Diptacus rubuscolum Trinidad, Duarte & Navia (Eriophyoidea: Diptilomiopidae) on blackberry (Rubus ulmifolius Schott) in Mexico: occurrence, complementary description, and biology
González-Domínguez, Sandra G.  1
; Santillán-Galicia, Ma. Teresa
1
; Santillán-Galicia, Ma. Teresa  2
; Guzmán-Franco, Ariel W.
2
; Guzmán-Franco, Ariel W.  3
; de Jesús García-Avila, Clemente
3
; de Jesús García-Avila, Clemente  4
; López-Buenfil, José Abel
4
; López-Buenfil, José Abel  5
and Romero-Rosales, Felipe6
5
and Romero-Rosales, Felipe6
1Postgrado en Fitosanidad-Entomología y Acarología. Colegio de Postgraduados, km 36.5 Carretera Mexico-Texcoco, Montecillo, Texcoco, Edo. de Mexico, 56264, Mexico.
2✉ Postgrado en Fitosanidad-Entomología y Acarología. Colegio de Postgraduados, km 36.5 Carretera Mexico-Texcoco, Montecillo, Texcoco, Edo. de Mexico, 56264, Mexico.
3Postgrado en Fitosanidad-Entomología y Acarología. Colegio de Postgraduados, km 36.5 Carretera Mexico-Texcoco, Montecillo, Texcoco, Edo. de Mexico, 56264, Mexico.
4Centro Nacional de Referencia Fitosanitaria, km 37.5, Carretera Federal México-Pachuca, Tecámac, Edo. de México, C.P. 55740, Mexico.
5Centro Nacional de Referencia Fitosanitaria, km 37.5, Carretera Federal México-Pachuca, Tecámac, Edo. de México, C.P. 55740, Mexico.
6 * Postgrado en Fitosanidad-Entomología y Acarología. Colegio de Postgraduados, km 36.5 Carretera Mexico-Texcoco, Montecillo, Texcoco, Edo. de Mexico, 56264, Mexico.
2023 - Volume: 63 Issue: 3 pages: 793-806
https://doi.org/10.24349/j7im-caraOriginal research
Keywords
Abstract
* The author died before the publication of this paper. This represent his last scientific contribution.
Introduction
Blackberries (Rubus ulmifolius Scott) have become increasingly popular with consumers due to their high levels of antioxidants (Schulz et al. 2019). Currently, Mexico is the main producer of blackberries worldwide (Stupková 2016) and it is among the most important fruit crops in Mexico; the state of Michoacan produces 60% of the Mexican blackberry crop (Strik et al. 2007; Servicio de Información Agroalimentaria y Pesquera [SIAP] 2020).
Mites from the family Tetranychidae and the superfamily Eriophyoidea (Eriophyidae, Phytoptidae and Diptilomiopidae) are among the most important arthropod pests affecting blackberry production (de Lillo and Duso 1996; Marchetti and Ferla 2011), and some Eriophyidae species have greater negative impact on cultivated plants because they transmit plant viruses (de Lillo et al. 2016; de Lillo et al. 2018). Despite the economic importance of this crop, there are few studies reporting species diversity and biology of associated eriophyoids in Mexico. The following eriophyoid mite species have been reported associated with Rubus spp., Acalitus essigi Hassan, A. orthomera Keifer, Chakrabartiella sp. and Diptacus rubuscolum Trinidad, Duarte & Navia in Brazil (Marchetti and Ferla 2011; Trinidad et al. 2018); Diptacus glaber (Huang and Wang 2009), Diptacus gigantorubra (Xin and Dong, 1983), Diptacus chihouensis (Wang et al. 2009) and Diptacus rubi (Kuang 2001) in China and Taiwan; and Rhynacus abronius (Keifer) (Cham. & Schltdl.), Apodiptacus rubi (Kuang) and Diptacus caeseius in Germany and Austria (Domes 2000).
To provide more information on species diversity of eriophyoid mites in Mexican blackberry crops, a field survey was carried out between April and November 2018, in six orchards from two municipalities in the state of Michoacán. We conducted both morphological and molecular identification, only D. rubuscolum was found and a complementary description of this species is provided. In adition, biological information on the duration of the different developmental stages of this mite species is presented.
Material and methods
Collection and identification of species
Monthly samples were taken between May and November 2018 from six blackberry (R. ulmifolius var. Tupy) orchards in the municipalities of Los Reyes de Salgado and Peribán, Michoacán. In each orchard 40 sampling points were randomly selected but covered the entire orchard. At each sampling point buds, flowers, leaves and fruits infested with mites were collected in plastic bags and transported to the Acarology Laboratory, Colegio de Postgraduados, Mexico state in a cool box. Mites were washed off the plant samples and collected after filtration through sieves with mesh numbers 200, 325 and 500, representing 74, 44 and 25 µm openings respectively.
Identification of mites was done using phase contrast microscopy (PCM), scanning electronic microscopy (SEM) and using sequence information from the D2 region of the 28S rRNA. For PCM evaluation, specimens were mounted in modified Keifer mounting liquid on glass slides (Amrine and Manson 1996). Slides were maintained at 40° C for 15 days before observations were made. All specimens were evaluated using an Olympus microscope (model BX41, Olympus Corporation of the Americas, PA, USA) with the 40X and 100X objectives. Photographs were taken using a digital camera (Olympus) attached to the microscope. For genus identification, we used the information reported by Amrine et al. (2003) and the genitalia anatomy reported by Lindquist (1996). We measured all the structures suggested for identification of eriophyid mites (De Lillo et al. 2010). All measurements (µM) were made using the software Image J v 1.5 (Abràmoff et al. 2004).
For SEM analysis, two females and one male, previously stored in 70% ethanol, were used. The specimens were freeze dried, to ensure all ethanol was removed, and then coated with colloidal gold by ionization (1 mA) for 100 seconds in a sputter coater (Fine-Coat Ion Sputter JFC-1100, JEOL Ltd., Tokyo, Japan). They were then observed and photographed in the vacuum chamber of a scanning electron microscope (JOEL JSM-6390, JEOL Ltd., Tokyo, Japan).
Molecular studies
DNA extraction, amplification, and sequencing
For molecular identification and characterization, partial sequences of the COI gene, 18S and two regions of the 28S of the rRNA were obtained from three specimens and deposited in GenBank. DNA was extracted from individual mites using the ZR Tissue and Insect DNA MicroPrep™ kit (ZYMO Research Corporation, Irving, CA, USA), following the manufacturer's instructions. The concentration of DNA in samples was estimated using a NanoDrop™ spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and the DNA then stored at -20 °C until required. COI sequences were obtained using the primers LCO1490 and HCO2198 (Folmer et al. 1994). The 18S region of the rRNA was amplified with the primers fw390 and rev960 (Dabert et al. 2010). Within the 28S rRNA gene, two regions were amplified, the D2-5 region using two pair of primers, ND2f /D2R (Campbell et al. 1994) and 28S3F/28S3R (Whiting et al. 1997), and the D9-10 region using the primers 28S9F and 28S9R (Hillis and Dixon 1991). All PCRs were conducted in a 30µL reaction volume containing 3 µL of 10X PCR buffer (Tris-Cl, KCl, (NH4)2SO4, 15mM MgCl2; pH 8.7), 0.3 µM of each primer, 0.2 mM of dNTPs, 0.5U of Taq DNA polymerase (QIAGEN, GmbH, Hilden, Germany), 1.2 µL of MgCl2 (25 mM), three µL of DNA (approx. 10 ng/µL) and 20.3 µL of 6% sterile trehalose. Amplifications were done in a MyCyclerTM thermal cycler (BIORAD Laboratories Inc., Hercules, CA, USA) using the following conditions: for the COI primers, one cycle of 60 s at 94 °C; five cycles of 60 s at 94 °C, 90 s at 45 °C and 90 s at 72 °C, followed by 35 cycles of 60 s at 94 °C, 90 s at 60 °C, 60 s at 72 °C and a final extension at 72 °C for 5 min. For the 18S and the D9-10 region (28S rRNA) primers, one cycle of 5 min at 96 °C, followed by 30 cycles of 30 s at 95 °C, 60 s at 50 °C, 60 s at 72 °C and a final extension at 72 °C for 5 min. For the other two pairs of primers, all conditions were the same except the annealing temperatures which were 52 and 55 °C for the ND2f/D2R and 28S3F/28S3R combinations, respectively. PCR products were visualized on 1.5% agarose gels in 1X TAE, stained with ethidium bromide (10 mg mL-1), and photographed to confirm amplifications. All amplicons obtained were sent to Macrogen Inc. (Geumchen-gu, Seoul, Korea) for direct Sanger sequencing.
Sequences edition, genetic distances and phylogenetic analyses
Resulting sequences were edited using BioEdit v.7.1.9 (Hall, 1999). Multiple alignments were made using Clustal W (Thompson et al. 1994) implemented in BioEdit. For analyses, we retrieved sequences from the families Diptilomiopidae that had been reported by Druciarek et al. (2019) and Li et al. (2014), including sequences of D. rubuscolum. Genetic distances and phylogenetic analyses were carried out using only sequences for the D2 region of the 28S rRNA, as they were the only ones available in GenBank. Analyses of the pairwise genetic distances between and within nucleotide sequences amongst samples studied, as well as the choice of the most appropriate evolutionary models for estimation of inter- and intra-lineage genetic variation were performed in Molecular Evolutionary Genetic Analysis (MEGA) v.7 (Tamura et al. 2016) using the Kimura 2-Parameters (K2P) model (Kimura 1980). Standard error estimates were obtained by a bootstrap procedure with 1000 replicates. Phylogenetic analysis was done using maximum likelihood in MEGA ver. 7, with the Close-Neighbour-Interchange algorithm. The robustness of branches was estimated by bootstrap analysis with 1000 repeated samplings of the data (Felsenstein, 1985).
Biological studies
For this study , adult D. rubuscolum mites were collected from blackberry plants at one sampling site selected randomly, and the development time of their progeny determined on leaf discs in Petri dish arenas. Leaf discs were cut from leaves harvested from four-month-old blackberry plants grown under glasshouse conditions in 20 L pots of compost (1:1:1 mix of peat moss: coconut fibre: vermiculite). Leaf discs (0.5 cm diameter) were cut from leaves avoiding leaf ribs, washed and rinsed with distilled water, and dried with a clean paper towel. Single leaf discs were placed in the base of 5 cm diameter Petri dishes containing damp sterile cotton wool. For ventilation the lid of the Petri dish had a 3 cm diameter hole covered with printing mesh. An individual adult female mite was placed, using a fine brush (size 15/0), into each arena (n=24) and incubated at 25 °C, 60% RH in a 12:12 light:dark regime for 10 hours during which time she laid eggs. The females and any additional eggs were then removed leaving only one egg in each arena. The cotton wool in each arena was moistened every 24 h with 3 mL of sterile distilled water and leaf discs replaced every four days. Observations were made every 12 h using a stereomicroscope (Nikon SMZ 1500, Nikon Instruments, Inc. Melville, NY) to determine when the larva hatched out of the egg and how many and how long each life stage was until the adult stage was reached. The duration of each developmental stage was estimated using the equations described by Perring et al. (1984). The duration of the egg stage was estimated using the following equation:
\[TE = \frac{Te_{1} + Te_{2} }{2} - T0\]
Where: Te1 = time before egg hatch, Te2 = time after egg hatch, T0 = time when oviposition took place, and TE = time to complete egg stage. The larval stage was considered to last until an individual became inactive (nymphochrysalis). The total larval developmental time was estimated using the equation:
\[TL = \frac{Tl_1 + Tl_2}{2} - TE\]
Where: Tl1 = time before the larva became quiescence (nymphochrysalis), Tl2 = time spent as a nymphochrysalis, TE = time spent as an egg, and TL = time spent as a larva. All other developmental stages were estimated using the same equation.
Results
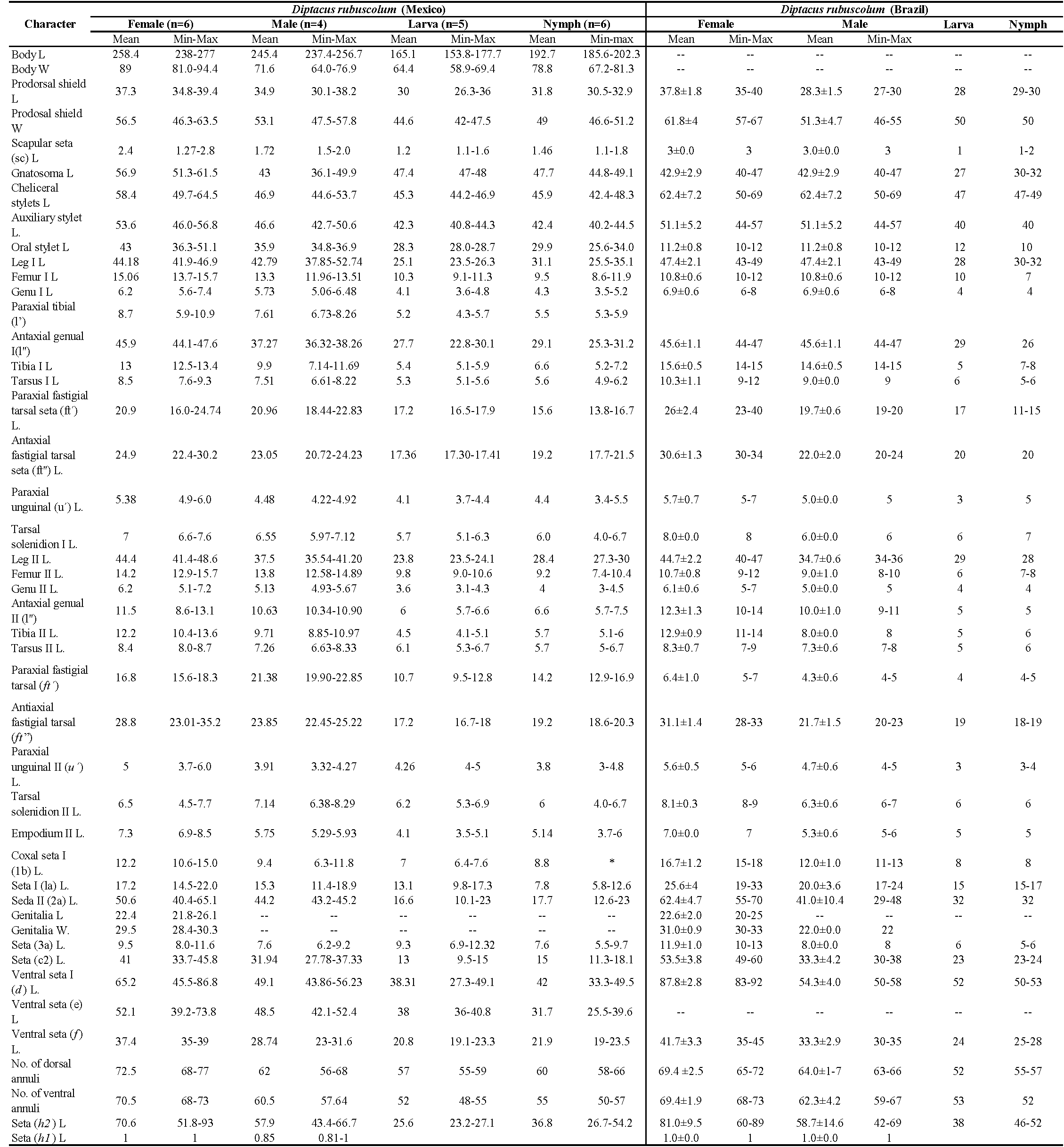
All 800 specimens mounted were identified as belonging to one species, D. rubuscolum. The morphological characteristics obtained matched the description of D. rubuscolum made by Trinidad et al. (2018); however, few differences and details on morphological structures observed through SEM are pointed out. Average measurements obtained from Mexican specimens are shown in Table 1, alongside data from Brazilian specimens provided by Trinidad et al. (2018) for comparison.


Complementary description
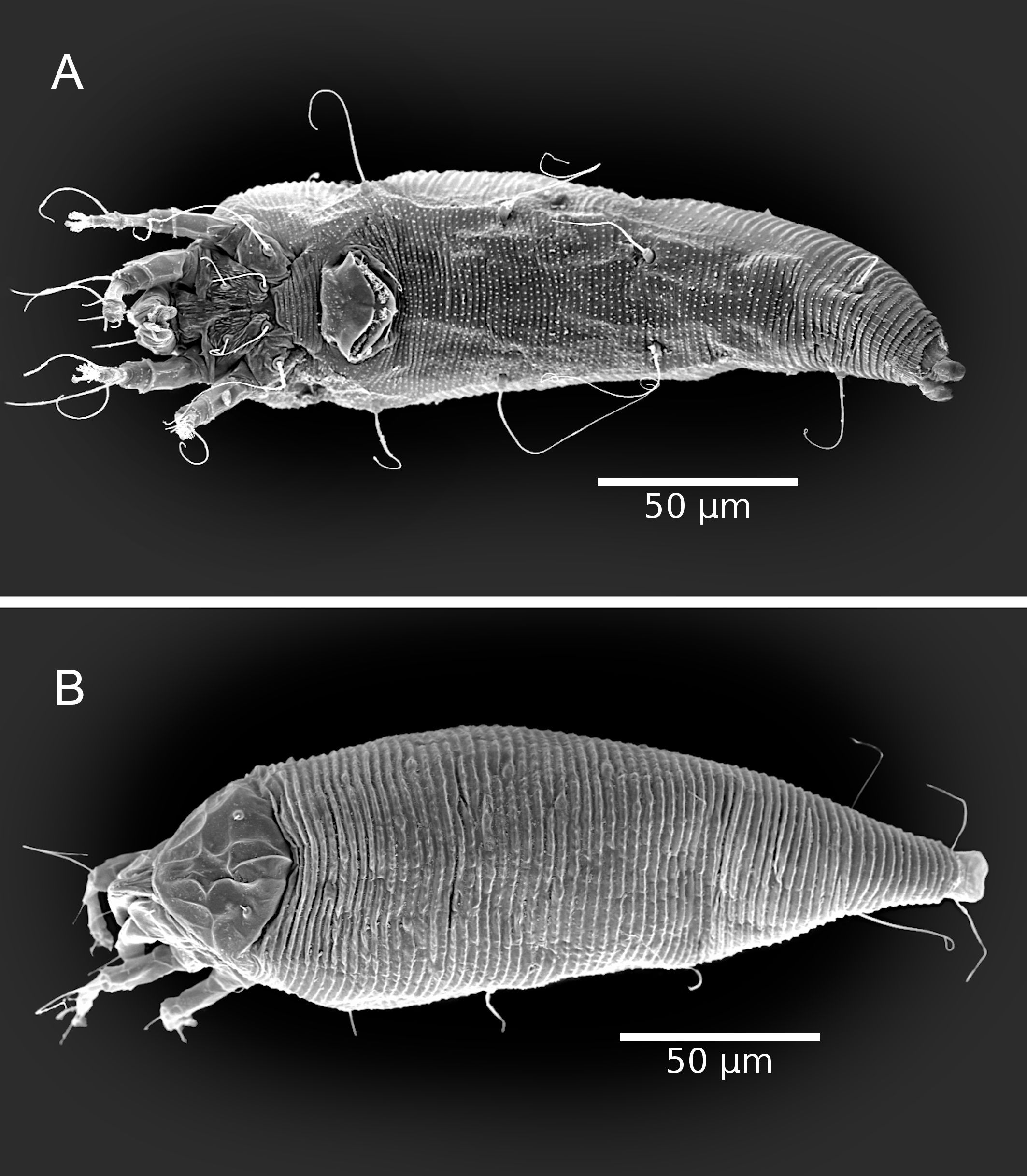


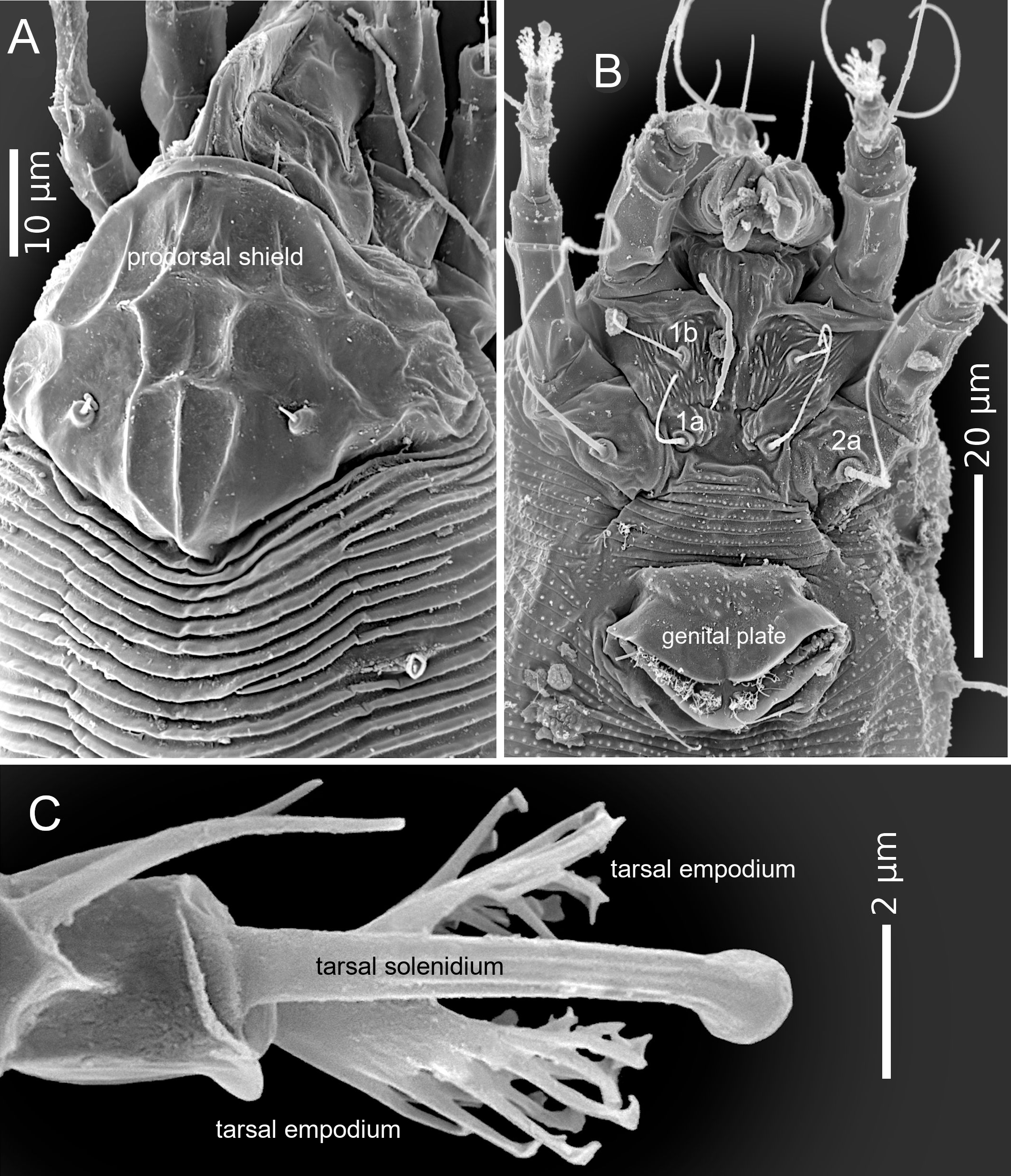


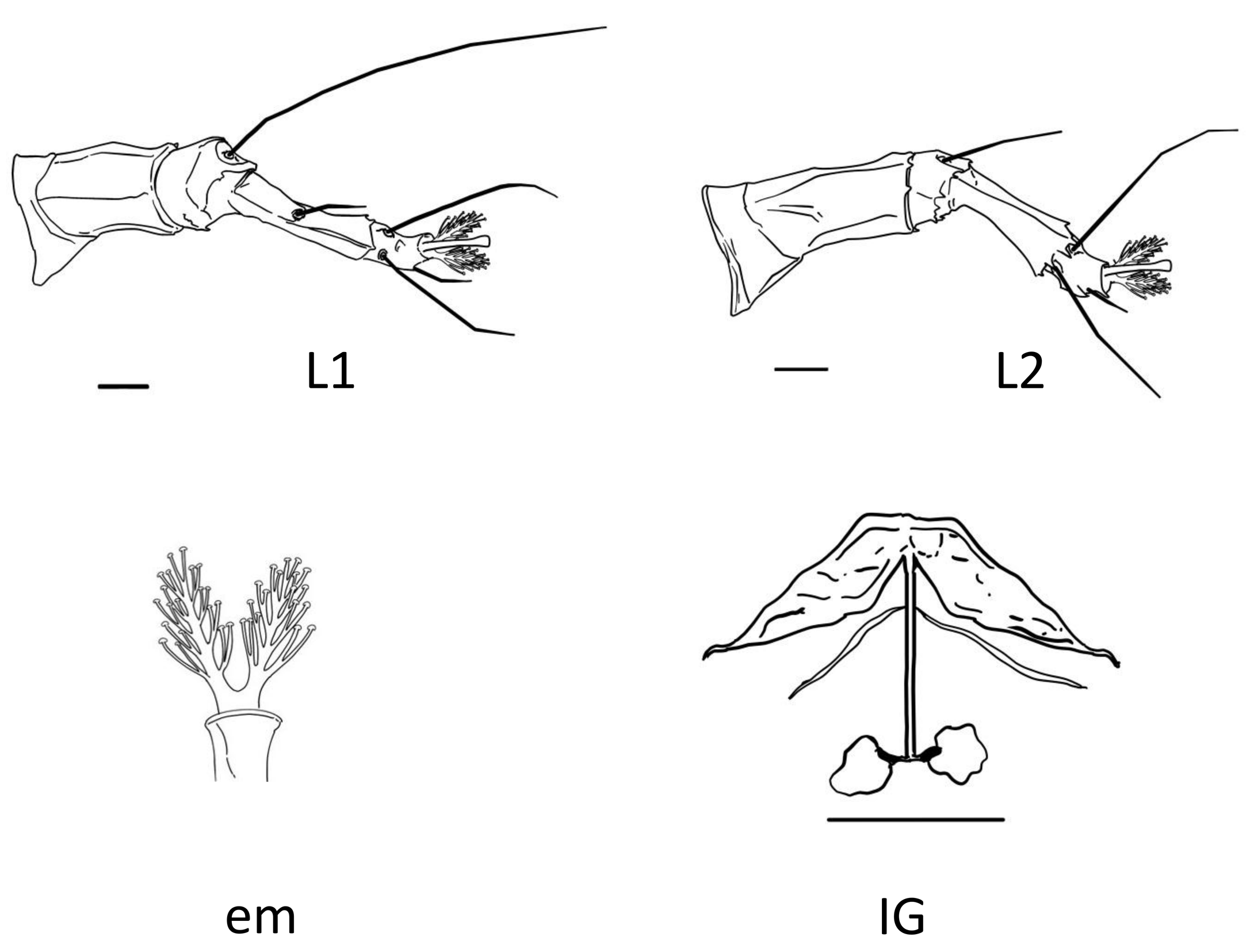


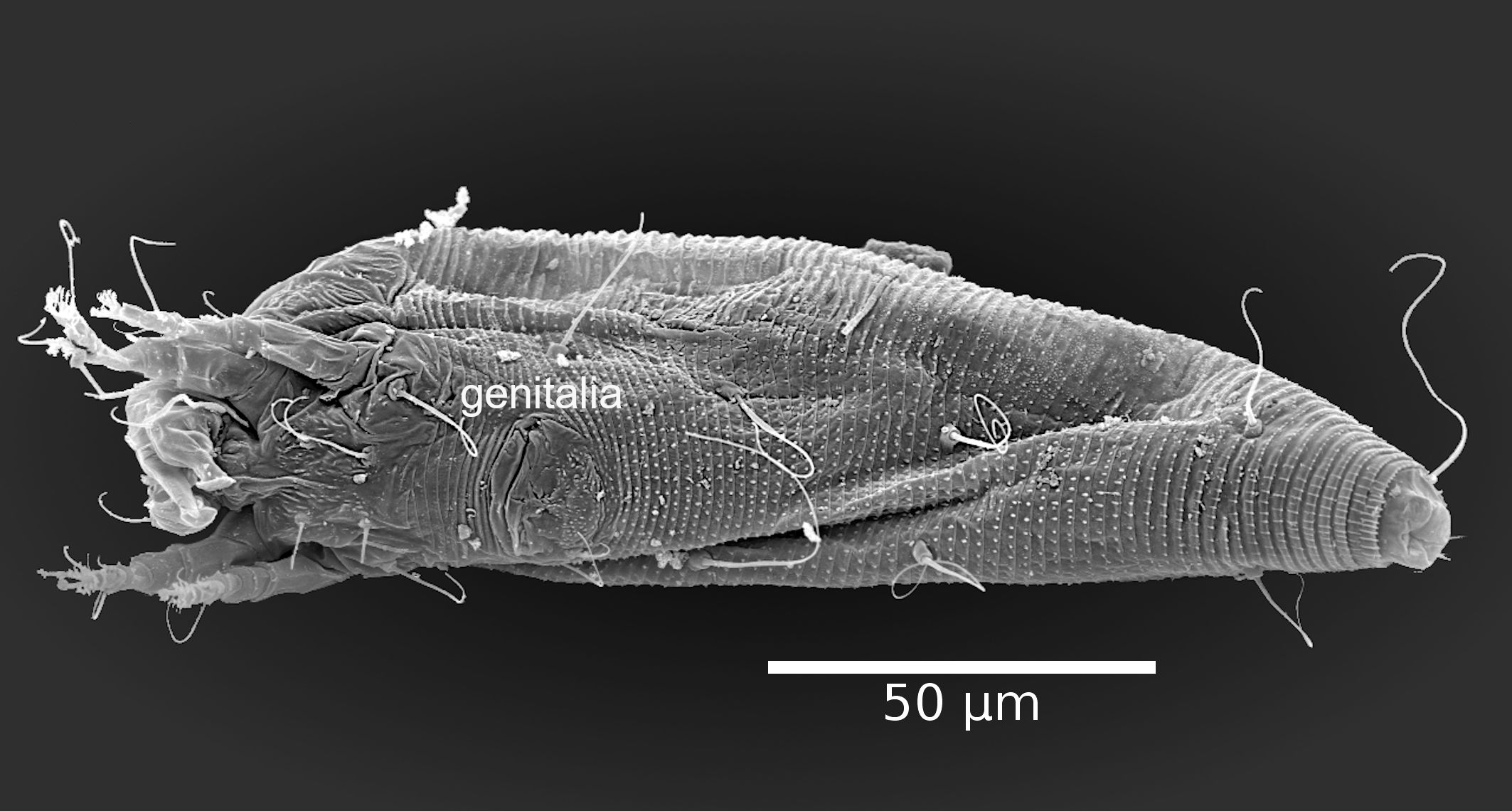


The adult female body is fusiform (Fig. 1A, B), with gnathosoma projecting downwards and strongly curved at the base; prodorsal shield with ornamentation net-like and scapular seta sc projecting forward ahead of rear shield margin (Fig. 2A); coxal plates with short lines and coxal plate I separated (Fig. 2B). In leg I, the tarsal empodium (em) is divided, each half of the empodium with five rays, terminal pair of simple rays (without subrays), and four basal pairs of complex rays (each ray with one additional medial subray); all rays were terminal with a tiny knob (Fig. 2C) and femoral setae bv absent (Fig. 3). In leg II, tarsal empodium (em) divided, each half of the empodium with five rays, terminal pair of simple rays (without subrays), and four basal pairs of complex rays (each ray with one additional medial subray); all rays were terminal with a tiny knob and femoral setae bv absent (Fig. 3); external genitalia had a coverflap with short irregular lines at the base (Fig. 2B); internal genitalia with anterior genital apodeme trapezoidal, the oblique apodeme under the anterior genital apodeme and a pre-spermathecal tube joined to the longitudinal bridge, spermathecal tube shorts, and a globose shaped spermatheca (Fig. 3); the opisthosoma with 68–77 subequal dorsoventral smooth annuli, and 68–73 ventral annuli with round microtubercles on rear annulus margin (Fig. 1A).
The adult male body is fusiform (Fig 4); gnathosoma projecting downwards and strongly curved at base; prodorsal shield with ornamentation net-like; coxal plates with short lines and coxal plate I separated; in leg I, tarsal solenidion slightly curved and knobbed, tarsal empodium (em) divided, each half of the empodium with five rays, terminal pair of simple rays (without subrays), and four basal pairs of complex rays (each ray with one additional medial subray); all rays were terminal with a tiny knob, and femoral setae bv absent; in leg II, tarsal solenidion slightly curved and knobbed and tarsal empodium (em) divided, each half of the empodium with five rays, terminal pair of simple rays (without subrays), and four basal pairs of complex rays (each ray with one additional medial subray); all rays terminal with tiny knob, and femoral setae bv absent; the opisthosoma with 56–68 dorsal subequal smooth annuli, and 57–64 ventral annuli with round microtubercles on rear annulus margin.
Nymphs with vermiform body; gnathosoma projecting downwards and strongly curved at base; prodorsal shield with ornamentation net-like and projecting forward ahead of rear shield margin; in leg I, tarsal solenidion slightly curved and knobbed, and tarsal empodium (em) divided with femoral setae bv absent; in leg II, tarsal empodium divided and tarsal solenidion slightly curved and knobbed; the opisthosoma with 58–66 dorsal annuli and 55–57 ventral annuli; both with round microtubercles on rear annulus margin.
Larva with vermiform body; gnathosoma projecting downwards and strongly curved at base; prodorsal shield with ornamentation net-like and scapular tubercles in the rear shield margin; in both legs the empodium is divided, the solenidion is slightly curved and knobbed and seta bv absent on both legs; the opisthosoma with 55–59 dorsal annuli, 48–55 ventral annuli, both with round microtubercles on rear annulus margin.
Molecular characterization
The final length of the mtCOI sequences were 652 bp and available from GenBank with the accession numbers MZ519880 and MZ519881; for the 18S they were 792 bp (MZ518817-MZ518818); for the D2-5 and D9-10 regions of the 28S rRNA they were 945 bp (MZ518781-MZ518782) and 739 bp (MZ518794-MZ518795), respectively.
Sequence diversity
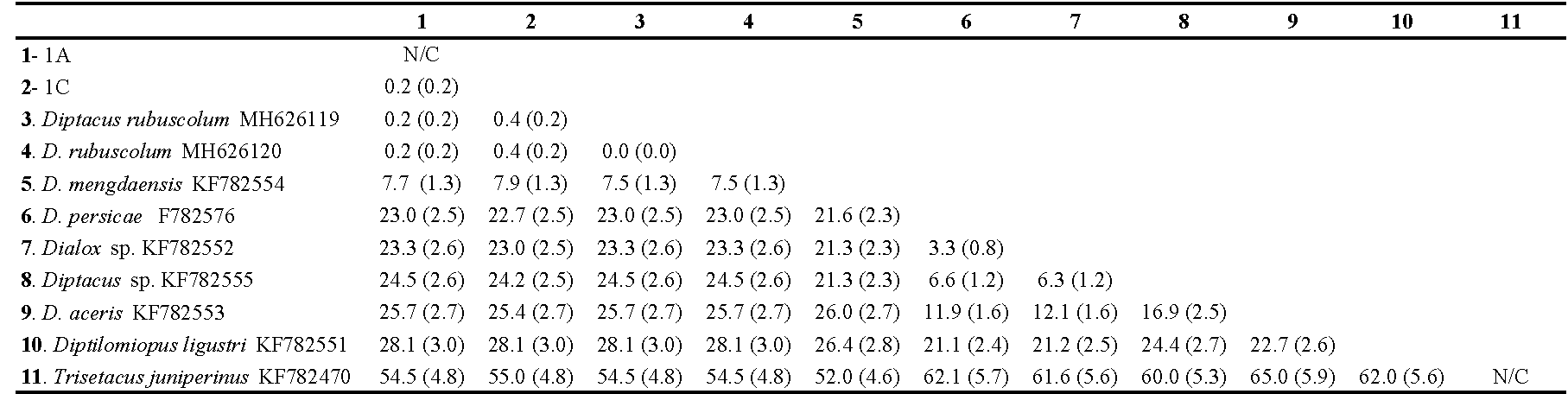
The sequence data from the D2 region of the 28S rDNA we used, comprised 11 aligned sequences of 950 bps, two obtained in this study from D. rubuscolum and nine retrieved from GenBank including one outgroup species. In the alignment, 128 sites were parsimony informative, and 268 sites were variable. The average mean divergence over all sequence pairs (including the outgroup taxa) was 25% (SE=2%) and ranged from 0.2 to 62.0%. The average mean divergence over the Diptacus spp. sequences was 15.0% (SE =1%) and ranged from 0.2 to 25% (Table 2). The average mean divergence between the D. rubuscolum sequence we obtained and that obtained from Genbank was 0.2 % (SE = 0.2%) confirming species identity (Table 2).


Phylogenetic analysis
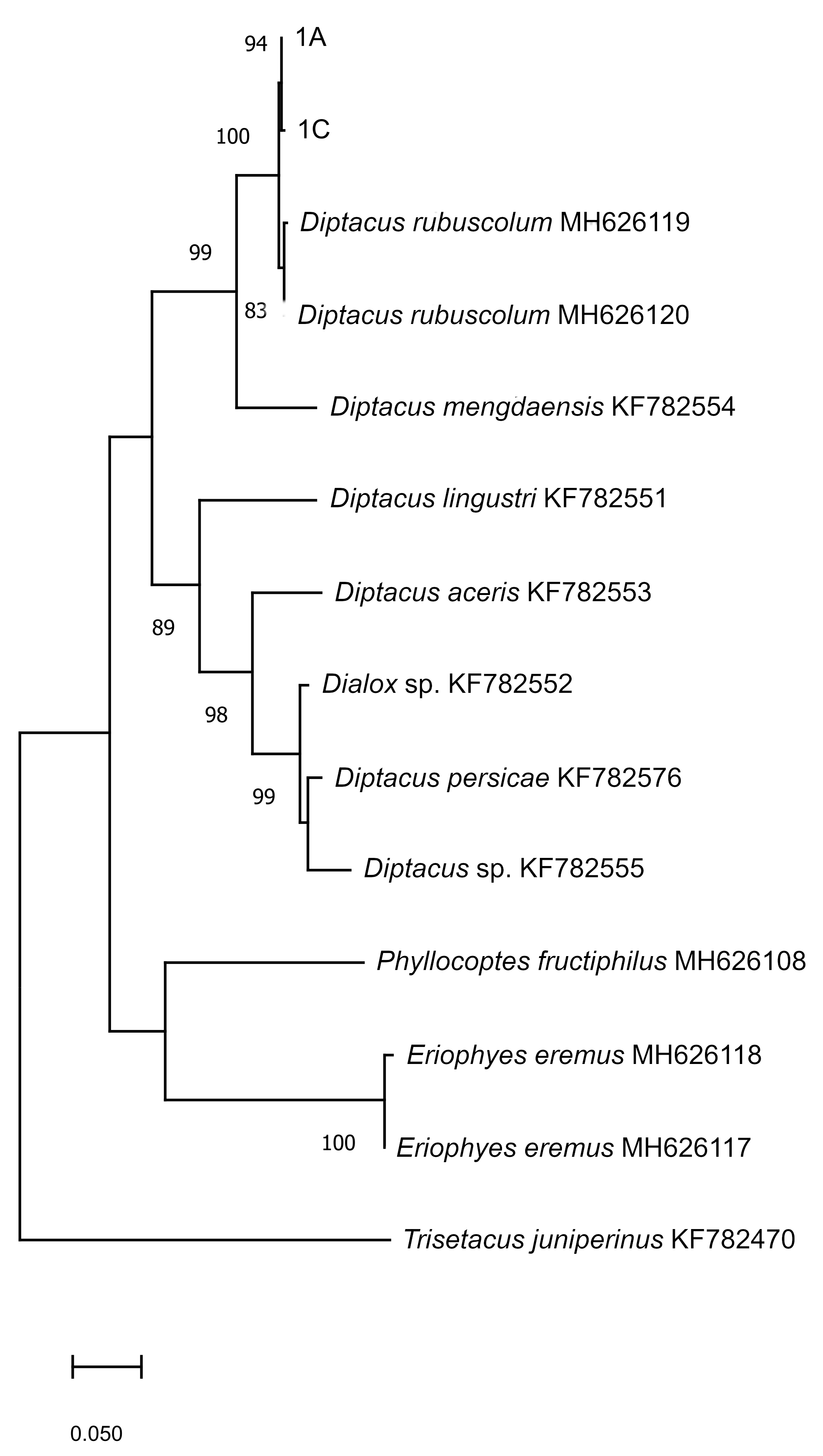
The phylogenetic tree inferred from the maximum likelihood analysis of the D2 region of the sequences studied, consisted of two well-supported clades, one containing D. rubuscolum and D. mengdaensis sequences; all D. rubuscolum samples were grouped with 99% probability but separated from D. mengdaensis with 89% probability. The other clade contained all other Diptacus species clustered separately with probabilities ranging from 96 to 99% (Fig. 5). This analysis successfully supported identification of D. rubuscolum in our samples.


Biology of Diptacus rubuscolum
Diptacus rubuscolum has a simple life cycle with the same developmental stages typical of the Eriophyioidea superfamily: egg, larva, nymph and adult. They had two quiescent stages; the first was between the larval stage and the nymphal stage (nymphochrysalis), and the second was between the nymphal stage and the adult stage (imagochrysalis). The crystalline colour of the egg became yellow and translucent, and it took on a semi-spherical shape. Fertilized females oviposited on leaf surfaces, particularly along the secondary ribs. The mean duration of the egg stage was 56.5±2 h. Larvae were crystalline in colour and became translucent before the first quiescent stage; the mean duration of this stage was 56.5±2 h. The first quiescent stage, or nymphochrysalis, was white in colour with a mean duration of 21.8±1.5 h. There was only one nymphal stage, white in colour, which showed greater mobility and feeding activity than the larva; the mean duration of this stage was 41.3±1.9 h. The second quiescent stage, or imagochrysalis, was white in colour, and occurred just before the adult stage and had a mean duration of 19.7±1.3 h. The adult was white in colour and the most active stage with a mean duration of 114.5±2.7 h.
Discussion
Morphological characteristics, genetic diversity and phylogenetic analyses confirmed the identity of this mite species in our samples. The small size of eriophoid mites, as well as the existence of potential cryptic species, make it difficult to rely only on morphological methods to identify these mite species. Therefore, a combination of morphological and molecular methods is considered the most accurate strategy for identification of eriophyoid mites (Duarte et al. 2019). Partial sequences of the D2 region have been reported to show low variation amongst species, compared with COI or ITS, sometimes being very similar amongst closely related species (Lee and Ó Foighil 2004). However, our results after analysing partial sequences of the D2 region confirmed a clear separation between D. rubuscolum and other Diptacus species, with genetic variations of 7–25%. Analyses of this region from other Eriophyidae species revealed variation of 2–19% amongst Abacarus species (Duarte et al. 2019), 30–36% amongst Aculops species (Duarte et al. 2023), 2% between Aceria tosichella Keifer and A. eximia Sukhareva (Skoracka et al. 2012), and 4% between Eriocaenus ramosissimi Petanović & Amrine and E. equiseti Farkas (Petanović et al. 2015).
In all our surveys and despite sampling all plant structures and on six different occasions, we did not find Acalitus essigi or A. orthomera, which have been reported previously on blackberry, R. ulmifolius from Michoacán, Mexico (Ayala-Ortega et al. 2019). Previously, A. essigi, has been reported causing uneven ripening of blackberry fruits in Australia (Scott et al. 2008), a symptom previously called the ′redberry disease′ (de Lillo and Cuso 1996). In our surveys, we did observe some blackberry fruits with uneven ripening, but the fact that we did not find A. essigi suggest more research is needed to determine more conclusively the relationship between A. essigi and the redberry disease (Low et al. 2020).
The morphological description we have provided, in addition to the existing description of the type material (Trinidad et al. 2018), represents an important contribution towards overall knowledge about this species. All morphological traits were in line with what has been reported previously for the type specimen; all our measurements fell within the range reported for the type species (Trinidad et al., 2018). However, we found some differences; the adult type specimen was considered to have a vermiform body, while we consider that the specimens observed in our study had fusiform bodies (Fig. 1). Having a fusiform body is considered an important and unique characteristic of members of the subfamily Diptilomiopinae (Hong and Shang 1997), which includes the genus Diptacus. Moreover, in fusiform mites, the dorsal tergites are morphologically differentiated from the ventral esternites (Linquist 1996), which is the case for the specimens we evaluated (Fig. 1). A more detailed observation through SEM photography, revealed the existence of a divided empodium, and in each half, a terminal pair of simple rays with four basal pair, and each one with an additional subray (Fig. 2C, 3).
Protogyne females of D. rubuscolum deposited their eggs close to the secondary leaf ribs, presumably for better access to nutrients (Royalty and Perring 1996). The average duration from egg to the end of the adult stage was 13 days, with two quiescent stages between larva and nymph and between nymph and adult, known as the nymphochrysalis and imagochrysalis, respectively (Sternlicht and Goldenberg 1971). Information regarding life cycle and longevity on Eriophydae are very scarce; some publications on longevity of mites in this family report mean values of 24 days for Rhyncaphytoptus ficifoliae Keifer (Trombidiformes; Diptilomiopidae) (Bahirai et al. 2019) and 19 days for Aculops guajavae (Abou-Awad et al. 2016), considered a synonym of Tegolophus guavae Boczek (Elhalawany 2018).
None of the active stages of D. rubuscolum caused visible damage when feeding on blackberry plants, as also reported for D. caseius on Rubus caeseius plants in Germany (Domes 2000); or any symptom of virus infection, potentially transmitted by D. rubuscolum. It has been reported that the effect of eriophyid mites, in most cases, is non-symptomatic where mechanical damage caused by feeding activity is normally insignificant (Lindquist and Oldfield 1996), but in high populations they may cause phytotoxemia (de Lillo et al. 2018). Potential explanations for the lack of visible damage to plant tissues are that either chloroplast particles or cell contents are not fully extracted during feeding, or that the cell wall repairs itself after the mite´s mouthparts are removed (Lindquist and Oldfield 1996). It has also been suggested that the lack of visible damage is because of low population densities of the mite, making damage to the plants negligible (Petanović and Kielkiewicz 2010). However, a recent study carried out on Mexican blackberries, showed larger populations of D. rubuscolum than T. urticae (González-Domínguez et al. 2023). Here we studied only one plant cultivar (Tupy), and damage caused by eriophyoid mites may be different depending on the plant cultivar, or even on the eriophyoid genotype, in addition to population density and environmental conditions (Petanović and Kielkiewicz 2010). Overall, more research is required to determine whether any damage is caused by D. rubuscolum when feeding on blackberry plants.
In conclusion, D. rubuscolum was the only eriophyoid mite found on blackberry plants in the studied areas in México. We have provided more information about the morphology of this species, which in combination with partial sequences of mtCOI, the 18S and 28S of the rRNA, will facilitate accurate identification of this species in future studies. This is the first report about the biology and duration of the different life stages of this species, contributing to the overall knowledge of Eriophyoidea.
Acknowledgements
SGGD received a scholarship from CONACYT for her PhD. This study was funded by Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA) within the project entitled'Dinámica espacio-temporal de ácaros plaga en el cultivo de zarzamora en Michoacán', grant number PM-18-4017. The authors are grateful to the growers that allowed the first author to take samples from their blackberry crops. We thank Dr P.E. Chetverikov for his valuable help with the morphology of the internal genitalia and empodium.
References
- Abou-Awad, B.A., Alazzazy, M.M., Afia, S.I. 2016. Biology of Aculops guajavae, a new species (Acari: Eriophyidae) infesting guava trees. Int. J. Chemtech Res., 9: 108-113.
- Abràmoff, D., Magalhães, P.J., Ram, S.J. 2004. Image processing with Image. J. Biophotonics Int., 11: 36-4.
- Amrine, J.W. Jr., Stasny, T.A.H., Flechtmann, C.H.W. 2003. Revised keys to the world genera of the Eriophyoidea (Acari:Prostigmata). West Bloomfield, Michigan, Indira Publishing House, pp. 244.
- Amrine, J.W. Jr., Manson, D.C.M. 1996. Preparation, mounting and descriptive study of eriophyoid mites. In: Lindquist EE, Sabelis MW, Bruin J (eds.) Eriophyoid mites-their biology, natural enemies and control. Elsevier, Amsterdam. World Crop Pests Vol. 6 p383-396 https://doi.org/10.1016/S1572-4379(96)80023-6
- Ayala-Ortega, J.J., Martínez-Castillo, A.M., Pineda-Guillermo, S., Figueroa-de la Rosa, J.I., Acuña-Soto, J., Ramos-Lima, M., Vargas-Sandoval, M. 2019. Ácaros asociados a la zarzamora (Rubus sp. cv. Tupy) en dos localidades del estado de Michoacán, México. Rev. Colomb. Entomol., 45, e8480. https://doi.org/10.25100/socolen.v45i2.8480
- Bahirai, F., Jafari, S., Lotfollahi, P., Shakarami, J. 2019. Effect of temperature on life table parameters of Rhyncaphytoptus ficifoliae Keifer (Trombidiformes; Diptilomiopidae). Syst. Appl. Acarol., 24: 1394-1405. https://doi.org/10.11158/saa.24.8.5
- Campbell, B.C., Steffen-Campbell, J.D., Werren, J.H., 1994. Phylogeny of the Nasonia species complex (Hymenoptera: Pteromalidae) inferred from an internal transcribed spacer (ITS2) and 28S rDNA sequences. Insect Mol. Biol., 2: 225-237. https://doi.org/10.1111/j.1365-2583.1994.tb00142.x
- Dabert, M., Witalinski, W., Kazmierski, A., Olszanowski, Z., Dabert, J., 2010. Molecular phylogeny of acariform mites (Acari, Arachnida): strong conflict between phylogenetic signal and long-branch attraction artifacts. Mol. Phylogenet. Evol., 56: 222-241. https://doi.org/10.1016/j.ympev.2009.12.020
- de Lillo, E., Duso, C. 1996. 3.2. 6 Currants and berries. In: Lindquist EE, Sabelis MW, Bruin J (eds.) Eriophyoid mites-their biology, natural enemies and control. Elsevier, Amsterdam. World Crop Pests Vol. 6 p583-591. https://doi.org/10.1016/S1572-4379(96)80037-6
- de Lillo, E., Craemer, C., Amrine, J. W., Nuzzaci, G. 2010. Recommended procedures and techniques for morphological studies of Eriophyoidea (Acari: Prostigmata). Exp. Appl. Acarol., 51: 283-307. https://doi.org/10.1007/978-90-481-9562-6_15
- de Lillo, E., Domenico, V., Pasquale, S. 2016. Attuali conoscenze sugli eriofioidei vettori di virus. Accademia nazionale italiana di entomología, 113-121.
- de Lillo, E., Pozzebon, A., Valenzano, D., Duso, C. 2018. An intimate relationship between Eriophyoid mites and their host plants - A review. Front. Plant. Sci. 9:1786. https://doi.org/10.3389/fpls.2018.01786
- Domes, R. 2000. Four new species of Eriophyiodea on Prunus domestica, Rosa canina, Rubus caesius and Prunus padus: Rhynophytoptus domestica N.SP., Paraphytoptus rosae N.SP., Diptacus caesius N.SP. and Eriophyes padi N.SP. Acarologia, 40: 307-319.
- Druciarek, T., Lewandowski, M., Tzanetakis, I. 2019. A new, sensitive and efficient method for taxonomic placement in the Eriophyoidea and virus detection in individual eriophyoids. Exp. Appl. Acarol., 78: 247-261. https://doi.org/10.1007/s10493-019-00382-4
- Duarte, M.E., de Mendonça, R.S., Skoracka, A., Silva, E.S., Navia, D. 2019. Integrative taxonomy of Abacarus mites (Eriophyidae) associated with hybrid sugarcane plants, including description of a new species. Exp. Appl. Acarol., 3:373-401. https://doi.org/10.1007/s10493-019-00388-y
- Duarte, M. E., Lewandowski, M., de Mendonça, R. S., Simoni, S., Navia, D. 2023. Genetic analysis of the tomato russet mite provides evidence of oligophagy and a widespread pestiferous haplotype. Exp. Appl. Acarol., 89: 171-199. https://doi.org/10.1007/s10493-023-00777-4
- Elhalawany, A. S. 2018. A new species, new synonymy and a new record of eriophyoid mites (Acari: Eriophyidae), from Egypt. Acarines: J. Egyp. Soc. Acarol., 12: 7-16. https://doi.org/10.21608/ajesa.2008.164281
- Felsenstein, J., 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783-791. https://doi.org/10.2307/2408678
- Folmer, O., Black, M., Hoech, W., Lutz, R., Vrijenhoek, R., 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol., 3: 294-299.
- González-Domínguez, S., Santillán-Galicia, M. T., Guzmán-Franco, A. W., Avila-García, C. D. J., López-Buenfil, J. A., Romero-Rosales, F. 2023. Species diversity, population dynamics and spatial distribution of mites on blackberry (Rubus ulmifolius Schott): A comparison between organic and conventionally-managed orchards. Phytoparasitica, 51: 241-253 https://doi.org/10.1007/s12600-023-01051-4
- Hall, T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser., 41: 95-98.
- Hillis, D.M., Dixon, M.T., 1991. Ribosomal DNA: molecular evolution and phylogenetic inference. Q. Rev. Biol., 66, 411-453. https://doi.org/10.1086/417338
- Hong, X., Zhang, Z.Q. 1997. Systematics and generic relationships of the mites in the subfamily Diptilomiopinae (Acari: Eriophyoidea: Diptilomiopidae). Syst. Entomol. 22, 313-331. https://doi.org/10.1046/j.1365-3113.1997.d01-48.x
- Huang, K.W., Wang, C.F. 2009. Eriophyoid mites (Acari: Eriophyoidea) of Taiwan: thirty-seven species from Yangmingshan, including one new genus and twenty-two new species. Zootaxa, 1986: 1-50. https://doi.org/10.11646/zootaxa.1986.1.1
- Kimura, M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120 https://doi.org/10.1007/BF01731581
- Kuang, H.Y. 2001. Two new species of Diptacus Keifer from China. Entomotaxon, 23: 153-156.
- Kumar, S., Stecher, G., Tamura, K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol., 33: 1870-1874. https://doi.org/10.1093/molbev/msw054
- Lee, T., Ó Foighil, D. 2004. Hidden Floridian biodiversity: mitochondrial and nuclear gene trees reveal four cryptic species within the scorched mussel, Brachidontes exustus, species complex. Mol. Ecol., 13: 3527-3542. https://doi.org/10.1111/j.1365-294X.2004.02337.x
- Li, H. S., Xue, X. F., Hong, X. Y. 2014. Homoplastic evolution and host association of Eriophyoidea (Acari, Prostigmata) conflict with the morphological-based taxonomic system. Mol. Phylogenet. Evol., 78: 185-198. https://doi.org/10.1016/j.ympev.2014.05.014
- Lindquist, E.E., Oldfield, G.N. 1996. Evolution of eriophyoid mites in relation to their host plant. In: Lindquist E.E., Sabelis, M.W., Bruin, J. (Eds), Eriophyoid mites: their biology, natural enemies and control, Elsevier, Amsterdam. p. 277-300. https://doi.org/10.1016/S1572-4379(96)80018-2
- Lindquist, E.E. 1996. External anatomy and notation of structures. In: Lindquist, E.E., Sabelis, M.W., Bruin, J. (Eds), Eriophyoid Mites: their biology, natural enemies and control. Elsevier, Amsterdam, p. 3-31. https://doi.org/10.1016/S1572-4379(96)80003-0
- Law, H. M., Allen, G. R., Davies, J. T., Corkrey, R., Buntain, M., Quarrell, S. R. 2020. Sampling, extraction and incidence of redberry mites (Acalitus essigi) on blackberries in Australia. Exp. Appl. Acarol., 81: 317-334. https://doi.org/10.1007/s10493-020-00509-y
- Marchetti, M., Ferla, N.J. 2011. Diversidade e flutuação populacional de ácaros (Acari) em amora-preta (Rubus fruticosus, Rosaceae) no estado do Rio Grande do Sul, Brasil. Iheringia, 101: 43-48. https://doi.org/10.1590/S0073-47212011000100005
- Petanović, R., Kielkiewicz, M. 2010. Plant-eriophyoid mite interactions: specific and unspecific morphological alterations. Part II. Exp. Appl. Acarol., 51: 81-91. https://doi.org/10.1007/978-90-481-9562-6_5
- Petanović, R.U., Amrine, J.W., Chetverikov, P.E., Cvrković, T.K. 2015. Eriocaenus (Acari: Trombidiformes: Eriophyoidea), a new genus from Equisetum spp. (Equisetaceae): morphological and molecular delimitation of two morphologically similar species. Zootaxa, 4013: 51-66. https://doi.org/10.11646/zootaxa.4013.1.3
- Perring, T.M., Holtzer, T.O., Toogle, J.L., Norman, J.M., Myers, G.L. 1984. Influences of temperature and humidity on pre-adult development of the Banks grass mite (Acari: Tetranychidae). Environ. Entomol., 13: 338-343. https://doi.org/10.1093/ee/13.2.338
- Royalty, R.N., Perring, T.M. 1996. Nature of damage and its assessment. In: Lindquist EE, Sabelis MW, Bruin J (Eds) Eriophyoid Mites: their biology, natural enemies and control. Elsevier, Amsterdam. p. 493-512. https://doi.org/10.1016/S1572-4379(96)80031-5
- Schulz, M., Seraglio, S.K.T., Betta, F.D., Nehring, P., Valese, A.C., Daguer, H., & Fett, R. (2019) Blackberry (Rubus ulmifolius Schott): Chemical composition, phenolic compounds and antioxidant capacity in two edible stages. Food Res. Int., 122: 627-634. https://doi.org/10.1016/j.foodres.2019.01.034
- Scott, J.K., Yeoh, P.B., Knihinicki, D.K. 2008. Redberry mite, Acalitus essigi (Hassan) (Acari: Eriophydae), an additional biological control agent for Rubus species (blackberry) (Rosaceae) in Australia. Aust. J. Entomol., 47: 261-264. https://doi.org/10.1111/j.1440-6055.2008.00654.x
- Servicio de Información Agroalimentaria y Pesquera [SIAP]. 2020. Cierre de la producción agrícola por estados de zarzamora y frambuesa. SAGARPA. Accessed March 2020.
- Skoracka, A., Lechosław, K., de Mendonça, R.S., Dabert, M., Szydło, W., Knihinicki, D., Truol, G., Navia, D. 2012. Cryptic species within the wheat curl mite Aceria tosichella (Keifer) (Acari: Eriophyoidea), revealed by mitochondrial, nuclear and morphometric data. Invertebr. Syst., 26: 417-433. https://doi.org/10.1071/IS11037
- Sternlicht, M., Goldenberg, S. 1971. Fertilisation, sex ratio and postembryonic stages of the citrus bud mite Aceria sheldoni (Ewing) (Acarina, Eriophyidae). Bull. Entomol. Res., 60: 391-397. https://doi.org/10.1017/S000748530004030X
- Strik, B.C., Clark, J.R., Finn, C.E., Bañados, M.P. 2007. Worldwide blackberry production. Hort. Technol., 17: 205-2013. https://doi.org/10.21273/HORTTECH.17.2.205
- Stupková, L.C. 2016. Global value chain in agro-export production and its socio-economic impact in Michoacán, México. Agris on-line Papers in Economics and Informatics, 8: 25-36. https://doi.org/10.7160/aol.2016.080103
- Thompson, J.D., Higgins, D.G., Gibson, T.J., 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positionsspecific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673-4680. https://doi.org/10.1093/nar/22.22.4673
- Trinidad, C.T.O., Duarte, M.E., Silva, Da C.U., Navia, D. 2018. Eriophyoid mites associated with the blackberry in Brazil-a new species in the genus Diptacus Keifer 1951 (Diptilomiopidae) and first report and supplementary description of Acalitus orthomerus (Keifer, 1951) (Eriophyidae). Syst. Appl. Acarol., 23: 1199-1216. https://doi.org/10.11158/saa.23.6.15
- Wang, Z., Xiao-Feng, X., Xiao-Yue, H. 2009. Four new species and a re-described species of the Diptilomiopinae (Acari: Eriophyoidea: Diptilomiopidae) from China. Int. J. Acarol., 35: 123-132. https://doi.org/10.1080/01647950902917601
- Whiting, M.F., Carpenter, J.C., Wheeler, Q.D., Wheeler, W.C., 1997. The Strepsiptera problem: phylogeny of the holometabolous insect orders inferred from 18S and 28S ribosomal DNA sequences and morphology. Syst. Biol., 46: 1-68. https://doi.org/10.1093/sysbio/46.1.1
- Xin, J-L., Dong, H-Q. 1983. Tree new species of Diptilomiopid mites found in China (Acarina: Eriophyioidea). Acarologia, 24: 181-185.



2022-05-14
Date accepted:
2023-06-06
Date published:
2023-06-27
Edited by:
Navia, Denise

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 González-Domínguez, Sandra G.; Santillán-Galicia, Ma. Teresa; Guzmán-Franco, Ariel W.; de Jesús García-Avila, Clemente; López-Buenfil, José Abel and Romero-Rosales, Felipe
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



