomofauna. An identification manual of insects, spiders and their allies. Leiden - Boston: Brill. p. 714-748.
- Makarova O.L. 2015c. Prostigmata (Actinendida). In: Böcher J., Kristensen N.P., Pape T., Vilhelmsen L (Eds). The Greenland Entomofauna. An identification manual of insects, spiders and their allies. Leiden - Boston: Brill. p. 754-789.
- Makarova O.L. 2019. Gamasid mites (Parasitiformes, Mesostigmata) in tundra soils of the Khibiny Mountains. In: Tembotova F.A. (Ed). The mountain ecosystems and their components; Makhachkala: ALEF. p. 136-138. (In Russian)
- Makarova O.L. 2023. Free-living mites (Acari) of the Franz Josef Land Archipelago, the coldest Old World territory: diversity, geographic distributions, assemblages. Acarologia, 63(4): 1163-1186. https://doi.org/10.24349/p6wb-pcni
- Makarova O.L., Böcher J. 2009. Diversity and geographical ranges of Greenland mites (Acari: Oribatida and Mesostigmata). In: Golovatch S.I., Makarova O.L., Babenko A.B., Penev L.D. (Eds). Species and Communities in Extreme Environments. Sofia - Moscow: Pensoft - KMK Scientific Press. pp. 165-186.
- Makarova O.L., Rosenfeld S.B. 2014. Gamasid mites (Parasitiformes, Mesostigmata) inhabiting goose nests on Kolguev Island, the Pechora Sea. In: Matishov G.G., Tarasov G.A. (Eds). Comprehensive studies of the Spitzbergen's nature and the adjacent shelf, Issue 12; Moscow: Geos p. 182-190. (In Russian)
- Makarova O.L., Behan-Pelletier V.M. 2015. Oribatida (=Cryptostigmata, Beetle mites). In: Böcher J., Kriestensen N.P., Pape T., Vilhelmsen L. (Eds). The Greenland Entomofauna. An identification manual of insects, spiders and their allies. Leiden - Boston: Brill. p. 802-845.
- Makarova O.L., Bizin M.S. 2020. Littoral mesostigmatic mites (Acari, Parasitiformes) from the Kola Peninsula. Polar Biol., 43 (10): 1503-1518. https://doi.org/10.1007/s00300-020-02724-0
- Makarova O.L., Anufriev V.V., Babenko A.B., Bizin M.S., Glazov P.M., Kolesnikova A.A., Marusik Yu.M., Tatarinov A.G. 2019. Fauna of the East European tundra: the input of ''Siberian'' species. Bulletin of the North-East Scientific Center, Russia Academy of Sciences Far East Branch, 1: 59-71. (In Russian) https://doi.org/10.34078/1814-0998-2019-1-59-71
- Makarova O.L., Aksenova O.V., Babenko A.B., Bespalaya Yu.B., Krasheninnikov A.B., Lantsov V.I., Nekhaeva A.A., Potapov G.S., Sorokina V.S., Spitsyn V.M. 2022. Terrestrial invertebrates. In: Baryshev I.B., Boyarsky P.V., Kuliev A.N. (Eds). Islands and Archipelagos of the Kara Sea, Yamal and Taymyr Peninsulas. Moscow: Institute of Heritage. pp. 428-457. (In Russian)
- Makarova O.L., Ermilov S.G., Yurtaev A.A., Mansurov R.I. 2015. The first data on the soil mites (Acari) of the Arctic Belyi Island (Northern Yamal, the Kara Sea). Entomol. Rev., 95(6): 805-810. https://doi.org/10.1134/S0013873815060147
- Malkova M.G. 2009. Zonal faunistic complexes and community structure of small mammals and associated arthropods of West Siberia [DSc Thesis]. Novosibirsk: Institute of Systematics and Ecology of Animals. pp.42. (In Russian)
- Marchenko I.I. 2010. Soil-inhabiting mites (Acari, Mesostigmata) of North-East Altai: transformation of taxonomic, geographical and population structure of communities along an altitudinal gradient. Euroasian Entomol. J., 9(4): 741-756. (In Russian)
- Marchenko I.I. 2011. Spatial-typological organization of the soil Gamasina mite (Acari, Mesostigmata) community of the Northeastern Altai. Communication I. Contemp. Probl. Ecol., 4(4): 379-387. https://doi.org/10.1134/S1995425511040059
- Marchenko I.I. 2012. Soil gamasid mites (Acari, Mesostigmata) of North Siberia. Euroasian Entomol. J., 11(6): 517-528. (In Russian)
- McAlpine J.F. 1965. Insect and related terrestrial invertebrates of Ellef Ringnes Island. Arctic, 18 (2): 73-103. https://doi.org/10.14430/arctic3455
- Melekhina E.N. 2011. Taxonomic diversity and areology of oribatid mites (Oribatei) of the European North of Russia. Izvestia Komi Scientific Center of the Russian Academy of Sciences, 2(6): 30-37. (In Russian)
- Melekhina E.N. 2020. Oribatid mites as inhabitants of lichens in the taiga zone of northeastern Europe: biotopic associations and ecological groups of species. Biol. Bull. Russ. Acad., 47 (5): 522-534. https://doi.org/10.1134/S1062359020050064
- Melekhina E.N., Zinovyeva A.N. 2012. First data on oribatid mites (Acari; Oribatida) of Pay-Khoy Ridge (Yugor Peninsula). Izvestia Komi Scientific Center of the Russian Academy of Sciences, 2(10): 42-50. (In Russian)
- Melekhina E.N., Matyukhin A.V., Glazov P.M. 2019. Oribatid mites in nests of the Lapland bunting (Calcarius lapponicus) on the arctic island of Vaygach (with analysis of the islands fauna). Proceedings of the Karelian Scientific Center of the Russian Academy of Sciences, 8: 108-122. (In Russian) https://doi.org/10.17076/bg892
- Minor M.A., Babenko A.B., Ermilov S.G., Khaustov A.A., Makarova O.L. 2016a. Effects of cushion plants on high-altitude soil microarthropod communities: cushions increase abundance and diversity of mites (Acari), but not springtails (Collembola). Arct. Antarct. Alp. Res., 48 (3): 485-500. https://doi.org/10.1657/AAAR0015-064
- Minor M.A., Ermilov S.G., Philippov D.A., Prokin A.A. 2016b. Relative importance of local habitat complexity and regional factors for assemblages of oribatid mites (Acari: Oribatida) in Sphagnum peat bogs. Exp. Appl. Acarol., 70: 275-286. https://doi.org/10.1007/s10493-016-0075-9
- Nekhaeva A.A. 2018. Spiders (Arachnida, Aranei) of the High Arctic Shokalsky Island (73°N), the Kara Sea, Russia. Arthropoda Sel., 27 (4): 367-372. https://doi.org/10.15298/arthsel.27.4.14
- Novichkova A. 2016. The first data on the freshwater microcrustaceans of Shokalsky Island (Russian Arctic). Biodivers. Data J., 4: e10930. https://doi.org/10.3897/BDJ.4.e10930
- Novichkova A.A., Chertoprud E.S. 2017. Cladocera and Copepoda of Shokalsky Island: new data from northwest Siberia. J. Nat. Hist., 51 (29-30): 1781-1793. https://doi.org/10.1080/00222933.2017.1355077
- Petrova A.D., Grechanichenko T.E. 1987. Soil-inhabiting mites. In: Rabotnov T.A. (Ed). Biogeocoenoses of alpine heaths (on the example of Northwestern Caucasus). Moscow: Nauka. pp. 48-56. (In Russian)
- R Core Team. 2021. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from: https://www.R-project.org/.
- Rebristaya O.V. 2002. Vascular plants of the Shokalsky Island (Kara Sea). Bot. Zhurn., 87(6): 29-40. (In Russian)
- Rounsewell D.E. 1977.The ecology of Pan-Antarctic mite Nanorchestes antarcticus. In: Llando G.A. (Ed). Adaptation within Antarctic Ecosystem. Houston: Gulf Publ. Co. p. 1023-1033.
- Rozhnov V.V., Lavrinenko I.A., Razzhivin V.Yu., Makarova O.L., Lavrinenko O.V., Anufriev V.V., Babenko A.B., Bizin M.S., Glazov P.M., Goryachkin S.V., Kolesnikova A.A., Matweeva N.V., Pestov S.V., Petrovsky V.V., Pokrovskaya O.B., Tanasevitch A.V., Tatarinov A.G. 2019. Biodiversity revision of a large arctic region as a basis for its monitoring and protection under conditions of active economic development (Nenetsky Autonomous Okrug, Russia). Nature Conservation Research. Zapovednaya Nauka, 4 (2): 1-28. (In Russian) https://doi.org/10.24189/ncr.2019.015
- Russell D.J., Alberti G. 2010. Actinedid mite community diversity in a succession gradient in continental sand-dune habitats of central Europe. In: Sabelis M., Bruin J. (Eds). Trends in Acarology. Dordrecht: Springer. p. 135-142. https://doi.org/10.1007/978-90-481-9837-5_21
- Ryabinin N.A. 2015. Oribatid mites (Acari, Oribatida) in soils of the Russian Far East. Zootaxa, 3914 (3): 201-244. https://doi.org/10.11646/zootaxa.3914.3.1
- Seniczak A., Seniczak S., Iturrondobeitia J.C., Sølhoy, Flatberg K.I. 2020a. Diverse Sphagnum mosses support rich moss mite communities (Acari, Oribatida) in mires of Western Norway. Wetlands, 40: 1339-1351. https://doi.org/10.1007/s13157-019-01236-w
- Seniczak A., Seniczak S., Schwarzfeld M.D., Coulson S.J., Gwiazdowicz D.J. 2020b. Diversity and distribution of mites (Acari: Ixodida, Mesostigmata, Trombidiformes, Sarcoptiformes) in the Svalbard Archipelago. Diversity, 12 (9): 323. https://doi.org/10.3390/d12090323
- Sidorchuk E.A. 2009. New Data on the fauna of oribatid mites (Acari, Oribatida) from the Polar Urals. Entomol. Rev., 89: 554- 563. https://doi.org/10.1134/S0013873809050054
- Strandtmann R.W. 1971. The eupodoid mites of Alaska. Pacific Insects, 13(1): 75-118.
- Tolstikov A.V., Gordeeva E.V., Petrova A.D., Fyodorov-Davydov D.G. 1996. Contributions to the knowledge of the polygonal tundra mite community. In: R. Mitchel, D.J. Horn, G.R. Needham, W.C. Welbourn. (Eds). Acarology IX: Volume 1, Proceedings. Ohio: Ohio Biological Survey. p. 615-619.
- Usher M.B., Booth R.G. 1984. Arthropod communities in a maritime Antarctic moss-turf habitat: three-dimensional distribution of mites and Collembola. J. Anim. Ecol., 53 (2): 427-441. https://doi.org/10.2307/4526
- Weather Archive of M.V. Popov Weather Station [Internet]. [26.06.2023]. Available from: https://rp5.ru/Архив_погоды_на_метеостанции_им._М.В.Попова. (In Russian)
- Weigmann G. 2006. Hornmilben (Oribatida). Die Tierwelt Deutschlands. Teil 76. Keltern: Goecke & Evers. ss. 520.
- Yurtsev B.A., Tolmachev A.I., Rebristaya O.V. 1978. Floristic boundaries and subdivision of the Arctic. In: Yurtsev B.A. (Ed). Arctic floristic region. Leningrad: Nauka. p. 9-104. (In Russian)
- Zacharda M., Kučera T. 2006. Diversity of predatory rhagidiid mites (Acari: Rhagidiidae) inhabiting montane stony debris in the Ötztal Alps, North Tyrol. Arct. Antarct. Alp. Res., 38 (2): 292-300. https://doi.org/10.1657/1523-0430(2006)38%5B292:DOPRMA%5D2.0.CO;2
- Zacharda M., Kučera T. 2010. The Rhagidiidae (Acari: Prostigmata) in NW Lapland: Could their assemblages be climate warming monitors related to environmental and habitat patterns? Pedobiologia, 54(1): 1-8. https://doi.org/10.1016/j.pedobi.2010.07.004
- Zenkova I.V., Zaitsev A.S., Zalish L.V., Liskovaya A.A. 2011. List of oribatid mites (Acariformes: Oribatida) in tundra and northern taiga soils of the Murmansk region. Proceedings of the Karelian Scientific Center of the Russian Academy of Sciences, 1: 54-67. (In Russian)



2023-10-13
Date accepted:
2024-01-11
Date published:
2024-02-01
Edited by:
Pfingstl, Tobias

This work is licensed under a Creative Commons Attribution 4.0 International License
2024 Bizin, Mikhail and Makarova, Olga L.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




