Ontogenetic instars of Ciprusenia troglobionta sp. nov. (Acari: Astigmata: Canestriniidae) from a cave of Zagros Mountains, Iran
Sadat-Shojaei, Mojgan  1
; Haitlinger, Ryszard
1
; Haitlinger, Ryszard  2
; Sadeghi, Saber
2
; Sadeghi, Saber  3
and Akrami, Mohammad Ali
3
and Akrami, Mohammad Ali  4
4
1Department of Biology, Faculty of Sciences, Shiraz University, Shiraz, Iran.
2Institute of Environmental Biology, Department of Invertebrate Systematics and Ecology, Wrocław University of Environmental and Life Sciences, Poland.
3✉ Department of Biology, Faculty of Sciences, Shiraz University, Shiraz, Iran.
4✉ Department of Plant Protection, School of Agriculture, Shiraz University, Shiraz, Iran
2023 - Volume: 64 Issue: 1 pages: 76-104
https://doi.org/10.24349/owvp-jv03ZooBank LSID: FDCBFA34-07A8-4F25-AB8A-B3AA3AB37918
Original research
Keywords
Abstract
Introduction
Astigmatic mites of the family Canestriniidae Berlese, 1884 include 370 species belonging to 101 genera. The species of this family are often reported as associated with 12 families of class Insecta including Blattidae, Carabidae, Cerambycidae, Chrysomelidae, Elateridae, Geotrupidae, Lucanidae, Passandridae, Passalidae, Scarabaeidae, Tenebrionidae and Zoopheridae as well as two families of class Diplopoda including Glomeridae and Odontopygidae. They may be phoretic or parasites (Beron 2021; Haitlinger 2022; Okabe et al. 2017; Trach and Khaustov 2011).
Genus Ciprusenia was proposed by Haitlinger in 2022 and he transferred five species of the genus Percanestrinia Berlese, 1911 to this new genus. In this study, we describe the sixth species of the genus and list all of them as follows: Ciprusenia troglobionta Sadat-Shojaei & Haitlinger sp. nov., C. carabicola (Berlese, 1882), C. izabelae (Haitlinger, 1992), C. kamelskyi (Samšiňák, 1970), C. sellnicki (Samšiňák, 1965), C. viviannae (Haitlinger, 1992) (Berlese 1911; Haitlinger 1992, 2022; Samšiňák 1965, 1970; Vitzthum 1924).
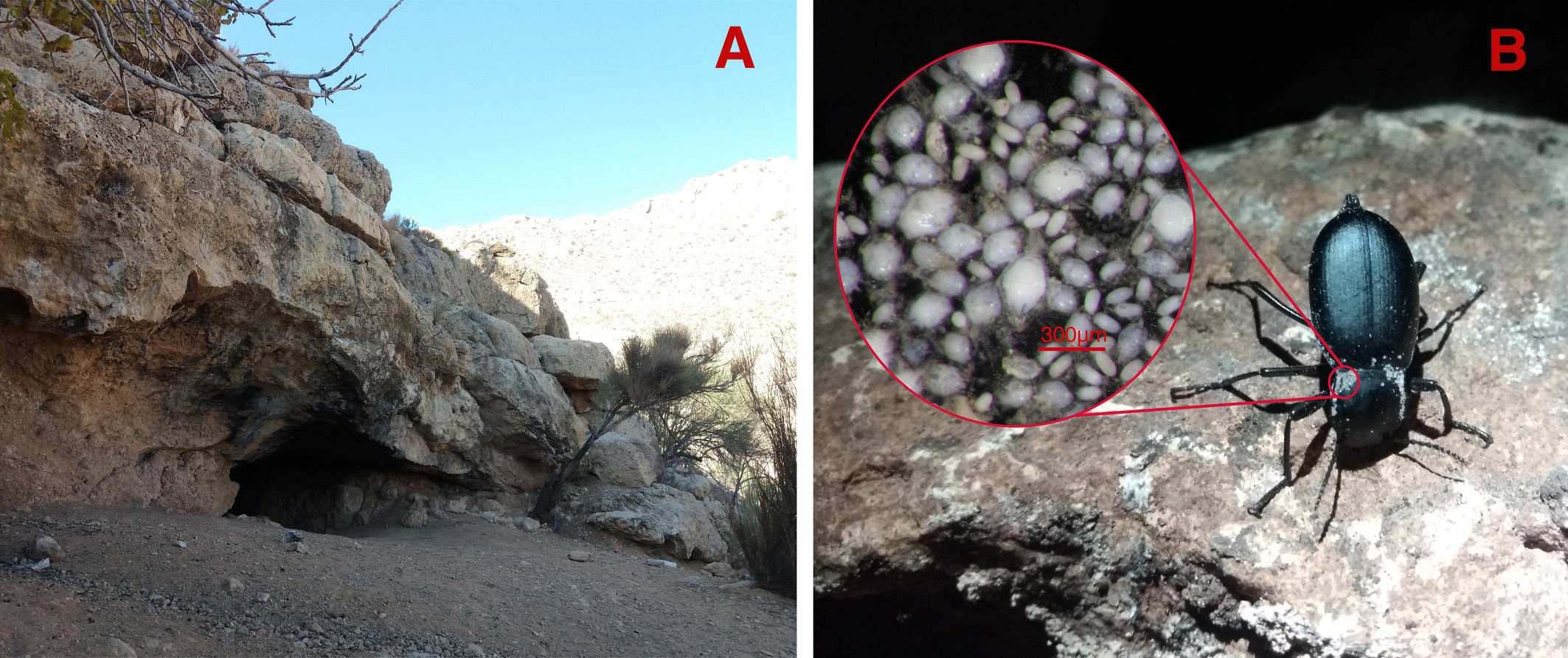
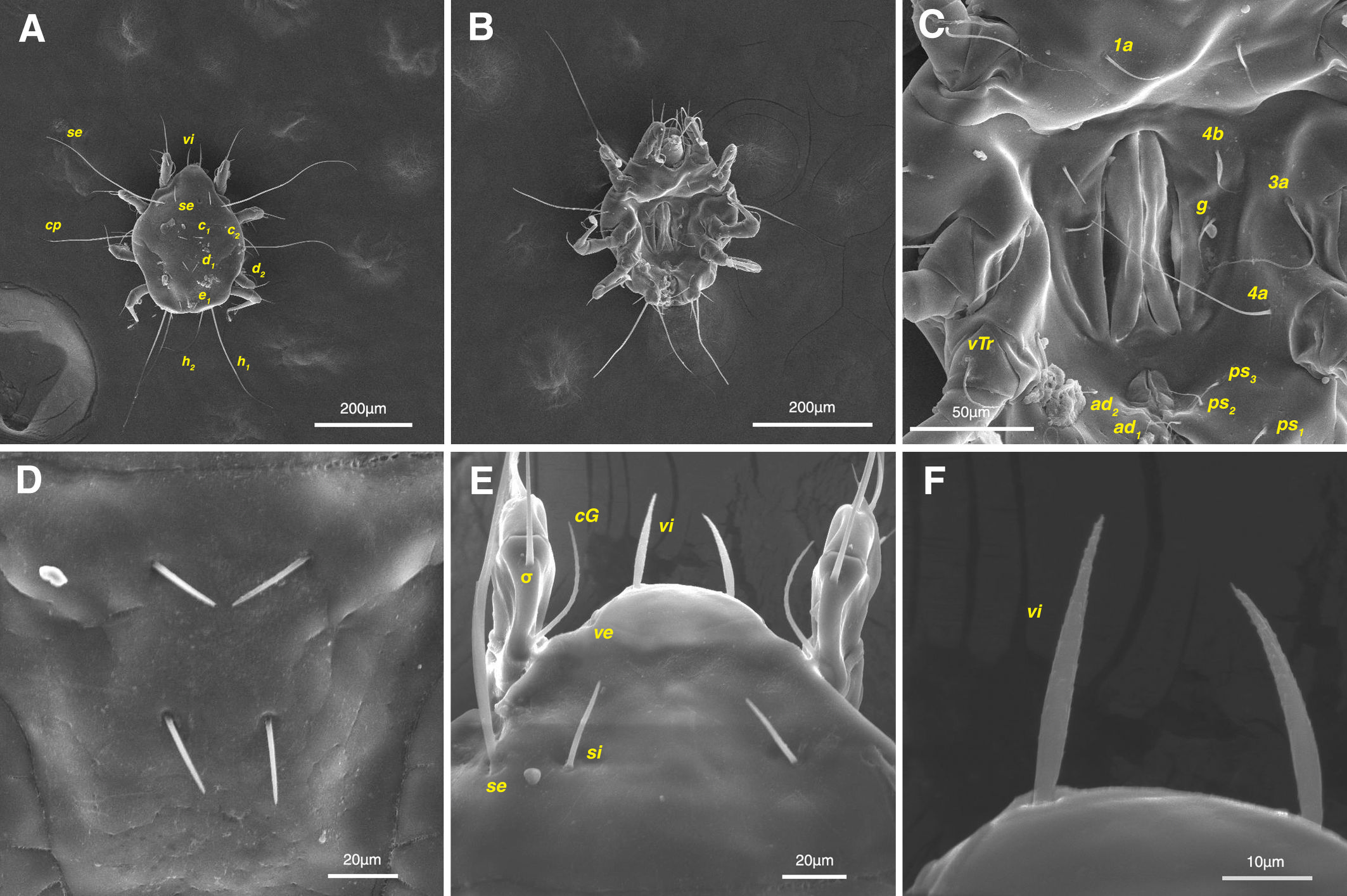
All of the mite life stages including male, female, tritonymph, protonymph and larva specimens were collected from the Blaps bushirensis Kaszab, 1959 (Fig. 1B). Only male and female forms were collected from the cave floor soil near the beetle host and not any stable population of the new species was extracted from the soil. Both males and females of the Blaps bushirensis were infected with this new mite species.
Usually, a relatively deep cave is divided into three main zones based on the amount of light penetration: the endogean zone or cave entrance area which receives the most amount of light, the para-hypogean zone or twilight area of the cave which receives less light than the entrance and the hypogean or dark area of the cave where no light penetrates (Lee et al. 2012).
The site of soil and host sampling was the transition zone of para-hypogean to hypogean of Sahlak Cave. The soil floor of this cave was mixed with bat guano in many parts. This cave is a natural karstic cave in the Zagros Mountains, with almost 330 meters in length which is located in Iran, Fars province, Darab county, Chah Kondar village, Sahlak canyon (Fig. 1A).
The Zagros Mountains form a vast orogenic range in Iran with approximately 2000 km long and 100 to 200 km wide in front of the Iranian and Turkish plateaus (Mouthereau et al. 2007; Ramsey et al. 2008). The biospeleology of many caves in the Zagros Mountains, especially the troglofauna of cave-dwelling Acari, has remained unknown.
Until now, Coleopterophagus baali Haitlinger, 1990 from Protaetia speciosa (Adams, 1817), Photia polymorpha Samšiňák, 1971 from Carabus persianus Roeschke, 1896 and an unidentified deutonymph of genus Camirohylla Haitlinger, 1991 from an unidentified species of Dila Fischer von Waldheim, 1844 have been recorded. Therefore, the Iranian fauna of the family Canestriniidae is weakly known (Ardeshir et al. 2016; Haitlinger 1990a; Samšiňák 1971). Ciprusenia troglobionta sp. nov. is the fourth record of the family and the first record of the genus for Astigmata mite fauna of Iran. The troglomorphic characteristics of this subterranean mite species as adaptations for living in a cave environment are discussed. The host species, Blaps bushirensis, is a new host record for the family Canestriniidae. The identification key of the world-known species of the genus Ciprusenia is provided. The adult male of C. viviannae (Haitlinger, 1992) is not listed in the male key due to lack of access. The holotype of this species is a female.


Material and methods
Specimens and Sampling
A sampling of the Blaps bushirensis and the soil of the cave floor near the beetles from the para-hypogean to the hypogean transition zone of Sahlak Cave was carried out on December 4, 2021 (Fig. 1A). Then in Entomology Research Laboratory (Biology Department), male and female mite specimens of the new species were extracted from the soil using Berlese-Tullgren funnels. Male, female, tritonymph, protonymph and larva specimens were collected from the beetle host with the help of fine laboratory tools using a ZEISS Stemi Sv11 stereomicroscope (Fig. 1B). To remove surface contamination, all of the specimens were preserved in 70% ethanol.
Observation and documentation
Since the color and general nature of the mite specimens often change after slide mounting, the male and female specimens were photographed in a cavity slide containing 70% ethanol using an Olympus CH2 Microscope equipped with a 7D Canon digital camera. To increase the quality of the output images, we used Zerene Stacker 2022 software (ver. 1.04, Zerene Systems, see https://zerenesystems.com/cms/home ![]() ). Detailed character images of male and female specimens were also taken using a VEGA3 TESCAN scanning electron microscope. The male, female, tritonymph, protonymph, and larva specimens were cleared in Nesbitt's fluid and mounted into Hoyer's medium (Walter and Krantz 2009). Detailed characters of the mounted mite slides were photographed using a Nikon Eclipse 80i microscope equipped with a digital camera. Manual line drawings of the mounted mite slides were made using an Olympus BX53 phase contrast microscope equipped with a drawing tube. The initial line drawings were processed in Adobe Photoshop 2023 software (ver. 24.1.0, Adobe, see https://www.adobe.com/au/
). Detailed character images of male and female specimens were also taken using a VEGA3 TESCAN scanning electron microscope. The male, female, tritonymph, protonymph, and larva specimens were cleared in Nesbitt's fluid and mounted into Hoyer's medium (Walter and Krantz 2009). Detailed characters of the mounted mite slides were photographed using a Nikon Eclipse 80i microscope equipped with a digital camera. Manual line drawings of the mounted mite slides were made using an Olympus BX53 phase contrast microscope equipped with a drawing tube. The initial line drawings were processed in Adobe Photoshop 2023 software (ver. 24.1.0, Adobe, see https://www.adobe.com/au/ ![]() ). All measurements are given in micrometers (µm) and taken using Fiji (ImageJ2) 2022 software (ver. 1.53t, ImageJ, see https://imagej.nih.gov/ij/index.html
). All measurements are given in micrometers (µm) and taken using Fiji (ImageJ2) 2022 software (ver. 1.53t, ImageJ, see https://imagej.nih.gov/ij/index.html ![]() ).
).
Abbreviations
The terminology and abbreviations followed by related literature (Grandjean 1939; Griffiths et al. 1990; Haitlinger and Šundić 2016; Norton 1998; Trach and Khaustov 2011).
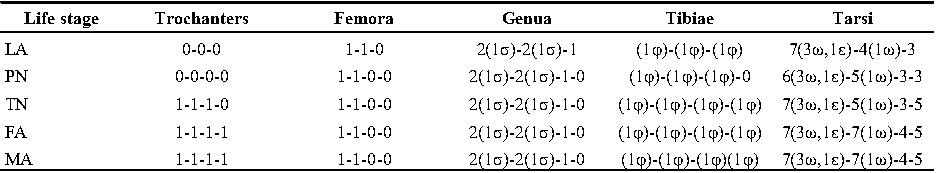
Additional abbreviations: IL, length of idiosoma; IW, width of idiosoma; GL, length of gnathosoma; CL, cheliceral length; MA, male adult; FA, female adult; TN, tritonymph; PN, protonymph; LA, larva; GO, Grandjean's organ.
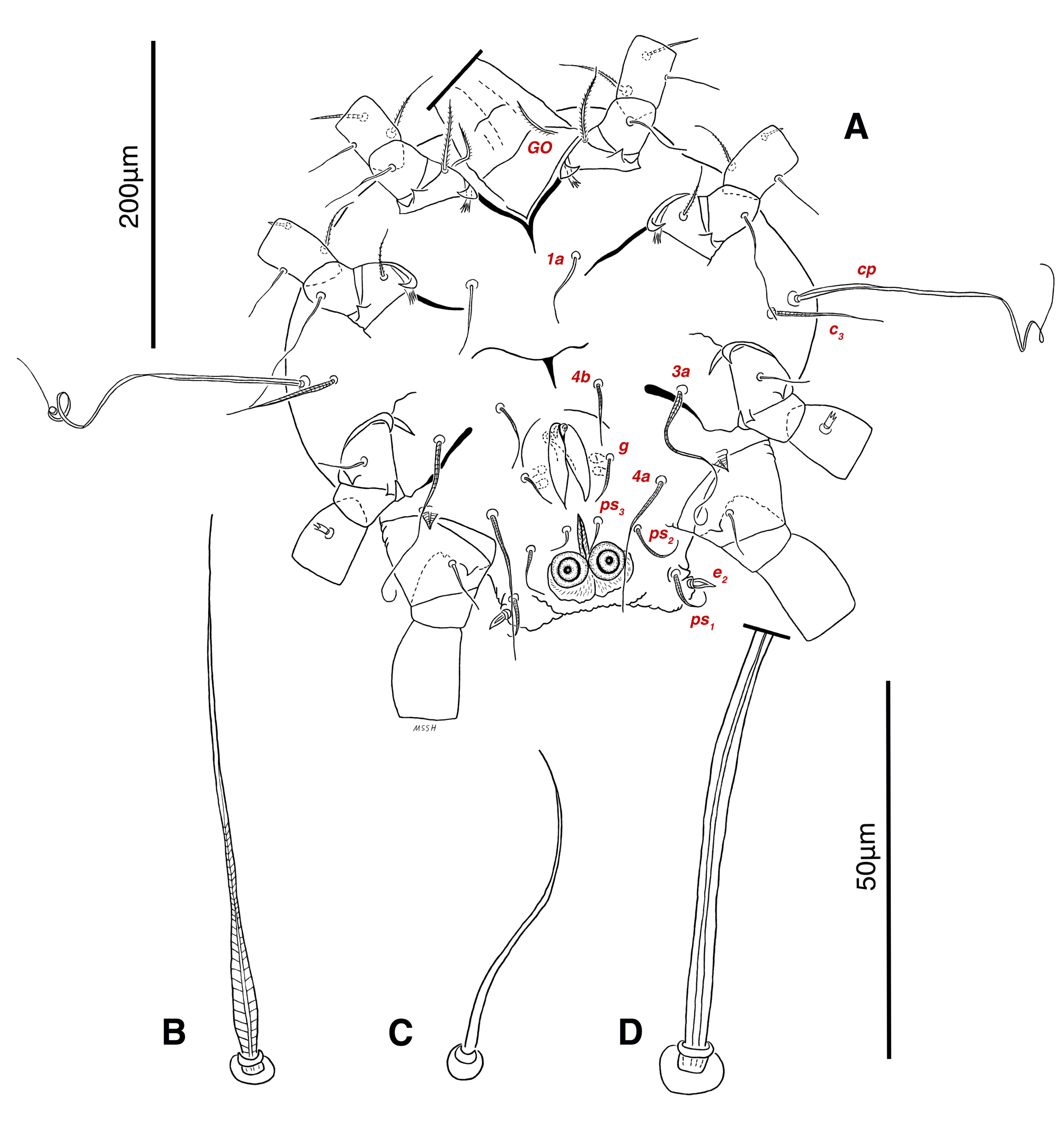
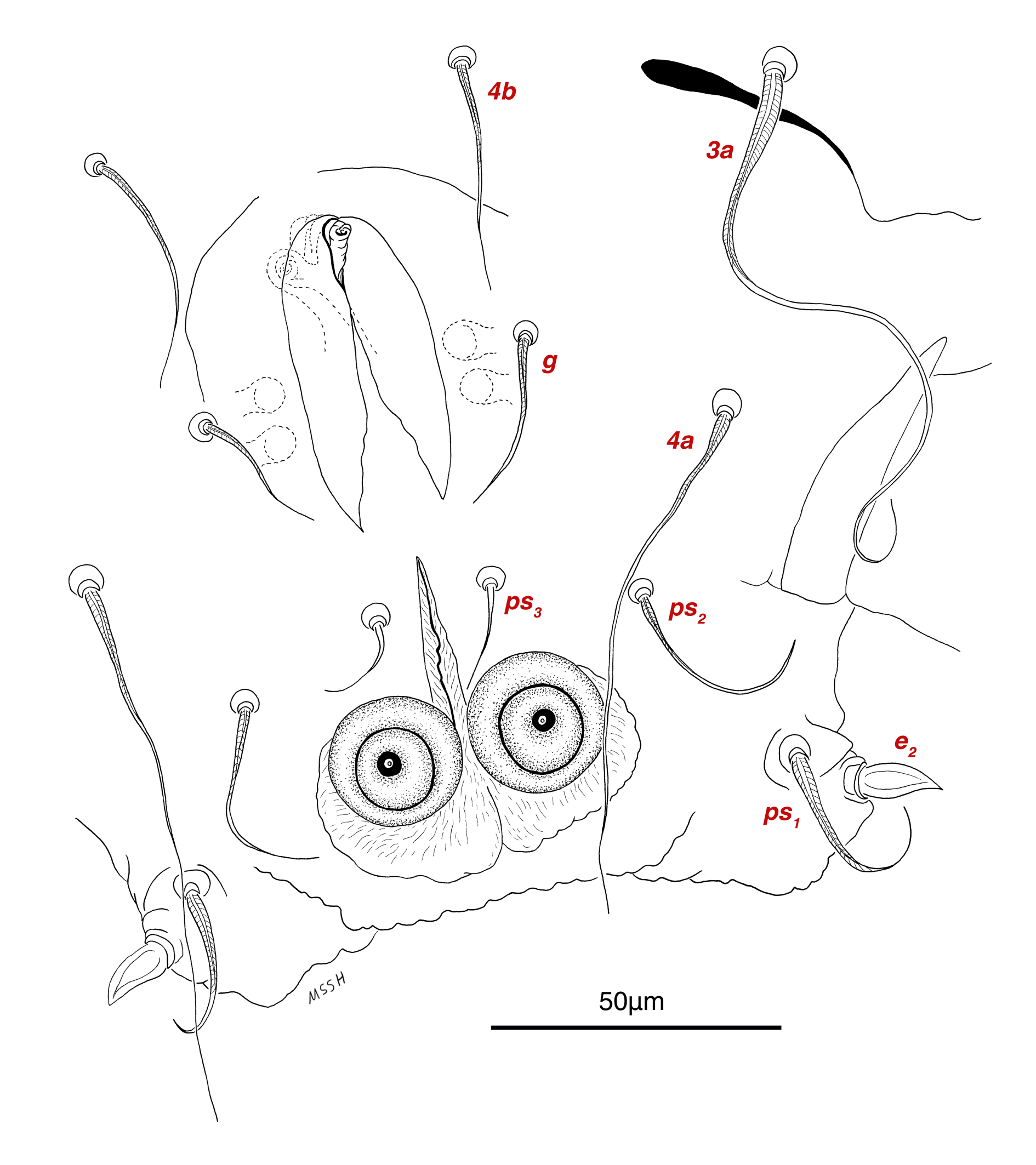
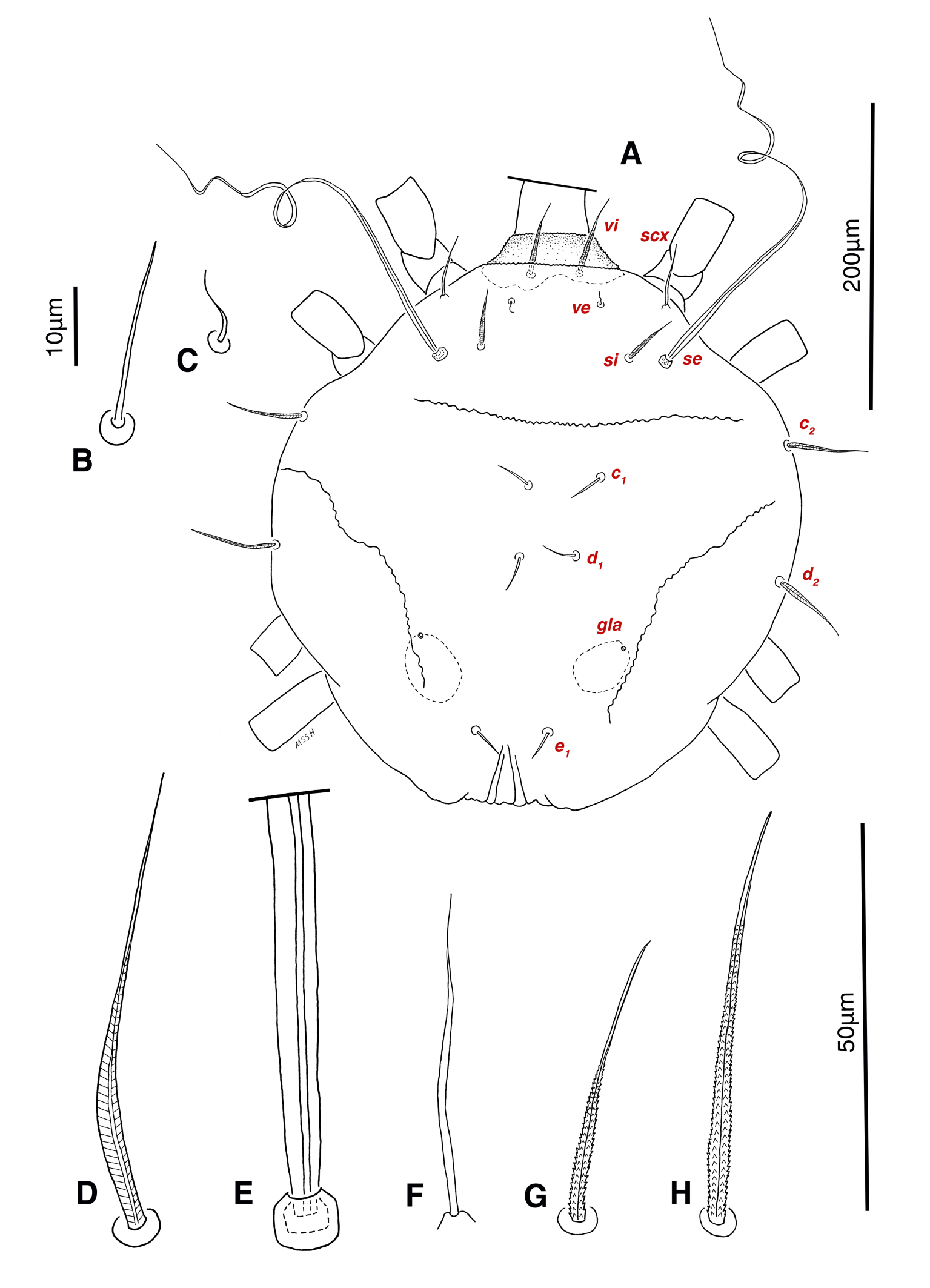
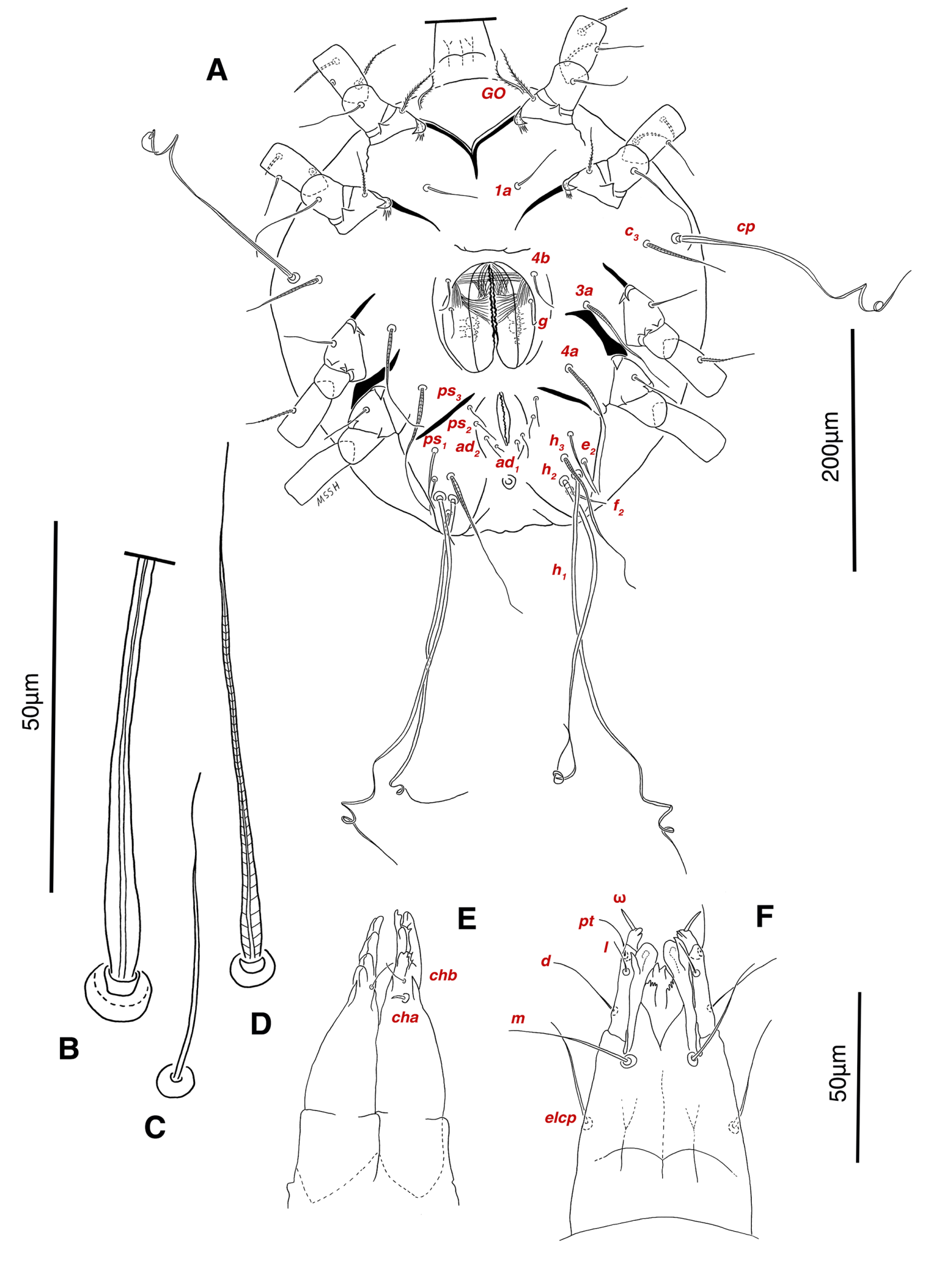
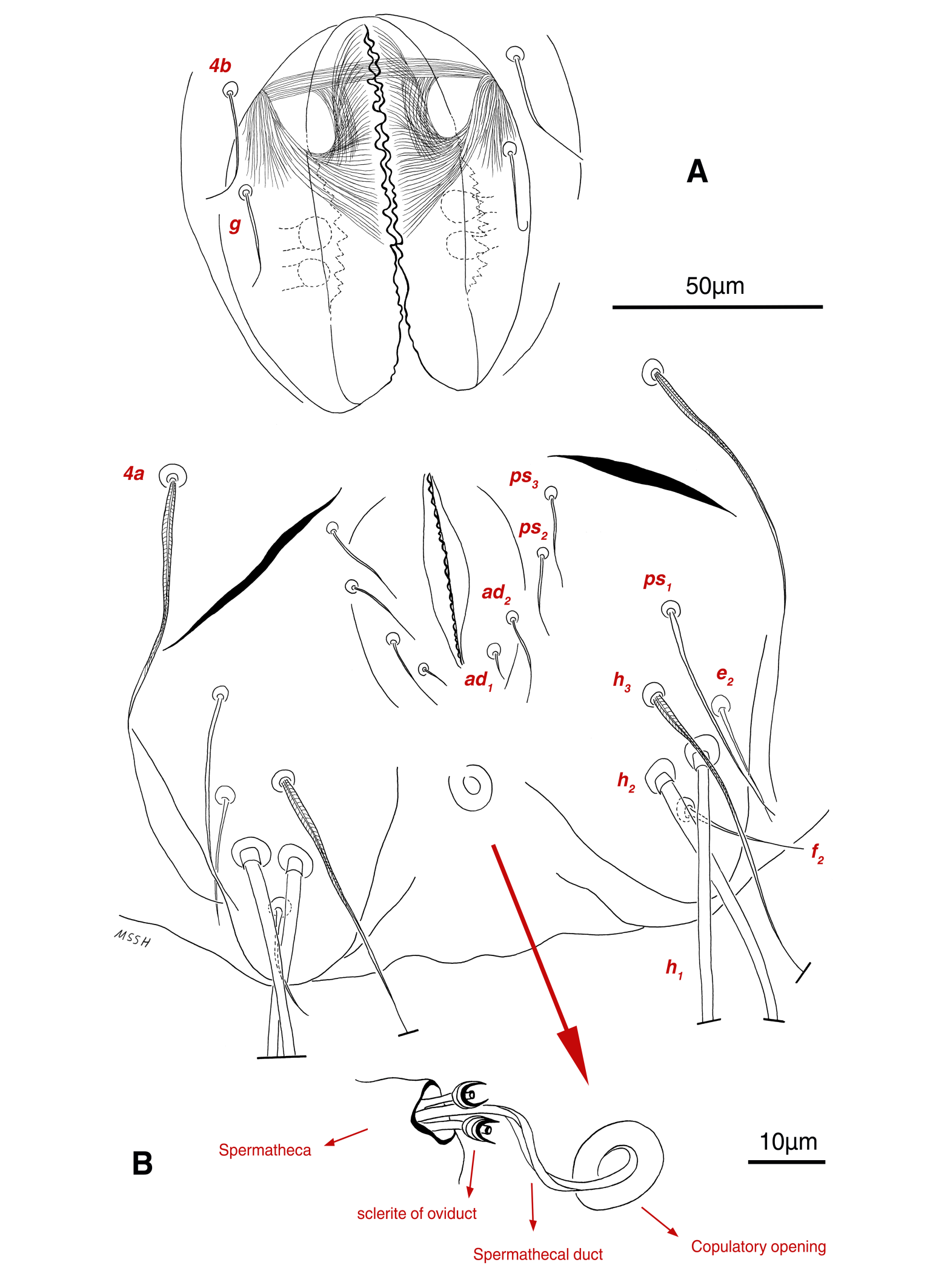
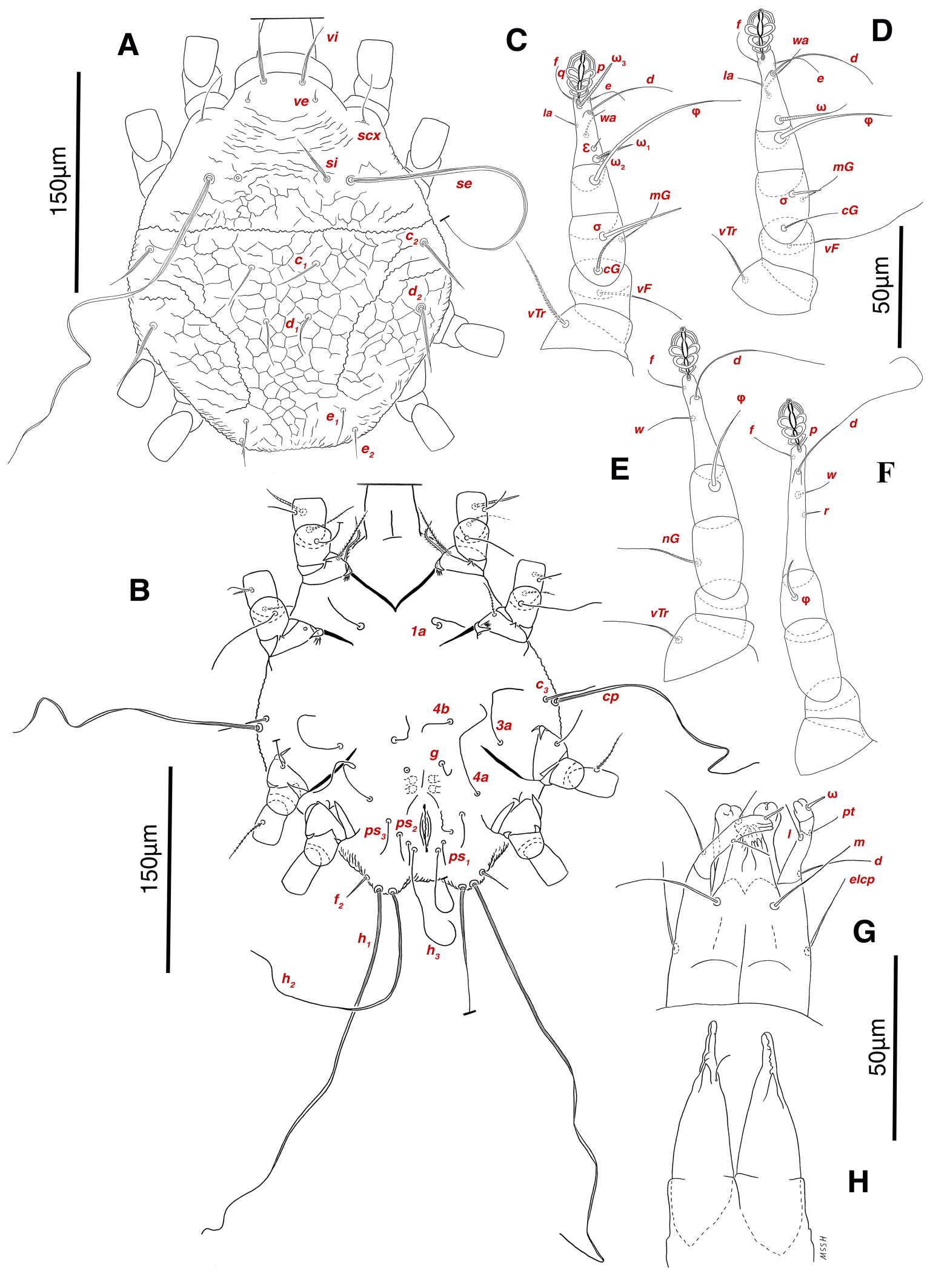
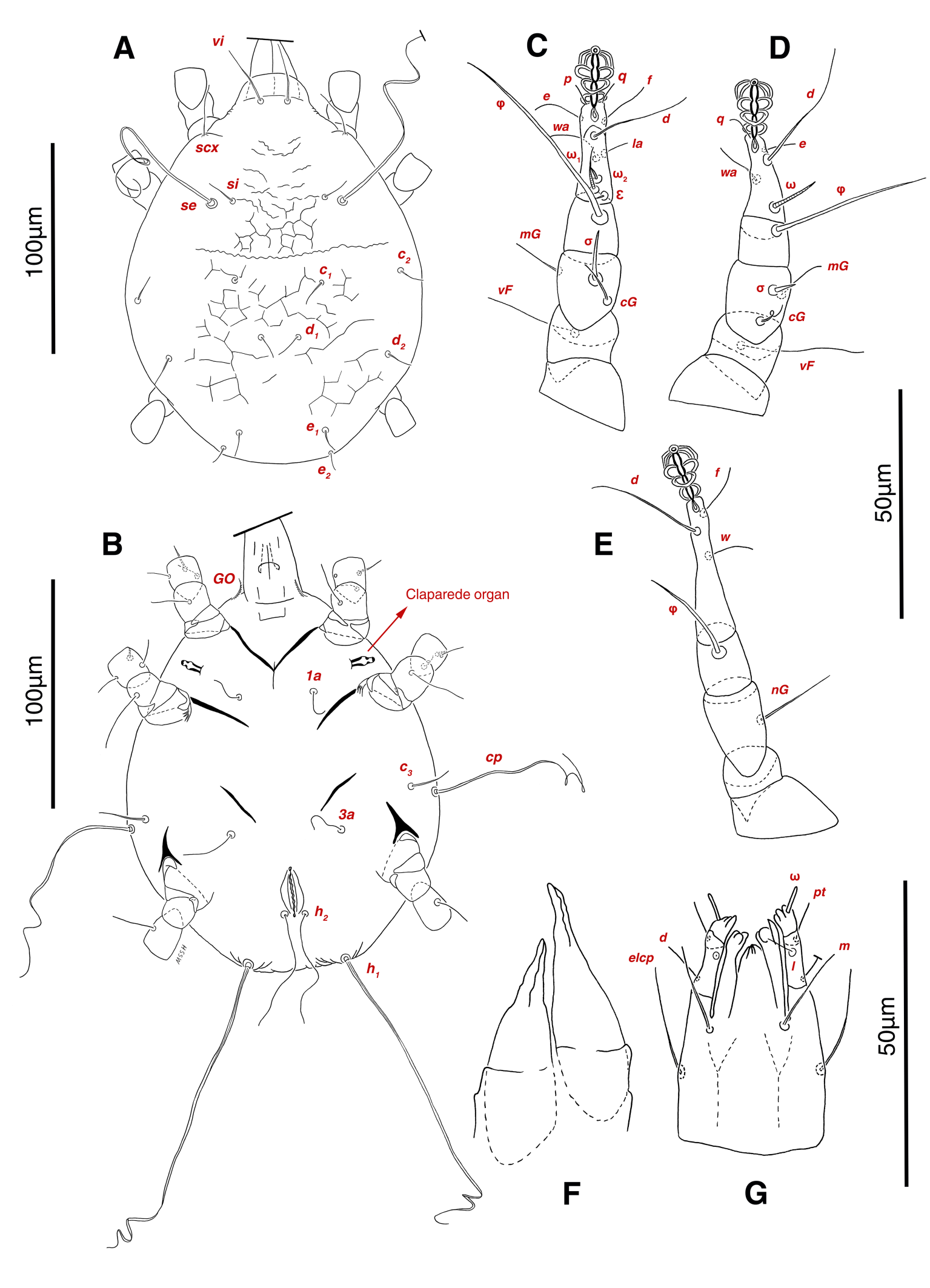
Abbreviations of dorsal and ventral setae: vi and ve, internal and external vertical setae; si and se, internal and external scapular setae; c1 , c2 , cp and c3 , first (innermost), second, third and fourth setae in the first row on hysterosoma; d1 and d2 , first (innermost) and second setae in the second row on hysterosoma; e1 and e2 , first (innermost) and second setae in the third row on hysterosoma; f2 , second setae in the forth row on hysterosoma; h1 , h2 and h3 , first (innermost), second and third setae in the fifth row on hysterosoma; scx, supracoxal setae; 1a, coxal-sternal setae associated with the bases of legs I; 3a, coxal-sternal setae associated with the bases of legs III; 4a and 4b, first (innermost) and second coxal-sternal setae associated with the bases of legs IV; g, genital setae; ad1 , ad2 and ad3 , first (posteriormost), second and third adanal setae; ps1 , ps2 and ps3 , first (posteriormost), second and third pseudoadanal setae.
Abbreviations of depositories: ZM-CBSU, Zoological Museum, Collection of Biology Department, Shiraz University, Shiraz, Iran; JAZM, Jalal Afshar Zoological Museum, Department of Plant Protection, University of Tehran, Karaj, Iran; MNHWU, the Museum of Natural History, Wrocław University, Wrocław, Poland.
Results
Taxonomy
Family Canestriniidae Berlese, 1884
Genus Ciprusenia Haitlinger, 2022
Ciprusenia Haitlinger, 2022, p. 24. Type species: Ciprusenia izabelae (Haitlinger, 1992).
Percanestrinia izabelae - Haitlinger, 1992, p. 536.
Ciprusenia troglobionta Sadat-Shojaei & Haitlinger, sp. nov.
ZOOBANK: 005F2E72-3105-47AE-B134-E2D84351437A ![]()
(Figures 1-21)
Material examined
Holotype — Male, Iran, Fars prov., Darab county, Chah Kondar vill., Sahlak canyon, Sahlak Cave; 28°32′51″N, 55°08′33″E; ex. Blaps bushirensis; 04 Dec 2021; leg. M. Sadat-Shojaei; microscopic slide, (ZM-CBSU Aca.00020).
Paratypes — Same collection data as holotype; 1 MA, 1 FA, microscopic slides, (MNHWU CTWP1, CTWP2); 1 MA, 1 FA, 1 TN, 1 LA, microscopic slides, (JAZM ARS-20230109-1b, ARS-20230109-1c, ARS-20230109-1d, ARS-20230109-1e) ; 2 MA, 2 FA, 3 TN, 3 PN, 3 LA, microscopic slides, (ZM-CBSU Aca.00021, Aca.00022, Aca.00029, Aca.00030, Aca.00036, Aca.00037, Aca.00038, Aca.00039, Aca.00040, Aca.00041, Aca.00042, Aca.00043, Aca.00044); 3 MA, 2 FA, mounted for SEM, (ZM-CBSU Aca.00023, Aca.00024, Aca.00025, Aca.00031, Aca.00032); 3 MA, 3 FA, preserved in ETOH. 70%, (ZM-CBSU Aca.00026, Aca.00027, Aca.00028, Aca.00033, Aca.00034, Aca.00035).
Etymology
`Troglobiont' refers to an obligatory cave-dwelling organism that is specialized for living in caves and exhibits troglomorphic characteristics.
Diagnosis
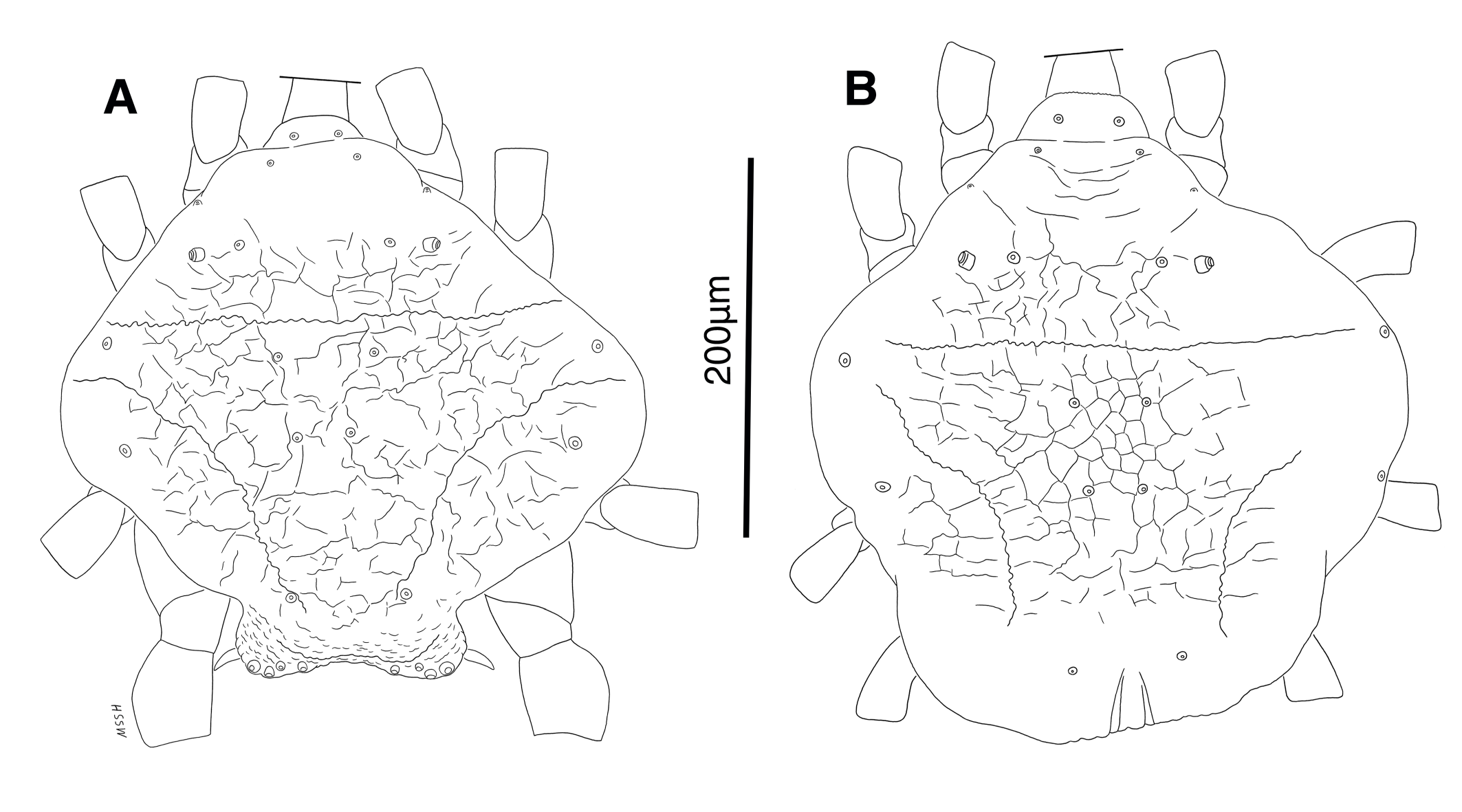
Male and female adults: white in color, transparent in some parts and without any pigmentation (Figs. 2A, B); propodosomal plate punctate; wavy sejugal furrow present, two diagonal wavy furrows on both sides of the hysterosoma; Grandjean's organs and setae scx well-developed; setae ve less than 20; setae vi and si spinulate; setae se and cp vascular-shaped; setae vTr I clearly pectinate.
Male: IL 250-341; setae e2 flame-shaped, setae nG tricarinate.
Female: IL 358 – 396; tarsi IV elongated > 90; fine regular fibers layer beneath the genital valves layer.
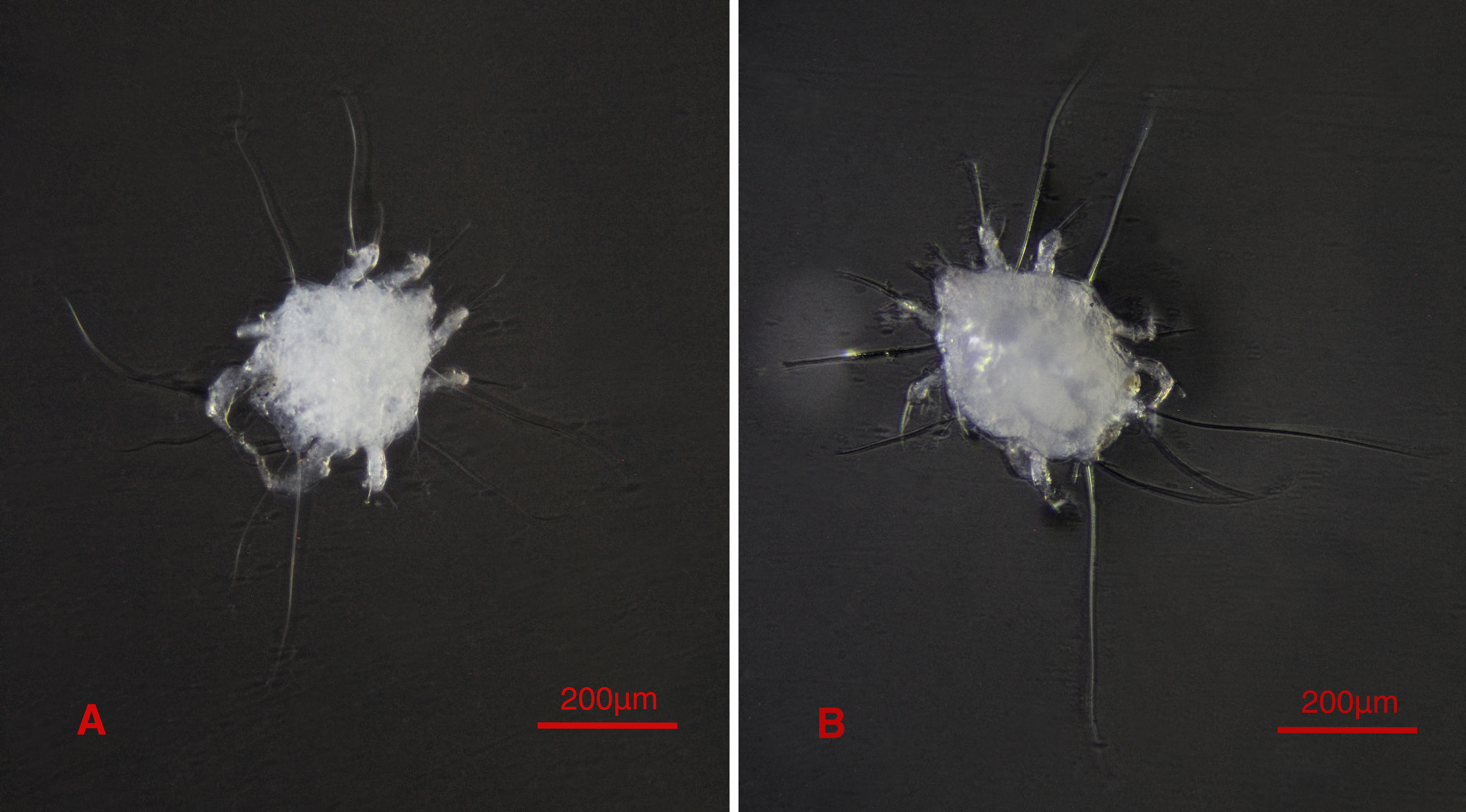


Description
Male (holotype and 10 paratypes)
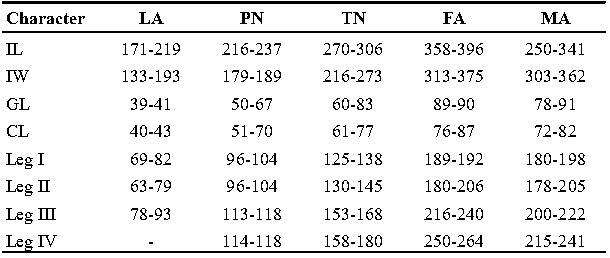
Measurements — (Paratypes in parentheses), IL 321 (250-341), IW 355 (303-362); GL 88 (78-91), CL 76 (72-82); leg I 191 (180-198), leg II 201 (178-205), leg III 222 (200-222), leg IV 241 (215-241) (Table 1).


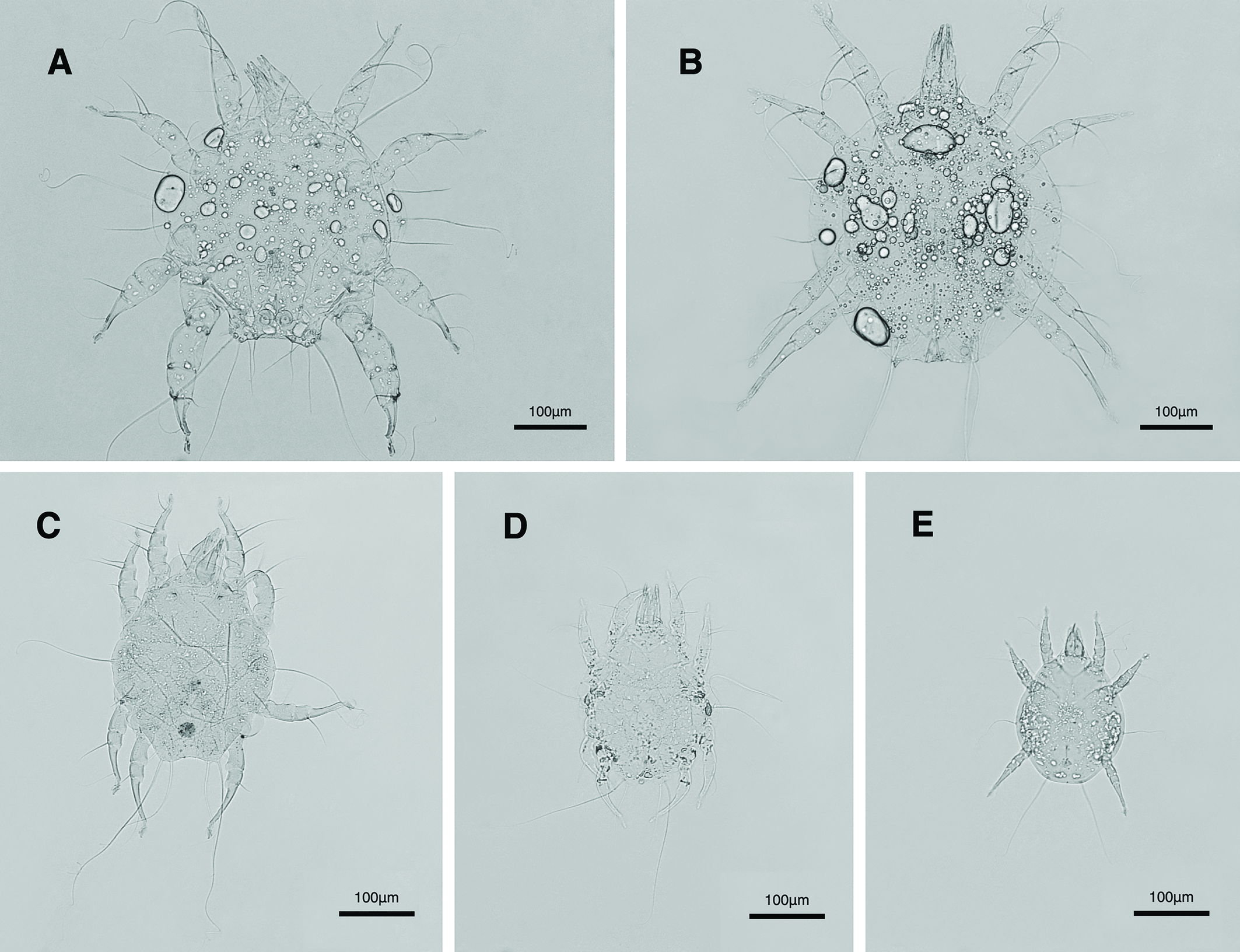


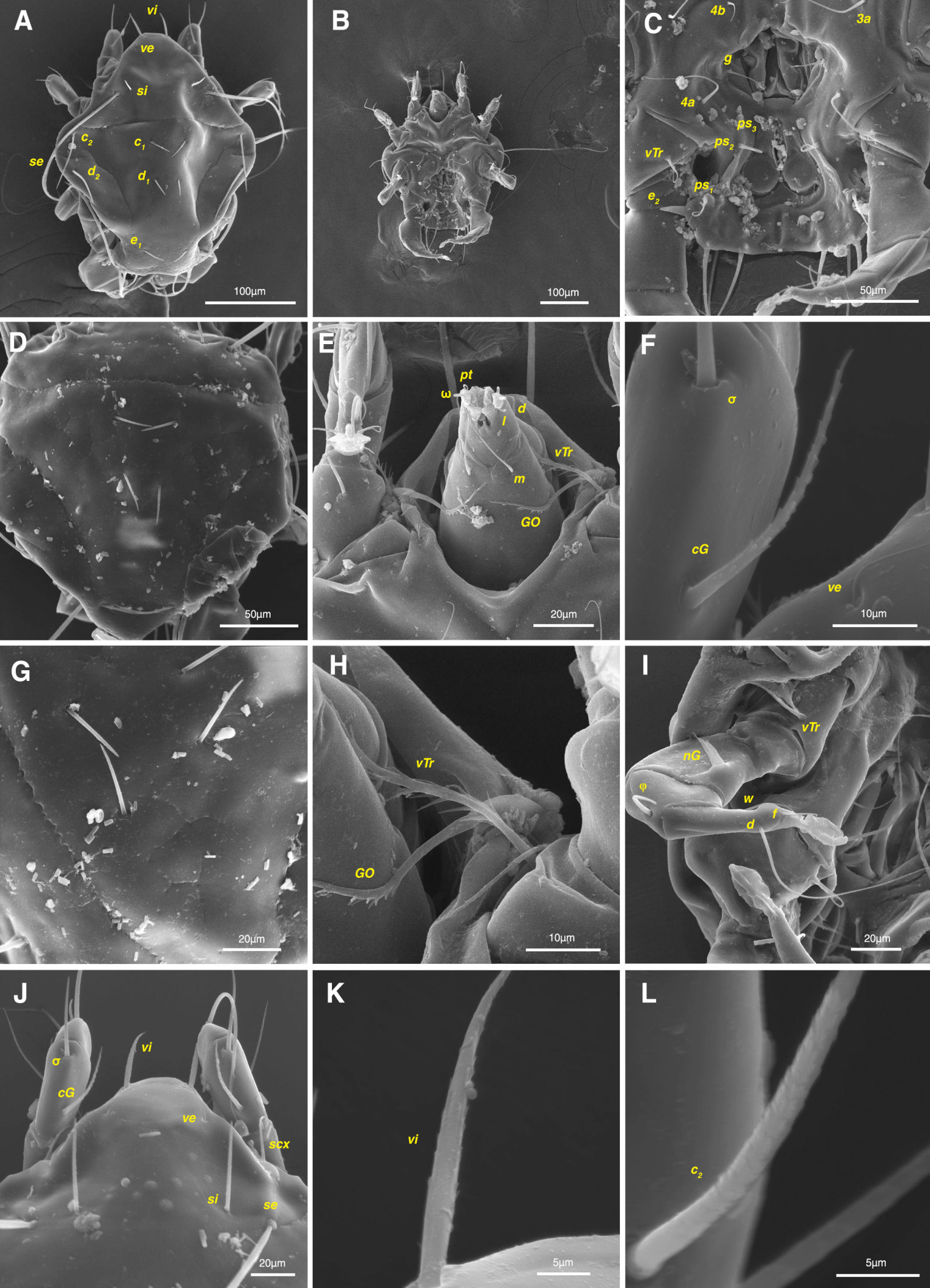


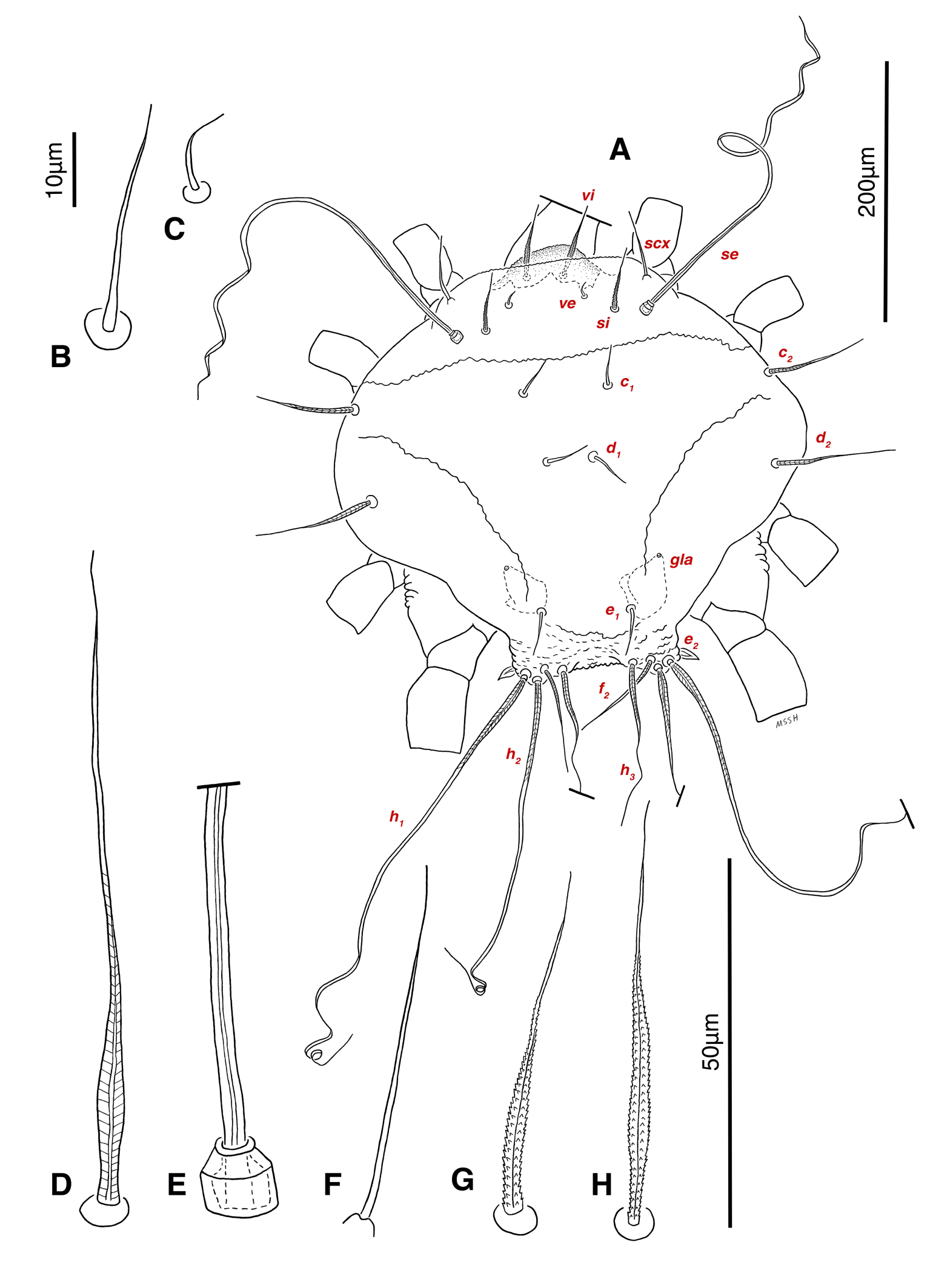






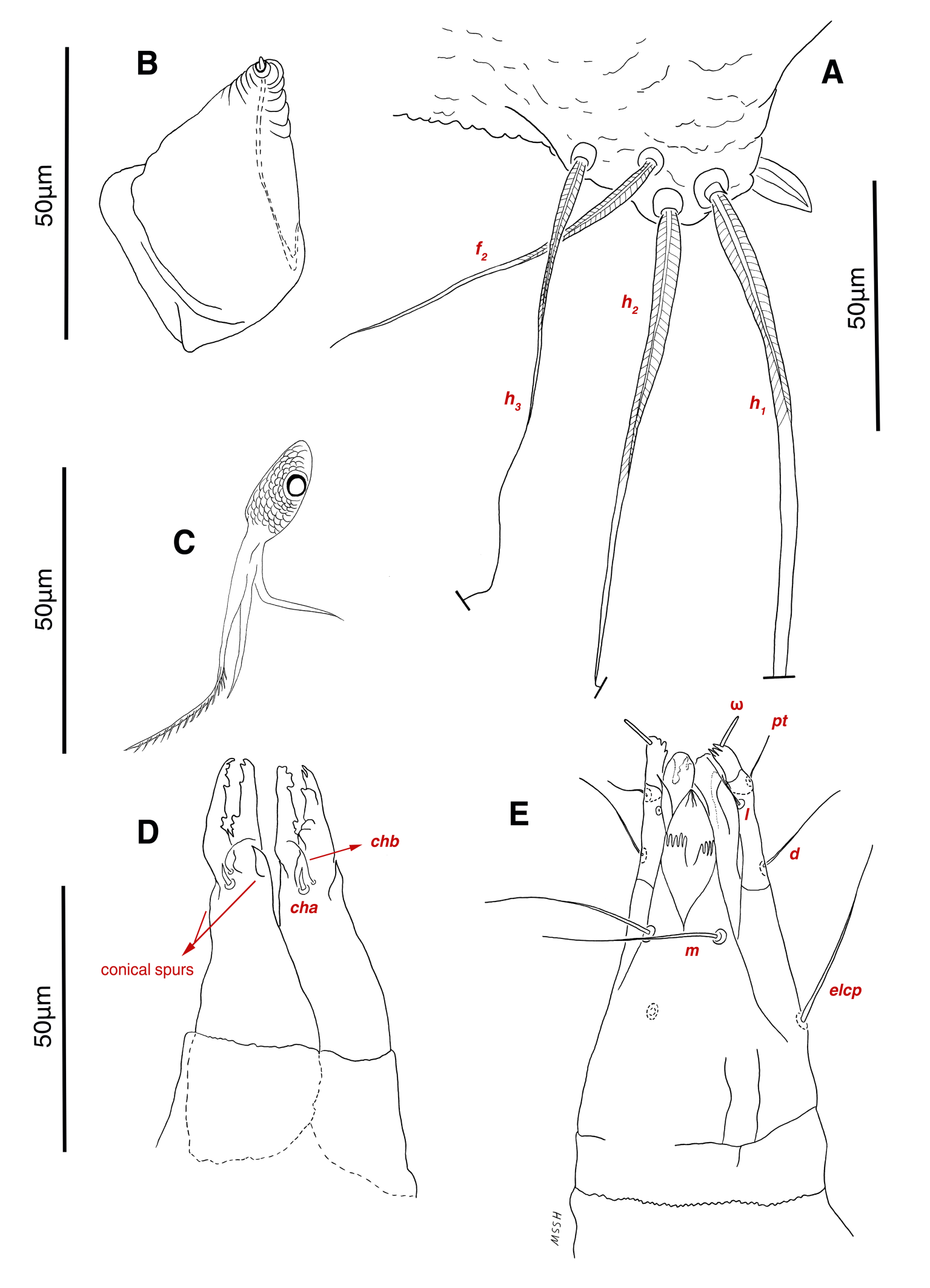






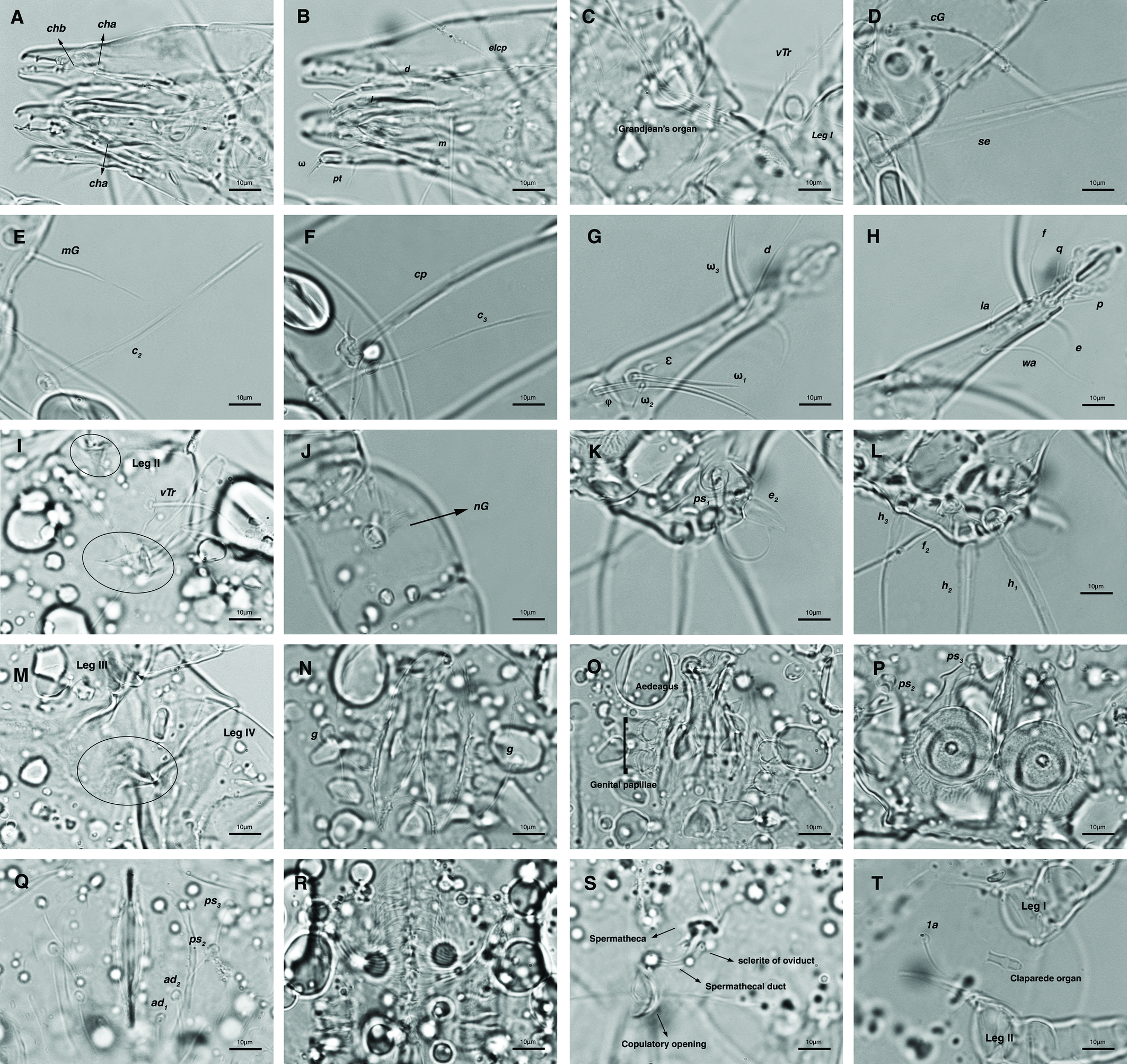


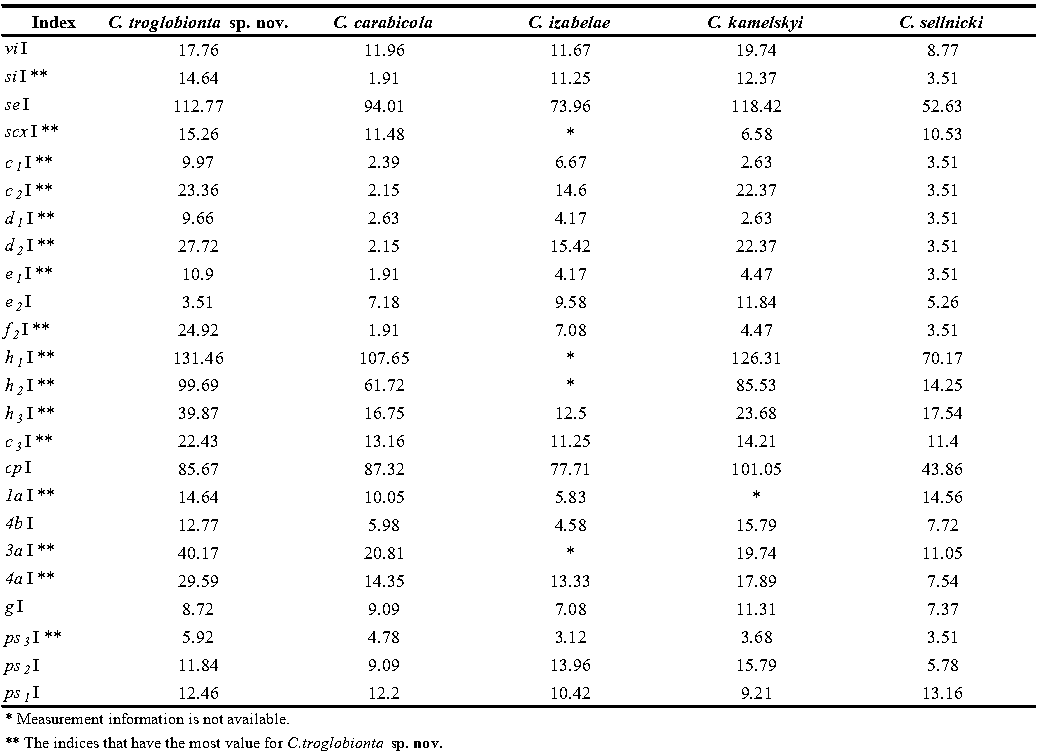
Dorsum — Idiosoma convex in middle part, maximum width between leg II and leg III, anterior margin finely wavy (Figs. 3A, 4A, 5A); idiosoma of smaller males with reticulate ornamentation, unlike larger ones and holotype (Figs. 4G, 13A); propodosomal plate punctate, with spinulate bulbiform setae vi, short smooth simple setae ve just behind the propodosomal plate (Figs. 4J, K, 5A, C, H); scapular setae si and se present, smooth simple setae scx anterolateral near legI (Figs. 4J, 5F), setae si spinulate bulbiform (Fig. 5G), setae se long and vascular-shaped, with cup-like bothridium (Figs. 5E, 11D); mild wavy sejugal furrow present, two diagonal mild wavy furrows symmetrically begin between setae c2 and d2 and continue near seta e1 (Figs. 4A, D, G, 5A); setae c2 and d2 leaf-shaped bulbiform (Figs. 4L, 5D, 11E); setae c1 , d1 and e1 smooth simple (Fig. 5B); hysterosoma contracted in opisthosomal border, opisthosoma with many fine wrinkled lines; setae f2 , h1 , h2 and h3 leaf-shaped bulbiform, all located on two lobes at end corners of body near the posterior margin of opisthosoma (Figs. 5A, 8A, 11L), h1 > h2 > h3 > f2 ; opisthonothal gland, gla, conical and slightly folded around opening (Fig. 8B); length of dorsal setae: vi 57 (51-62), ve 12 (11-18), scx 49 (44-50), si 47 (41-51), se 362 (320-362), c1 32 (24-32), c2 75 (56-75), d1 31 (25-33), d2 89 (82-89), e1 35 (30-35), f2 80 (70-80), h1 427 (414-427), h2 320 (282-320), h3 128 (110-128).
Venter — Grandjean's organ well developed at the base of leg I close to gnathosoma, include three or four branches connected to an oval structure with a distinct opening and reticulated surface, longest branch just pectinate usually close to vTr I (Figs. 4E, H, 6A, 8C, 11C); coxal- sternal setae 1a, 3a, 4a, 4b present, setae 1a near base of leg I and smooth simple, long setae 3a at base of leg III and leaf-shaped bulbiform, long seta 4a at base of leg IV and leaf-shaped bulbiform, setae 4b above genital region and leaf-shaped (Figs. 4B, C, 6A, C, 7); setae c3 leaf-shaped bulbiform near to long vascular-shaped setae cp (Figs. , 4B, 6A, B, D, 11F); aedeagus observed beneath genital valves layer (Figs. 4C, 6A, 7, 11O); leaf-shaped genital setae g, close to genital papillae (Figs. 6A, 7, 11N, O); anal valve with fine striations, anal suckers well-developed, a layer with fine striations around lateral and posterior parts of anal suckers (Figs. 4C, 6A, 7, 11P); short setae ps3 smooth simple, setae ps2 and ps3 leaf-shaped, ps1 > ps2 > ps3 ; flame-shaped setae e2 close to setae ps1 all located on two lobes at end corners of body (Figs. 4C, 6A, 7, 11K, 11P); posterior margin of body delicately folded (Figs. 6A, 7, 11P); length of ventral setae: 1a 47 (40-50), 3a 129 (100-129), 4a 95 (90-100), 4b 41 (33-41), c3 72 (63-72), cp 275 (245-290), g 28 (24-29), ps1 40 (40-45), ps2 38 (38-41), ps3 19 (15-22), e2 15 (15-17).
Gnathosoma — Conical and mostly covered by idiosoma (in natural form and not in microscopic slide) (Figs. 2A, 4A, B, E); gnathosomal palps two segmented, palpal tarsi solenidion setae ω and pulpal tarsi setae pt present, palpal tibia setae l and d present, long supracoxal setae elcp and long subcapitular setae m observed dorsally and ventrally respectively (Figs. 4E, 8E, 11B); chelicerae chelate-dentate with 4-5 teeth, cheliceral setae chb filiform, cha spiniform, two conical spurs on each chelicera (Figs. 8D, 11A); length of gnathosomal setae: ω 6 (6-7), pt 9 (8-11), l 11 (7-11), d 20 (18-20), elcp 36 (30-36), m 34 (30-34), cha 4 (3-4), chb 10 (9-10).
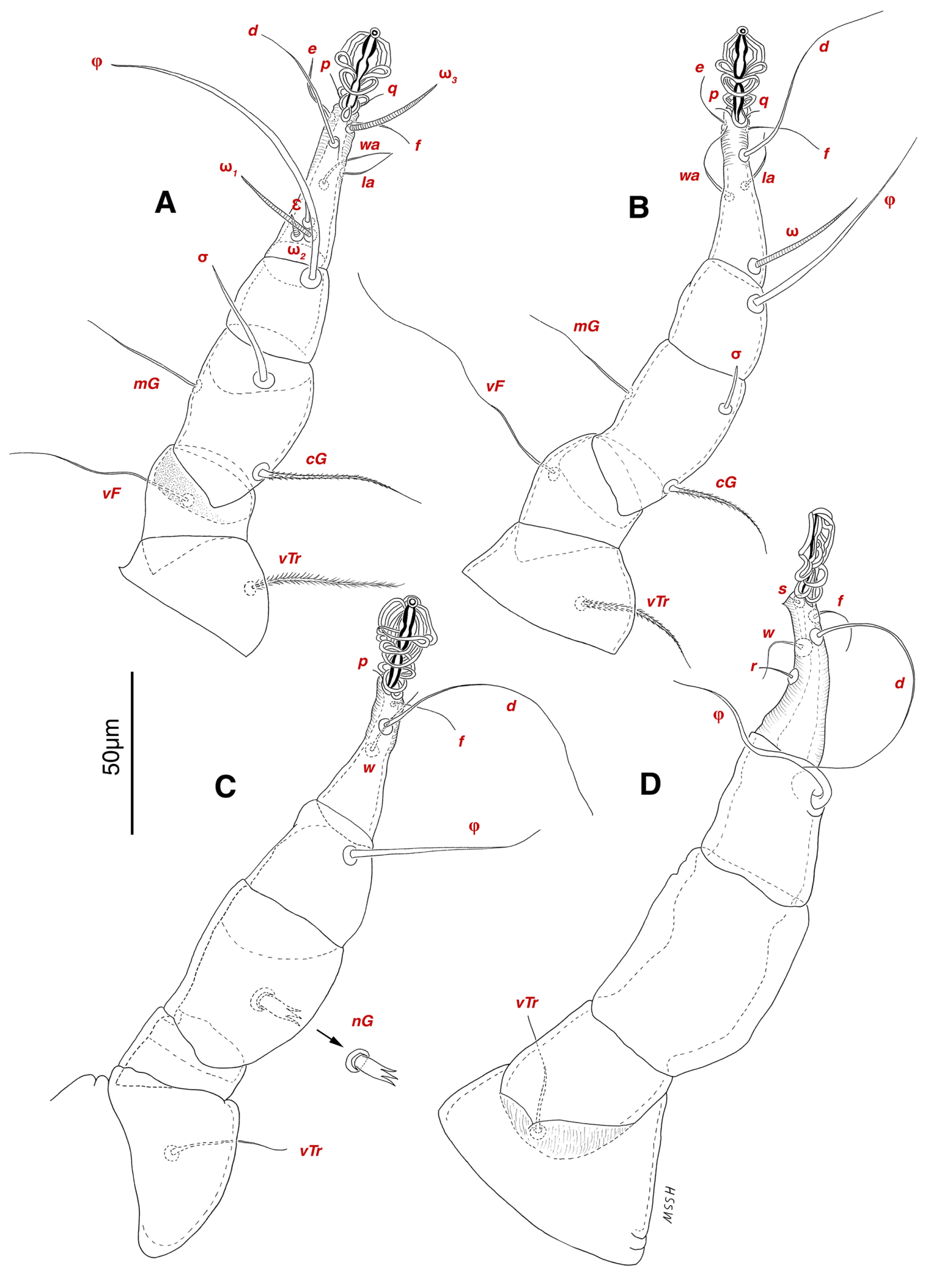
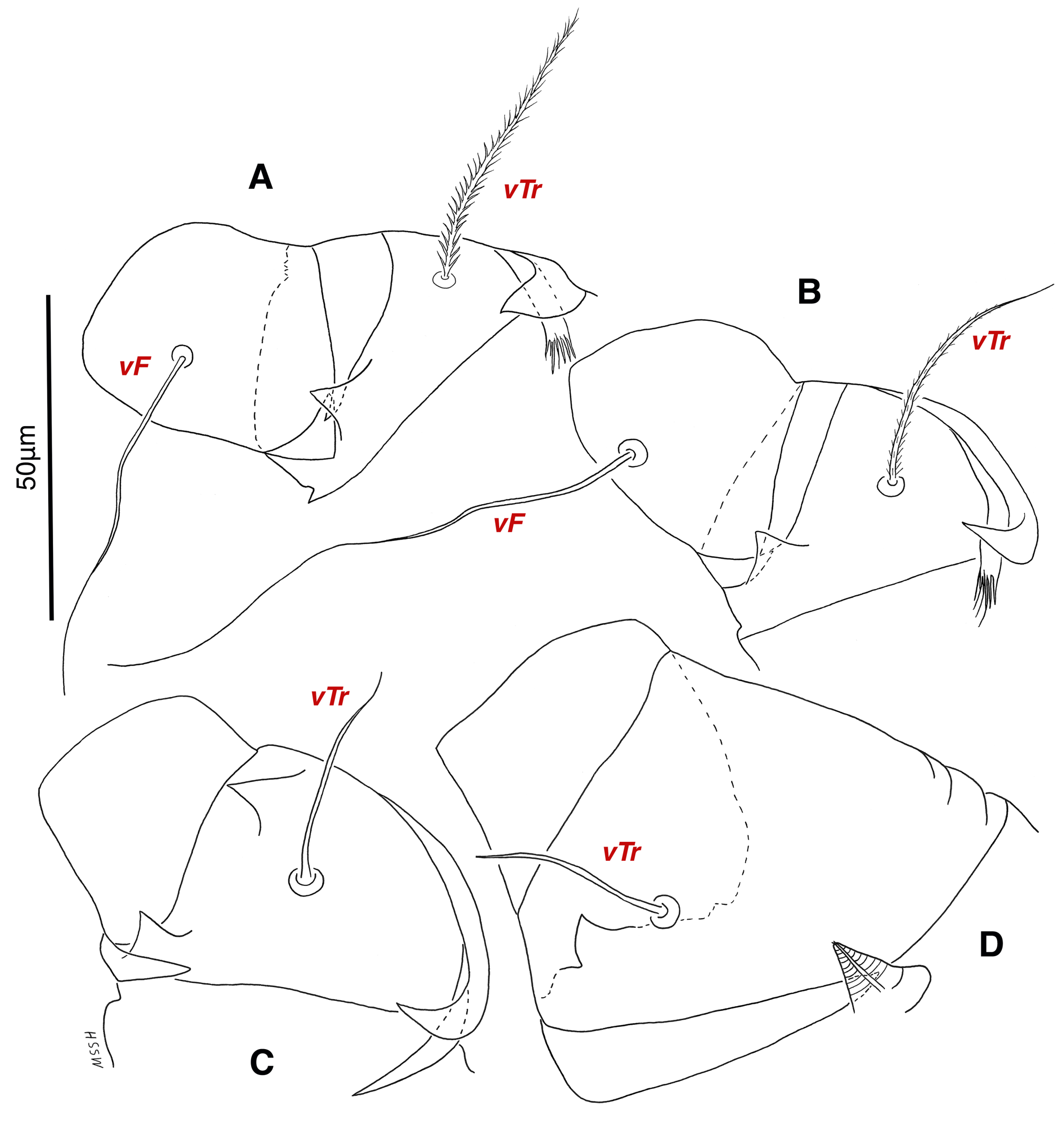
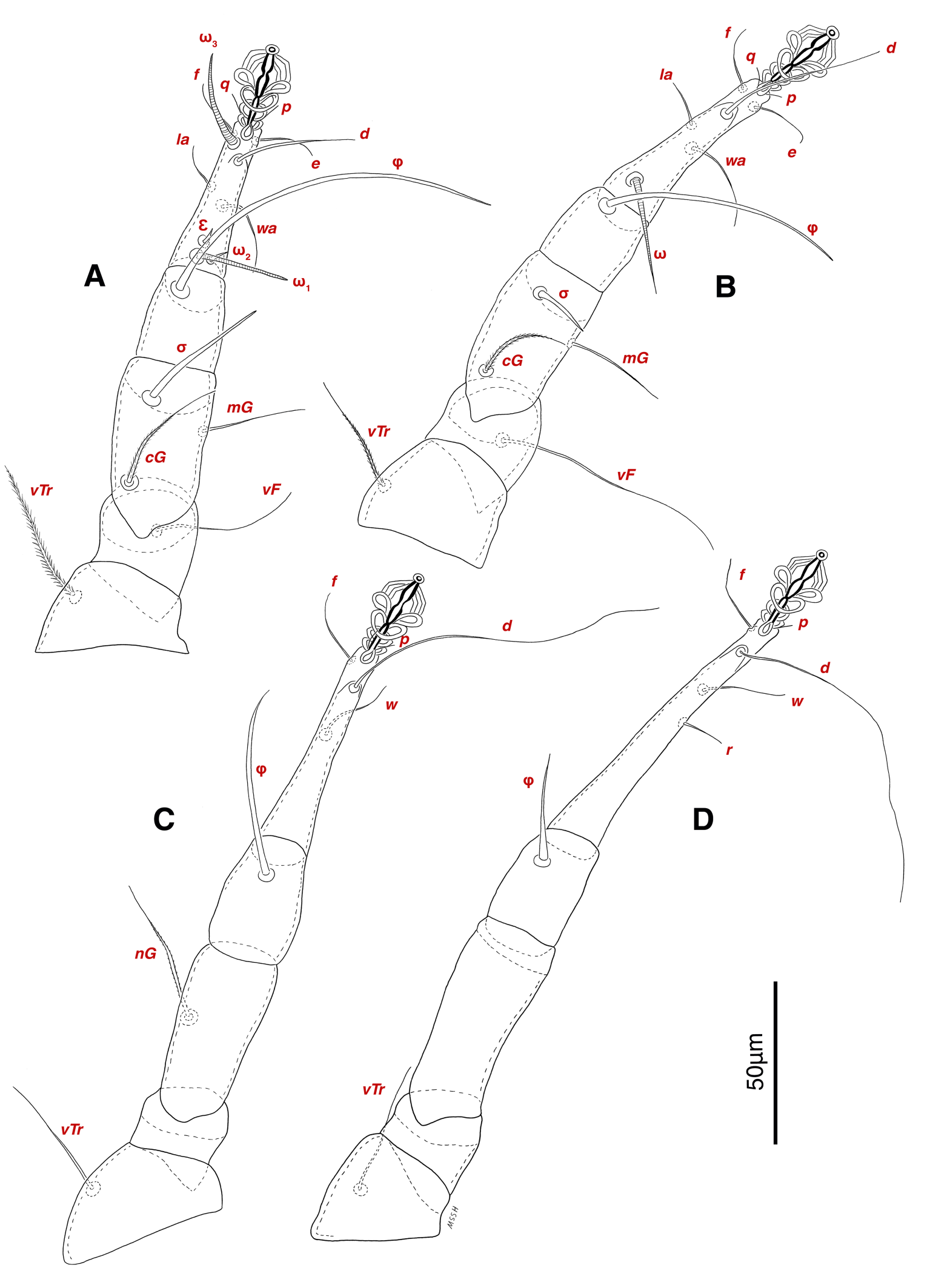
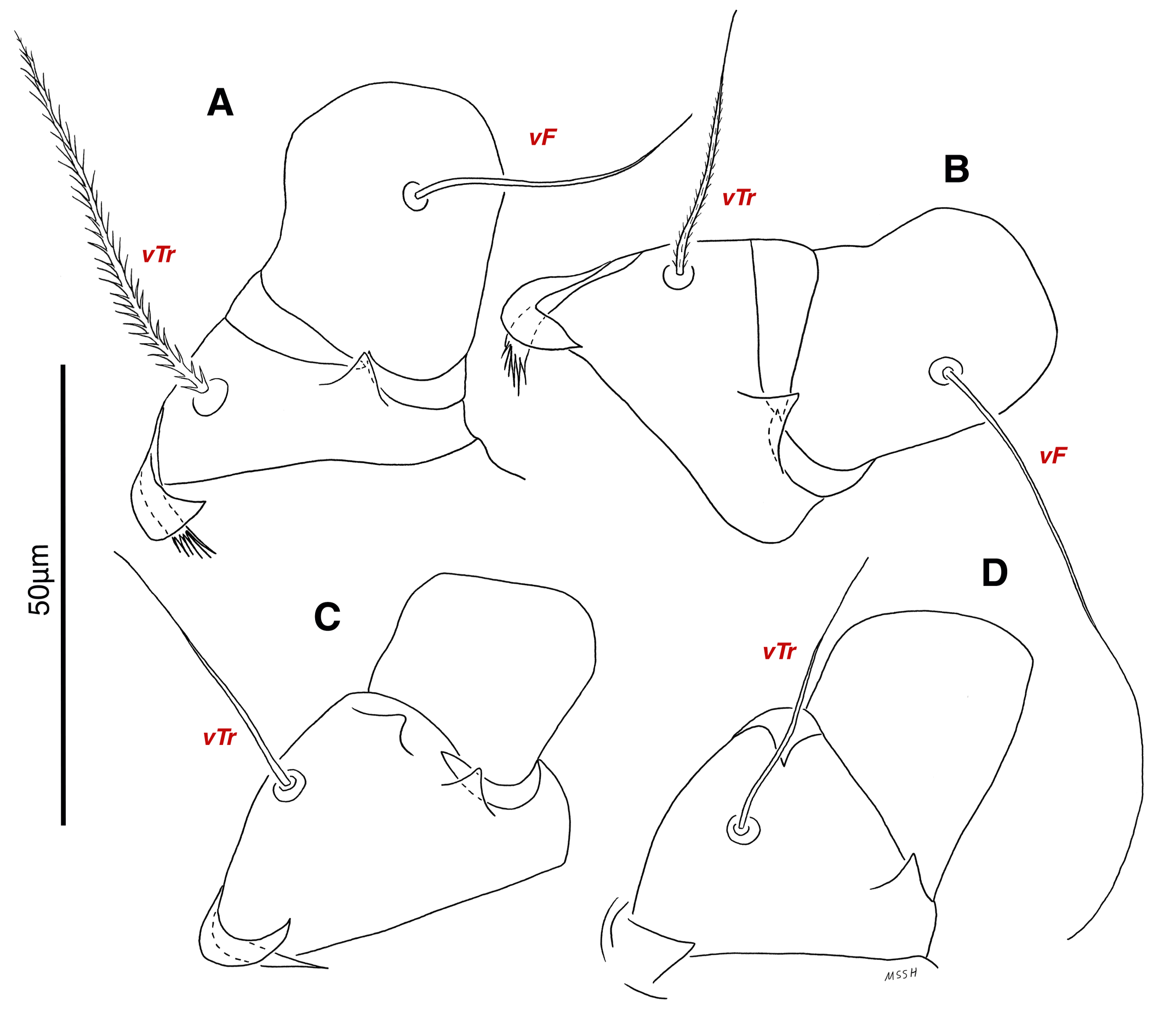
Legs — Ambulacral disc, empodial claw and condylophore well-developed in all pretarsi; last half tarsi I-III with fine transverse striations on dorsolatraly; tarsi IV with fine transverse striations on dorsolaterally in complete; setae vTr I clearly pectinate; setae cG I, cG II and vTr II barbed; setae nG tricarinate; postaxial femur I partly punctate dorsally; trochanter IV partly with fine striations dorsally in junction of femur; (Figs. 4F, I, J, 9A-D, 10A-D, 11G, H, I, M); chaetotaxy of legs (I-1V): trochanters 1-1-1-1, femora 1-1-0-0, genua 2(1σ)-2(1σ)-1-0, tibiae (1φ)-(1φ)-(1φ)(1φ), tarsi 7(3ω,1ε)-7(1ω)-4-5, (Table 2); measurements of leg segments and setae (length of tarsi excluding pretarsi): leg I 191 (180-198), Tr I 36 (30-36), vTr 47 (46-48), Fe I 41 (37-41), vF 57 (53-62), Ge I 57 (44-57), cG 51 (45-52), mG 39 (30-41), σ 39 (37-42), Ti I 30 (29-32), φ 102 (97-109), Ta I 58 (52-64), ω1 28 (21-28), ω2 7 (7-9), ε 6 (6), wa 23 (22-24), la 16 (16-20), d 41 (40-43), ω3 30 (26-30), e 21 (20-22), f 19 (15-19), p 10 (10), q 10 (10); leg II 201 (178-205), Tr II 31 (30-37), vTr 41 (38-41), Fe II 45 (38-45), vF 88 (80-90), Ge II 52 (50-56), cG 39 (39-49), mG 38 (30-44), σ 13 (13-17), Ti II 31 (30-32), φ 72 (67-73), Ta II 56 (54-57), ω 37 (32-41), wa 27 (26-27), la 18 (14-18), d 65 (62-65), e 17 (15-17), f 23 (20-23), p 7 (6-7), q 8 (7-8); leg III 222 (200-222), Tr III 54 (47-55), vTr 35 (30-38), Fe III 31 (29-31), Ge III 54 (50-55), nG 14 (12-16), Ti III 33 (31-34), φ 59 (50-59), Ta III 53 (51-58), w 22 (22-25), d 89 (80-90), f 21 (19-21), p 6 (6); leg IV 241 (215-241), Tr IV 55 (47-55), vTr 37 (31-43), Fe IV 45 (40-45), Ge IV 65 (56-65), Ti IV 42 (38-42), φ 69 (60-69), Ta IV 58 (50-58), r 11 (9-11), w 21 (17-22), d 89 (84-90), f 21 (17-21), s 4 (3-4).


Female (9 paratypes)
















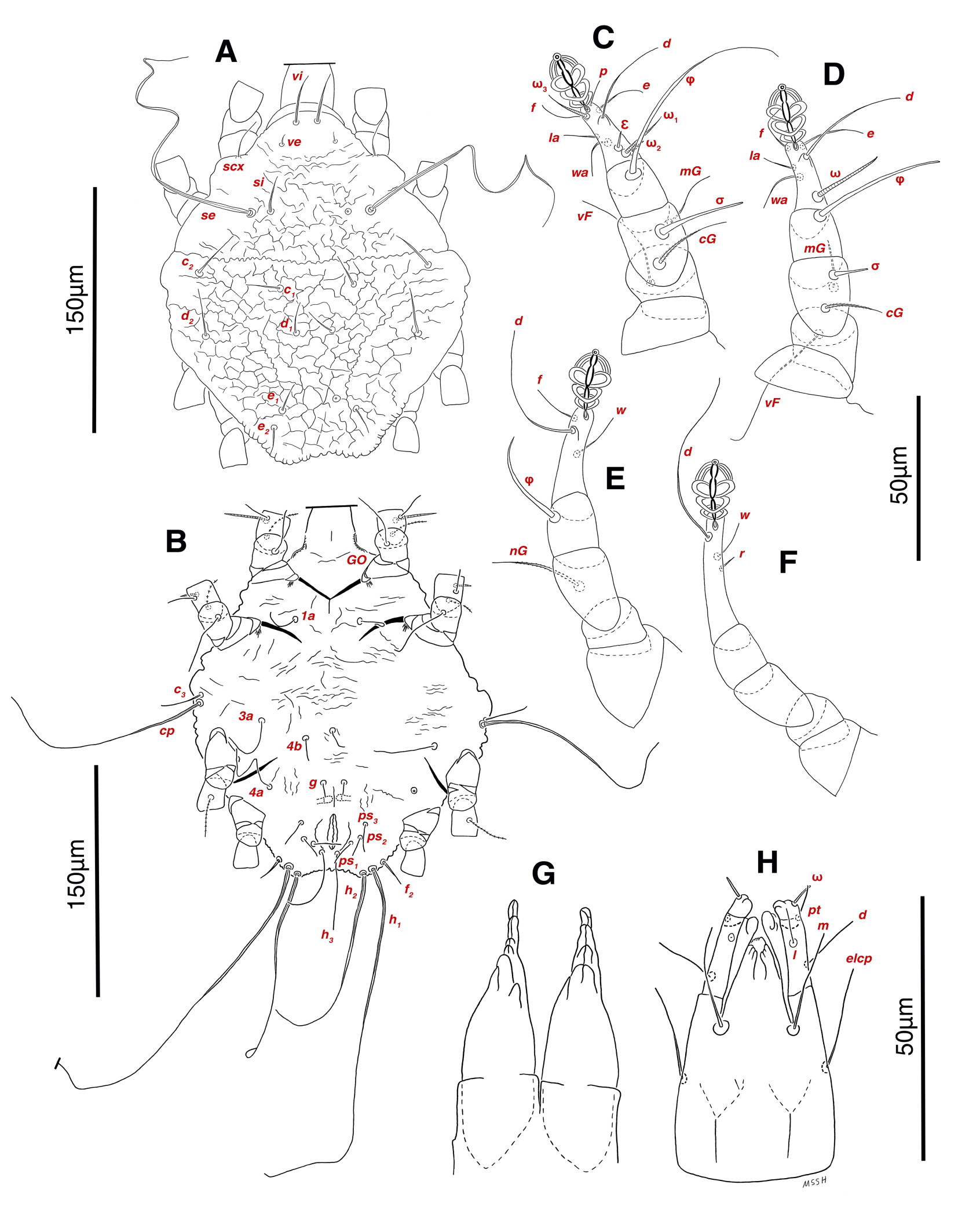




Measurements — IL 358-396, IW 313-375, GL 89-90, CL 76-87; leg I 189-192, leg II 180-206, leg III 216-240, leg IV 250-264 (Table 1).
Dorsum — Idiosoma moderately convex in middle part, maximum width between leg II and leg III, anterior margin finely wavy (Figs. 3B, 12A, 14A); idiosoma of smaller females with reticulate ornamentation, unlike larger ones (Figs. 12D, 13B); propodosomal plate punctate, with spinulate bulbiform setae vi, short smooth simple setae ve just behind the propodosomal plate (Figs. 12A, E, F, 14A, C, H,); scapular setae si and se present, smooth simple setae scx anterolateral near leg I, setae si spinulate bulbiform, setae se long and vascular-shaped, with cup-like bothridium (Figs. 12A, E, 14A, E, F, G); mild wavy sejugal furrow present, two diagonal mild wavy furrows symmetrically begin between setae c2 and d2 and continue near seta e1 (Figs. 12A, D, 14A); setae c2 and d2 leaf-shaped bulbiform (Figs. 14A, D); setae c1 , d1 and e1 smooth simple (Figs. 12A, D, 14A, B); terminal margin of opisthosoma with some vertical folding; opisthonothal gland, gla, oval form (Fig. 14A); length of dorsal setae: vi 50-59, ve 10-14, scx 42-44, si 38-45, se 332-337, c1 25-33, c2 50-61, d1 24-35, d2 60-67, e1 14-22.
Venter — Grandjean's organ well-developed at the base of leg I close to gnathosoma; Grandjean's organ similar to male (Fig. 15A); coxal- sternal setae 1a, 3a, 4a, 4b present, setae 1a near base of leg I and smooth simple, long setae 3a at base of leg III and leaf-shaped bulbiform, long seta 4a at base of leg IV and leaf-shaped bulbiform, smooth simple setae 4b next to genital region (Figs. 12B, C, 15A, C, 16A); setae c3 leaf-shaped bulbiform near to long vascular-shaped setae cp (Figs. 15A, B, D); fine regular fibers layer beneath the genital valves layer, leaf-shaped genital setae g, close to genital papillae (Figs. 11R, 12C, 15A, 16A); four pairs of smooth simple setae around anal region, shortest one setae ad1 , others setae ad2 , setae ps2 and setae ps3 ; long smooth simple setae ps1 little farther toward corner of body, ps1 > ps3 > ps2 (Figs. 11Q, 15A); setae e2 and f2 smooth simple; two pairs long thick setae h1 and h2 , leaf-shaped bulbiform setae h3 , setae e2 and setae f2 all located on two lobes at end corners of body (Figs. 15A, 16A); bursa copulatrix below anal region and between two lobes, copulatory opening ring-shaped, spermathecal duct thick and twisted, two U-shaped sclerites of oviduct end to spermatheca (Figs. 11S, 16A, B); terminal margin of body wrinkled (Figs. 15A, 16A). Length of ventral setae: 1a 41-46, 3a 85-95, 4a 110-133, 4b 28-37, c3 64-70, cp 248-270, g 22-25, ps1 46-53, ps2 20-24, ps3 22-28, e2 25-30, f2 22-31, h1 374-392, h2 321-335, h3 111-128.
Gnathosoma — General structure and setae similar to male (Figs. 15E, F); length of gnathosomal setae: ω 7, pt 12-14, l 9-10, d 19-23, elcp 32-39, m 29-34, cha 3-4, chb 7-8.
Legs — Ambulacral disc, empodial claw and condylophore well-developed in all pretarsi; setae vTr I clearly pectinate; setae cG I, cG II, vTr II barbed; setae nG weakly barbed; transverse lines on all leg solenidiae setae exclude φ I and φ II; broom-shaped structure includes a cover-like part and a delicate multi-branched appendage below it at each ventral bases of trochanters I, II; one conical structure at each ventral bases of femur I, II and IV, two conical structures at ventral base of femur III; tarsi IV elongated > 90 (Figs. 17A-D, 18A-D); chaetotaxy of legs (I-1V): trochanters 1-1-1-1, femora 1-1-0-0, genua 2(1σ)-2(1σ)-1-0, tibiae (1φ)-(1φ)-(1φ)-(1φ), tarsi 7(3ω,1ε)-7(1ω)-4-5, (Table 2); measurements of leg segments and setae (length of tarsi excluding pretarsi): leg I 189-192, Tr I 26-32, vTr 45-51 Fe I 37-44, vF 39-45, Ge I 44-55, cG 35-47, mG 32-40, σ 38-43, Ti I 27-30, φ 106-110, Ta I 57-62, ω1 28 -33, ω2 6-8, ε 5, wa 26-28, la 14-17, d 37-41, ω3 29-30, e 19-22, f 19-22, p 9-10, q 9-10; leg II 180-206, Tr II 28-35, vTr 37-44, Fe II 36-41, vF 64-83, Ge II 49-54, cG 30-33, mG 30-36, σ 17-18, Ti II 25-29, φ 68-74, Ta II 54-65, ω 36-42, wa 25-30, la 14-19, d 51-54, e 20-23, f 20-23, p 7, q 7; leg III 216-240, Tr III 37-42, vTr 39-45, Fe III 28-32, Ge III 49-58, nG 42-50, Ti III 38-42, φ 53-59, Ta III 72-74, w 23-25, d 90-99, f 23-26, p 7; leg IV 250-264, Tr IV 35-18, vTr 40-50, Fe IV 28-32, Ge IV 60-68, Ti IV 35-40, φ 33-34, Ta IV 92-95, r 11-13, w 23-27, d 102-104, f 20-24, p 7.
Tritonymph (4 paratypes)
Measurements — IL 270-306, IW 216-273, GL 60-83, CL 61-77; leg I 125-138, leg II 130-145, leg III 153-168, leg IV 158-180 (Table 1).
Dorsum — Idiosoma oval form, maximum width in middle part between leg II and leg III; propodosomal plate not punctate; anterior margin smooth, lateral and terminal margins wrinkled; anterior propodosoma with wrinkled lines, hysterosoma and posterior propodosoma with reticulate ornamentation; setae vi weakly spinulate bulbiform; short setae ve smooth simple; smooth simple setae scx anterolateral near leg I; setae si weakly spinulate bulbiform; long setae se vascular-shaped; mild wavy sejugal furrow and two diagonal mild wavy furrows well-developed; setae c2 and d2 leaf-shaped without transverse veins; all setae c1 , d1 , e1 and e2 smooth simple; opisthonothal gland, gla, unclear (Figs. 3C, 19A); length of dorsal setae: vi 41-42, ve 7-8, scx 33-36, si 34-37, se 271-285, c1 26-33, c2 40-46, d1 25-31, d2 46-47, e1 21-26, e2 19-26.
Venter — Grandjean's organ well-developed at the base of leg I close to gnathosoma; filiform coxal- sternal setae 1a, 3a, 4a, 4b present, setae 1a near base of leg I, long setae 3a at base of leg III, long seta 4a at base of leg IV, setae 4b above genital setae g; smooth simple setae c3 near to long vascular-shaped setae cp; filiform genital setae g, close to genital papillae; four pairs of filiform setae around anal region, longest one setae h3 , others setae ps1 , ps2 and ps3 , setae ps1 nearest and setae ps3 furthest to anal region; long vascular-shaped setae h1 , h2 and smooth simple setae f2 all located on two lobes at end corners of body (Fig. 19B); length of ventral setae: 1a 29-34, 3a 60-72, 4a 65-85, 4b 25-28, c3 36-41, cp 203-225, g 14-18, ps1 18-24, ps2 22-28, ps3 25-30, f2 20-22, h1 320-338, h2 200-250, h3 104-113.
Gnathosoma — General structure and setae similar to adult form, setae cha not observed (Figs. 19G, H); length of gnathosomal setae: ω 5-7, pt 9-10, l 8-10, d 15-19, elcp 23-28, m 25-30, chb 6-7.
Legs — Ambulacral disc, empodial claw and condylophore developed in all pretarsi; setae vTr pectinate; setae cG I, cG II, vTr II barbed; setae nG weakly barbed (Figs. 19B-F); chaetotaxy of legs (I-IV): trochanters 1-1-1-0, femora 1-1-0-0, genua 2(1σ)-2(1σ)-1-0, tibiae (1φ)-(1φ)-(1φ)-(1φ), tarsi 7(3ω,1ε)-5(1ω)-3-5, (Table 2); measurements of leg segments and setae (length of tarsi excluding pretarsi): leg I 125-138, Tr I 20-25, vTr 36-40, Fe I 31-34, vF 34-38, Ge I 30-37, cG 27-34, mG 22-27, σ 31-35, Ti I 21-25, φ 72-90, Ta I 41-45, ω1 16-27, ω2 5-8, ε 5, wa 14-20, la 9-16, d 37-40, ω3 22-30, e 12-17, f 9-17, p 9, q 9; leg II 130-145, Tr II 21-28, vTr 23-30, Fe II 29-33, vF 47-58, Ge II 30-35, cG 23-28, mG 17-22, σ 12-15, Ti II 18-22, φ 47-60, Ta II 42-44, ω 28-34, wa 20-25, la 11-13, d 41-51, e 17-20, f 16-18; leg III 153-168, Tr III 31-35, vTr 32-34, Fe III 23-24, Ge III 34-40, nG 33-36, Ti III 23-27, φ 34-45, Ta III 45-58, w 19-24, d 63-74, f 16-18; leg IV 158-180, Tr IV 23-32, Fe IV 24-26, Ge IV 35-37, Ti IV 26-30, φ 14-22, Ta IV 59-70, r 7-9, w 14-22, d 54-84, f 17-21, p 5.
Protonymph (3 paratypes)
Measurements — IL 216-237, IW 179-189, GL 50-67, CL 51-70; leg I 96-104, leg II 96-104, leg III 113-118, leg IV 114-118 (Table 1).
Dorsum — Idiosoma oval form, maximum width in middle part between leg II and leg III; propodosomal plate not punctate; anterior margin smooth, lateral and terminal margins wrinkled; anterior propodosoma with wrinkled lines, hysterosoma and posterior propodosoma with reticulate ornamentation; spinulation setae vi and si not yet developed; short setae ve smooth simple; smooth simple setae scx anterolateral near leg I; long setae se weakly vascular-shaped; mild wavy sejugal furrow and two diagonal mild wavy furrows well-developed; all setae c1 , c2 , d1 , d3 , e1 and e2 smooth simple; opisthonothal gland, gla, unclear (Figs. 3D, 20A); length of dorsal setae: length of dorsal setae: vi 26-30, ve 6-7, scx 24-28, si 22-26, se 192-220, c1 20-23, c2 26-28, d1 19-22, d2 28-32, e1 17-20, e2 16-19.
Venter — Wrinkled lines on ventral surface of idiosoma; Grandjean's organ developed; filiform coxal-sternal setae 1a, 3a, 4a, 4b present; filiform setae c3 near to semi-long weakly vascular-shaped setae cp; filiform genital setae g, close to one pair of genital papillae; four pairs of filiform setae around anal region, longest one setae h3 , others setae ps1 , ps2 and ps3 , setae ps1 nearest and setae ps3 furthest to anal region; long weakly vascular-shaped setae h1 , h2 and filiform setae f2 all located on two small lobes at end corners of body (Fig. 20B); length of ventral setae: 1a 22-24, 3a 39-40, 4a 55-56, 4b 13-14, c3 26-29, cp 143-180, g 8-9, ps1 14-15, ps2 15-17, ps3 18-19, f2 14-18, h1 230-243, h2 125-135, h3 49-50.
Gnathosoma — General structure and setae similar to adult form, setae cha and chb not observed (Figs. 20G, H); length of gnathosomal setae: ω 4-6, pt 6-9, l 5-7, d 11-13, elcp 19-21, m 15-19.
Legs — Ambulacral disc, empodial claw and condylophore developed in all pretarsi; setae cG I and cG II barbed; setae nG weakly barbed (Figs. 20B-F); chaetotaxy of legs (I-IV): trochanters 0-0-0-0, femora 1-1-0-0, genua 2(1σ)-2(1σ)-1-0, tibiae (1φ)-(1φ)-(1φ)-0, tarsi 6(3ω,1ε)-5(1ω)-3-3, (Table 2); measurements of leg segments and setae (length of tarsi excluding pretarsi): leg I 96-104, Tr I 17-20, Fe I 24-27, vF 28-34, Ge I 24-26, cG 23-24, mG 17-19, σ 24-26, Ti I 17-20, φ 53-65, Ta I 30-32, ω1 15-20, ω2 5-6, ε 5, wa 12-14, la 9-10, d 37-40, ω3 27-28, e 13-15, f 14-15, p 6-8; leg II 96-104, Tr II 14-18, Fe II 23-25, vF 33-34, Ge II 24-27, cG 16-17, mG 13-14, σ 10-13, Ti II 16-17, φ 39-42, Ta II 28-32, ω 17-23, wa 12-16, la 9-10, d 34-40, e 17-19, f 13-16; leg III 113-118, Tr III 25-28, Fe III 17-20, Ge III 26-27, nG 27-28, Ti III 20-22, φ 26-30, Ta III 34-38, w 11-15, d 42-50, f 12-14; leg IV 114-118, Tr IV 20-23, Fe IV 17-18, Ge IV 23-27, Ti IV 18-22, Ta IV 41-43, r 4-5, w 11-12, d 40-55.
Larva (4 paratypes)
Measurements — IL 171-219, IW 133-193, GL 39-41, CL 40-43; leg I 69-82, leg II 63-79, leg III 78-93 (Table 1).
Dorsum — Idiosoma oval form, maximum width in middle part between leg II and leg III; propodosomal plate not punctate; anterior propodosoma with some wrinkled lines, hysterosoma and posterior propodosoma with uncomplete reticulate ornamentation; smooth simple setae vi present; setae ve absent; filiform setae scx anterolateral near leg I; long smooth setae se close to filiform setae si; mild wavy sejugal furrow weakly developed; two diagonal furrows not yet developed; all setae c1 , c2 , d1 , d2 , e1 and e2 filiform; opisthonothal gland, gla, unclear (Figs. 3E, 21A); vi 25-28, scx 15-23, si 12-13, se 129-140, c1 10-12, c2 10-14, d1 9-13, d2 13-14, e1 9-11, e2 9-10.
Venter — Grandjean's organ developed; filiform coxal-sternal setae 1a, 3a present, setae 4a, 4b absent; Claparede organ well-developed; filiform setae c3 near to semi-long smooth setae cp; filiform setae h2 below anal region; long weakly vascular-shaped setae h1 located on very small two lobes at end corners of body; setae h3 absent; genital and pseudoanal setae not yet developed (Fig. 21B); length of ventral setae: 1a 14-17, 3a 24-36, c3 18-21, cp 90-110, h1 160-180, h2 54-65, Claparede organ 10-12.
Gnathosoma — General structure and setae similar to adult form, setae cha and chb not observed (Figs. 21F, G); length of gnathosomal setae: ω 4-5, pt 6-7, l 4-5, d 7-10, elcp 14-18, m 13-16.
Legs — Ambulacral disc, empodial claw and condylophore developed in all pretarsi; no setae yet barbed (Figs. 21B-E); chaetotaxy of legs (I-IV): chaetotaxy of legs (I-III): trochanters 0-0-0, femora 1-1-0, genua 2(1σ)-2(1σ)-1, tibiae (1φ)-(1φ)-(1φ), tarsi 7(3ω,1ε)-4(1ω)-3, (Table 2); measurements of leg segments and setae (length of tarsi excluding pretarsi): leg I 69-82, Tr I 13-16, Fe I 12-19, vF 20-21, Ge I 17-20, cG 10-18, mG 11-16, σ 11-13, Ti I 13-16, φ 42-47, Ta I 22-28, ω1 10-17, ω2 3-5, ε 2, wa 9-12, la 4-6, d 19-23, e 11-12, f 8-11, p 9-10, q 9-10; leg II 63-79, Tr II 13-15, Fe II 17-19, vF 26-33, Ge II 17-21, cG 10-15, mG 11-13, σ 4-5, Ti II 11-13, φ 35-36, Ta II 23-25, ω 11-17, wa 8-11, d 29-33, e 7-10; leg III 78-93, Tr III 16-20, Fe III 14-16, Ge III 19-22, nG 17-20, Ti III 14-16, φ 22-28, Ta III 32-33, w 8-11, d 25-40, f 12-13;
Remarks
The distinguishable characters of Ciprusenia troglobionta sp. nov. are mentioned in the diagnosis section. Additional comparisons are as follows: C. troglobionta sp. nov. differs from C. kamelskyi (Samšiňák, 1970), males, in shorter IL (250-341 vs. 380), vi (51-62 vs. 75), ve (11-18 vs. 85), c2 (56-75 vs. 85), e2 (15-17 vs. 45), ps2 (38-41vs. 60), longer scx (44-50 vs. 25), c1 (24-32 vs. 10), d1 (25-33 vs. 10), e1 (30-35 vs. 17), f2 (70-80 vs. 17), ps1 (40-45 vs. 35), h3 (110-128 vs. 90), females, shorter vi (50-59 vs. 80), ve (10-14 vs. 90), c2 (50-61 vs. 85), d2 (60-67 vs. 85), e2 (25-30 vs. 50); C. troglobionta sp. nov. differs from C. sellnicki (Samšiňák, 1965), males, in shorter IL (250-341 vs. 570), IW (303-362 vs. 380), scx (44-50 vs. 60), longer si (41-51 vs. 20), se (320-362 vs. 300), c1 (24-32 vs. 20), c2 (56-75 vs. 20), d1 (25-33 vs 20), d2 (82-89 vs. 20), f2 (70-80 vs. 20), h1 (414-427 vs. 400), h2 (282-320 vs. 250), h3 (110-128 vs. 100), females, shorter IL (358-396 vs. 880), IW (313-375 vs. 600), vi (50-59 vs. 80), ve (10-14 vs. 30), scx (42-44 vs. 90), c1 (25-33 vs. 45), d1 (24-35 vs. 45), e1 (14-22 vs. 45), h1 (374-392 vs. 550), h2 (321-335 vs. 350), h3 (111-128 vs. 190), f2 (22-31 vs. 45), longer c2 (50-61 vs. 45), d2 (60-67 vs. 45); C. troglobionta sp. nov. differs from C. izabelae (Haitlinger, 1992), males, in shorter IL (250-341 vs. 480), IW (303-362 vs. 424), e2 (15-17 vs. 46), longer d2 (82-89 vs. 74), e1 (30-35 vs. 20), f2 (70-80 vs. 34), c3 (63-72 vs. 54), 4a (90-100 vs. 64), females, shorter IL (358-396 vs. 544), IW (313-375 vs. 416), e2 (25-30 vs. 44), longer, c3 (64-70 vs. 48); C. troglobionta sp. nov. differs from C. viviannae (Haitlinger, 1992), females, in shorter IL (358-396 vs. 424), f2 (22-31 vs. 48), longer c3 (64-70 vs. 44), description of the adult male of C. viviannae (Haitlinger, 1992) is not available; C. troglobionta sp. nov. differs from C. carabicola (Berlese, 1882), males, in shorter IL (250-341 vs. 418-440), longer IW (303-362 vs. 281-291), females, shorter IL (358-396 vs. 438-534).
Discussion
One of the most abundant and diverse invertebrates found in caves associated with bat guano are mites, which together with other microarthropods play an effective role in the food chain of caves, so known as subterranean plankton (Palacios-Vargas et al. 2011; Thurgate et al. 2011). The most reported mite species richness related to bat guano belongs to the Trombidiformes, Sarcoptiformes and Mesostigmata orders of subclass Acari (Palacios-Vargas et al. 1998; Pellegrini and Ferreira 2013).
Except for a few families, not much data is available about the morphological cave adaptation of many groups of mites, but according to literature, obligatory cavernicoles (troglobionts) expose some troglomorphic characteristics such as loss of eyes, lack of pigmentation, reduction in body size, increase in the number and length of sensory organs, reduction in metabolic rate and increase in lifespan (Culver and Pipan 2013; Romero 2009). These characteristics may vary greatly in some families. The body size of some subterranean mite species is larger than that of soil species (Ducarme 2004).
Lack of eyes is common in all members of the family Canestriniidae. Although Vitzthum, 1924 had reported nut brown color for Canestrinia sardica (syn. of Ciprusenia carabicola), the lack of pigmentation has been observed in most of the family members including Ciprusenia troglobionta sp. nov. Moreover, all life stages of the new species have transparency in most body parts. With comparison in the male holotype of the genus members, C. troglobionta sp. nov. has the most reduction in idiosomal length along with an increase in the most of dorsal and ventral setae as the sensory organs. To logically comparison of measurements, we defined setae indices that indicate the percentage ratio of dorsal or ventral setae length to idiosomal length: (seta length/IL) * 100, as shown in Table 3. Due to a lack of access to C. viviannae adult males (the holotype is a female), indices have not been calculated for this species. Some measurements required for indices have been calculated based on the available drawings. According to the results of Table 3, among the total number of 24 setae compared, setae si, scx, c1 , c2 , c3 , d1 , d2 , e1 , f2 , h1 , h2 , h3 , 1a, 3a, 4a, ps3 equal to 66.67% of all have the most index value in the new species that means the most setae elongation compared to other genus species. This value for other species is as follows: C. carabicola 0%, C. izabelae 0%, C. kamelskyi 29.17%, C. sellnicki 4.17%.


Key to the world-known species of the genus Ciprusenia Haitlinger, 2022
Male
1. Idiosomal length < 400; setae d2 > 80
...... 2
— Idiosomal length > 400; setae d2 < 80
...... 3
2. Hysterosoma without two symmetric diagonal furrows
...... C. kamelskyi (Samšiňák, 1970)
— Hysterosoma with two symmetric diagonal furrows
...... C. troglobionta sp. nov.
3. Setae c2 longer than c1 (at least twice)
...... C. izabelae (Haitlinger, 1992)
— Setae c2 not longer than c1
...... 4
4. Idiosomal length > 500
...... C. sellnicki (Samšiňák, 1965)
— Idiosomal length < 500
...... C. carabicola (Berlese, 1882)
Female
1. Idiosomal width > 400
...... 2
— Idiosomal width < 400
...... 3
2. Idiosomal length > 700; Setae vi > 60
...... C. sellnicki (Samšiňák, 1965)
— Idiosomal length < 700; Setae vi < 60
...... C. izabelae (Haitlinger, 1992)
3. Hysterosoma with two symmetric diagonal furrows
...... C. troglobionta sp. nov.
— Hysterosoma without two symmetric diagonal furrows
...... 4
4. Setae si < 25; setae d2 < 35
...... C. carabicola (Berlese, 1882)
— Setae si > 25; setae d2 > 35
...... 5
5. Setae vi < 70; opisthosomal margin with obvious lobes
...... C. viviannae (Haitlinger, 1992)
— Setae vi > 70; opisthosomal margin without obvious lobe
...... C. kamelskyi (Samšiňák, 1970)
Note: Lombardini (1950) described two species: Coleoglyphus rosascostai and Grandiella rosascostai. Haitlinger (1990c) transferred C. rosascostai to the genus Hatohylla Haitlinger, 1990. In Haitlinger's work (2022) G. rosascostai was confused with H. rosascostai and excluded from the genus Grandiella Lombardini, 1939. It is an error, G. rosascostai is a good species (Haitlinger 1990b, 2022; Lombardini 1950).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Special thanks to Mohammad Javad Ghasempoor and Dr. Yaser Bakhshi (Shiraz University, Iran) for accompanying the first author in caving and sampling. We are very grateful to Dr. Maxim Nabozhenko (Daghestan Federal Research Centre, Russia) for his kind help in identifying the host beetle. We are grateful to the anonymous reviewers for their valuable comments. The current study has been partly supported by Iran National Science Foundation (INSF) under grant number 95849576.
References
- Ardeshir F., Hasanvand I., Haitlinger R., Jafari S. 2016. Camirohylla (Acari: Astigmata: Canestriniidae), a new mite genus to fauna of Iran. Persian Journal of Acarology, 5: 159-160. https://doi.org/10.22073/pja.v5i2.20412
- Berlese A. 1911. Alcuni Acari entomofili nuovi. Redia, 7: 183-186.
- Beron P. 2021. Acarorum catalogus IX. Acariformes, Acaridida, Schizoglyphoidea (Schizoglyphidae), Histiostomatoidea (Histiostomatidae, Guanolichidae), Canestrinioidea (Canestriniidae, Chetochelacaridae, Lophonotacaridae, Heterocoptidae), Hemisarcoptoidea (Chaetodactylidae, Hyadesiidae, Algophagidae, Hemisarcoptidae, Carpoglyphidae, Winterschmidtiidae). Bulgaria: Pensoft & National Museum of Natural History, Sofia. https://doi.org/10.3897/ab.e68613
- Culver D., Pipan T. 2013. Subterranean ecosystems. In: Levin S.A., (Ed). Encyclopedia of biodiversity. Academic Press. p. 49-62. https://doi.org/10.1016/B978-0-12-384719-5.00224-0
- Ducarme X., Wauthy G., André H., Lebrun P. 2004. Survey of mites in caves and deep soil and evolution of mites in these habitats. Canadian Journal of Zoology, 82: 841-850. https://doi.org/10.1139/z04-053
- Grandjean F. 1939. La chaetotaxie des pattes chez les Acaridiae. Bulletin de la Société Zoologique de France, 64: 50-60.
- Griffiths D., Atyeo W., Norton R., Lynch C. 1990. The idiosomal chaetotaxy of astigmatid mites. Journal of Zoology, 220: 1-32. https://doi.org/10.1111/j.1469-7998.1990.tb04291.x
- Haitlinger R. 1990a. The genus Coleopterophagus Berlese, 1882 (Acari, Astigmata, Canestriniidae) with descriptions of seven new species and key for species determination. Annales Zoologici, 43: 327-341.
- Haitlinger R. 1990b. Hatohylla n. Gen. (Acari, Astigmata, Canestriniidae) with the descriptions of three new species. Revista Iberica de Parasitologia, 50: 307-312.
- Haitlinger R. 1992. The genus Percanestrinia Berlese, 1911 (Acari, Astigmata, Canestriniidae) with descriptions of three new species. Zoiologische Jahrbucher, Abteilung fur Systematik, Okologie und Geographie, 119: 535-542.
- Haitlinger R. 2022. A review of host-commensal associations between canestriniid mites (Astigmata: Canestriniidae) and Insecta with keys and descriptions of the new genera. The European Zoological Journal, 89: 22-86. https://doi.org/10.1080/24750263.2021.2000049
- Haitlinger R., Šundić M. 2016. Ibizella balearica n. Gen., n. Sp. (Astigmata Canestriniidae) from Balearic Islands, Spain. Redia, XCIX: 75-82. https://doi.org/10.19263/Redia-99.16.09
- Lee N., Meisinger D., Aubrecht R., Kovacik L., Saiz-Jimenez C., Baskar S., Baskar R., Liebl W., Porter M., Engel A. 2012. Caves and karst environments. In: Bell E.M., (Ed). Life at extremes: Environments, organisms and strategies for survival. CAB International. p. 320-344. https://doi.org/10.1079/9781845938147.0320
- Lombardini G. 1950. Canestriniidae dell′America del sud (Acarina). Arthropoda, 1: 279-290.
- Mouthereau F., Lacombe O., Tensi J., Bellahsen N., Kargar S., Amrouch K. Year of Conferenc. Mechanical constraints on the development of the Zagros folded belt (Fars). In: Lacombe O., Roure F., Lavé J., Vergés J., (Eds). Thrust Belts and Foreland Basins; Berlin, Heidelberg: Springer. p. 247-266. https://doi.org/10.1007/978-3-540-69426-7_13
- Norton R. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Experimental and Applied Acarology, 22: 559-594. https://doi.org/10.1023/A%3A1006135509248
- Okabe K., Masuya H., Kanzaki N. 2017. Unintentional introductions of microscopic organisms associated with forest insects. Biological Invasions, 19: 3229-3242. doi: 10.1007/s10530-017-1507-0 https://doi.org/10.1007/s10530-017-1507-0
- Palacios-Vargas J., Castaño-Meneses G., Estrada-Bárcenas D. 2011. Diversity and dynamics of microarthropods from different biotopes of Las Sardinas cave (Mexico). Subterranean Biology, 9, 113. https://doi.org/10.3897/subtbiol.9.2514
- Palacios-Vargas J., Decu V., Javorski V., Hutzu M., Juberthie C. 1998. Cave terrestrial Acari. In: Juberthie C., Decu V., (Eds). Encyclopedie biospeologique. Saint-Girons: CNRS-Fabbro. p. 929-252.
- Pellegrini T., Ferreira R. 2013. Structure and interactions in a cave guano-soil continuum community. European Journal of Soil Biology, 57: 19-26. https://doi.org/10.1016/j.ejsobi.2013.03.003
- Ramsey L., Walker R., Jackson J. 2008. Fold evolution and drainage development in the Zagros mountains of Fars province, SE Iran. Basin Research, 20: 23-48. https://doi.org/10.1111/j.1365-2117.2007.00342.x
- Romero A. 2009. Cave biology: Life in darkness. Cambridge University Press. pp. 291. https://doi.org/10.1017/CBO9780511596841
- Samšiňák K. 1965. Die auf Procrustes (Col. Carab.) lebenden milben (Acari) und ihre bedeutung für die zoogeographie. Mitteilungen aus dem Museum für Naturkunde in Berlin. Zoologisches Museum und Institut für Spezielle Zoologie (Berlin), 41: 137-155. https://doi.org/10.1002/mmnz.19650410202
- Samšiňák K. 1970. Die auf Blaps (Col. Tenebrionidae) lebenden milben der gattung Canestrinia Berlese, 1881 (Acari). Entomologische Mitteilungen aus dem Zoologischen Museum Hamburg, 4: 71-78.
- Samšiňák K. 1971. Die auf Carabus-Arten (Coleoptera, Adephaga) der Palaearktischen region lebenden milben der unterordnung Acariforrnes (Acari); ihre taxonomic und bedeutung fur die losung zoogeographischer, entwicklungsgeschichtlicher und parasitophyletischer fragen. Entomologische Abhandlungen Dresden, 38: 145-234.
- Thurgate M., Gough J., Spate A., Eberhard S. 2001. Subterranean biodiversity in New South Wales: From rags to riches. Records of the Western Australian Museum, Supplement, 64: 37-47. https://doi.org/10.18195/issn.0313-122x.64.2001.037-047
- Trach V., Khaustov A. 2011. A review of the genus Coleopterophagus Berlese, 1882 (Acari: Astigmata: Canestriniidae) of Ukraine. Acarina, 19: 213-230.
- Vitzthum H. 1924. Acarologische beobachtungen. 8. Reihe Archiv für Naturgeschichte, 90: 41-51.
- Walter D.E., Krantz G.W. 2009. Collection, rearing and preparing specimens. In: Krantz G.W., Walter D.E., (Eds). A manual of Acarology. Texas Tech University Press. p. 83‐96.



2023-02-23
Date accepted:
2023-11-13
Date published:
2024-01-11
Edited by:
Akashi Hernandes, Fabio

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Sadat-Shojaei, Mojgan; Haitlinger, Ryszard; Sadeghi, Saber and Akrami, Mohammad Ali
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



