Two new feather mite species (Acarina: Psoroptidia) from the Chestnut Bulbul, Hemixos castanonotus (Passeriformes: Pycnonotidae), in China
Constantinescu, Ioana Cristina  1
; Chișamera, Gabriel Bogdan
1
; Chișamera, Gabriel Bogdan  2
; Motoc, Rozalia
2
; Motoc, Rozalia  3
; Gustafsson, Daniel R.
3
; Gustafsson, Daniel R.  4
; Zou, Fasheng
4
; Zou, Fasheng  5
; Chu, Xingzhi
5
; Chu, Xingzhi  6
and Costică, Adam
6
and Costică, Adam  7
7
1✉ 'Grigore Antipa' National Museum of Natural History, Sos. Kiseleff no.1, 011341 Bucharest, Romania.
2'Grigore Antipa' National Museum of Natural History, Sos. Kiseleff no.1, 011341 Bucharest, Romania & Institute of Biology – Bucharest, Romanian Academy, 296 Splaiul Independenței, 060031, Bucharest, P.O. Box 56-53, Romania.
3'Grigore Antipa' National Museum of Natural History, Sos. Kiseleff no.1, 011341 Bucharest, Romania.
4Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Library of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, 105 Xingang West Road, Haizhu District, Guangzhou, 510260, Guangdong Province, China.
5✉ Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Library of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, 105 Xingang West Road, Haizhu District, Guangzhou, 510260, Guangdong Province, China.
6Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Library of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, 105 Xingang West Road, Haizhu District, Guangzhou, 510260, Guangdong Province, China.
7'Grigore Antipa' National Museum of Natural History, Sos. Kiseleff no.1, 011341 Bucharest, Romania.
2023 - Volume: 63 Issue: 3 pages: 637-657
https://doi.org/10.24349/8srk-wv1jZooBank LSID: BAA63CD8-60B5-4342-9200-A0FBF20BAA37
Original research
Keywords
Abstract
Introduction
Feather mites are commensals or ectoparasites of birds. So far, over 2500 species of feather mites have been described, but the currently known species may represent less than 20% of the extant species (Mironov 2003). According to the current phylogenetic concept, the cohort Psoroptidia is subdivided into three superfamilies: Analgoidea and Pterolichoidea representing bird-associated mites, and Sarcoptoidea, the mammal-associated mites (OConnor 2009). Wang and Fan (2010) and Constantinescu et al. (2019) summarized the data on the diversity of feather mites in China poorly studied to date, which includes only about 100 known species.
In the present paper, we describe two new species of feather mites belonging to two genera widely spread in Asia: Trouessartia Canestrini, 1899 (Fam. Trouessartiidae) and Pteroherpus Gaud, 1981 (Fam. Pteronyssidae).
The feather mite genus Trouessartia comprises about 147 described species associated predominantly with birds of the order Passeriformes (Mironov 2022); however, recent estimates suggest that the actual number of species in the genus may be around 500–700 species (Hernandes 2014).
The only revision of this genus was performed by Santana (1976), encompassing the 71 species known at the time; other presently known species were described in the subsequent 40 years by various authors (Mauri and De Alzuet 1968; Černý 1979; Černý and Lukoschus 1975; Gaud 1977; Gaud and Atyeo 1986, 1987; Mironov 1983, 2021a, 2021b; Mironov and Kopij 1996, 2000a; Mironov and Galloway 2002, 2019; Mironov and González-Acuña 2013; Mironov and Overstreet 2016; Mironov and Palma 2016; Mironov and Bermúdez 2017; Mironov and Chandler 2020; Mironov and Zabashta 2022; Mironov et al. 2021; OConnor et al. 2005; Carleton and Proctor 2010; Constantinescu et al. 2013, 2016a, 2016b, 2017, 2018a, 2018b, 2021; Hernandes 2014, 2017, 2022, 2023; Hernandes and Valim 2015; Hernandes and OConnor 2017; Hernandes et al. 2022).
A detailed generic revision of the family Pteronyssidae (considered as a subfamily of Avenzoariidae) was published by Faccini and Atyeo (1981), and the genus Pteroherpus included at that time 12 described species. Subsequent taxonomic papers (Mironov 1992; Mironov and Kopij 2000b; Mironov and Wauthy 2006) and a recent revision of Pteroherpus (Mironov and Wauthy 2008) have raised the number of known species to 18, which were arranged in four species-groups: hoplophorus (10 species), diploplax (6 species), josephi (1 species) and nicator (1 species). In the past decade, five more species of the genus have been described: P. garrulacis Mironov & Proctor, 2011 and P. pomatorhinae Constantinescu, et al., 2019 (diploplax group), P. meghalayensis Constantinescu, 2014 and P. chinensis Constantinescu et al., 2019 (hoplophorus group), and P. surmachi Mironov, 2011 not referred to any of recognized species groups, because of having a distinct combination of characters both in females and males (Mironov 2011; Mironov and Proctor 2011; Constantinescu et al. 2014, 2019).
Material and methods
The material used in the present paper was collected from the Chestnut Bulbul, Hemixos castanonotus Swinhoe, 1870, in Guangdong Province in China. The bird was captured, identified and visually checked for the presence of mites; after mites were collected, it was released back into the wild. Mite specimens were placed in tubes with 95% ethanol, and later, in the laboratory, were cleared in lactic acid and mounted on microscope slides in Hoyer's medium. Drawings were made using an Olympus CX21 microscope equipped with a camera lucida drawing device.
For scanning electron microscope study (SEM), feather mites were cleaned in an ultrasonic cleaner (Evo Sonic) for 10–30 seconds to remove debris and then fixed in 2.5% glutaraldehyde in sodium cacodylate buffer (0.1M, pH 7.4) in a refrigerator at 4° for 4h. The specimens were dehydrated in an ascending alcohol series (30%, 50%, 70%, 80%, 95%, and 100% ethanol; 10 min. each) and then in hexamethyldisilazane (HMDS) for 1h (Han et al. 2019a; Han and Min 2019b). After being air-dried, samples were mounted on aluminum stubs covered with conductive double-sided adhesive carbon tabs. The samples were sputter-coated with gold for 30 seconds. The feather mites were analysed and photographed using a Phenom Pro scanning electron microscope (Phenom-World, Thermo Fisher Scientific, The Netherlands), at 10kV acceleration voltage.
The nomenclature of body setation follows that of Griffiths et al. (1990) with the coxal setae modifications made by Norton (1998), and that of the legs follows Gaud and Atyeo (1996). The measuring techniques of particular structures in Trouessartia description were detailed by Mironov and Chandler (2020), with the changes introduced by Mironov regarding the term DHA (dorsal hysterosomal apertures) (Mironov 2022). Description of new Pteroherpus species is given according to the current format used for mites of the family Pteronyssidae (Mironov 1992; Mironov and Wauthy 2006, 2008).
The bird specimens were identified according to Arlott (2017), but the taxonomy of the birds follows Clements et al. (2022), which differs in species limits used. We give the full set of measurements for a holotype (male) and range of measurements for all corresponding paratypes. All measurements are in micrometers (μm).
The type specimens of the new species are deposited in the Institute of Zoology, Guangdong Academy of Science, Guangzhou, China (IZGAS, previously the Guangdong Institute of Applied Biological Resources, GIABR) and in the Acarological Collection of the ''Grigore Antipa» National Museum of Natural History, Bucharest, Romania (MGAB). Specimens with the collection number GD-ACARI are in the IZGAS, and those with the collection number ANA are in MGAB.
Results
Family Trouessartiidae Gaud, 1957
Genus Trouessartia Canestrini, 1899
Trouessartia bulbuli Constantinescu, sp. nov.
ZOOBANK: C60F27E8-EAA0-4CE5-AB99-79FBA67163E7 ![]()
(Figures 1–7)
Type material
From Hemixos castanonotus Swinhoe (Passeriformes: Pycnonotidae): male holotype (ANA1845), 4 male (ANA1842–1844, ANA1846) and 5 female (ANA1847–1851) paratypes, in MGAB; 5 male (GD-ACARI-39–43) and 5 female (GD-ACARI-44–48) paratypes, in IZGAS, CHINA, Guangdong Province, Dinghu District, Dinghushan National Nature Reserve, 22°09′34″N, 112°32′17″E, 14 March 2019; coll. C. Adam.
Description












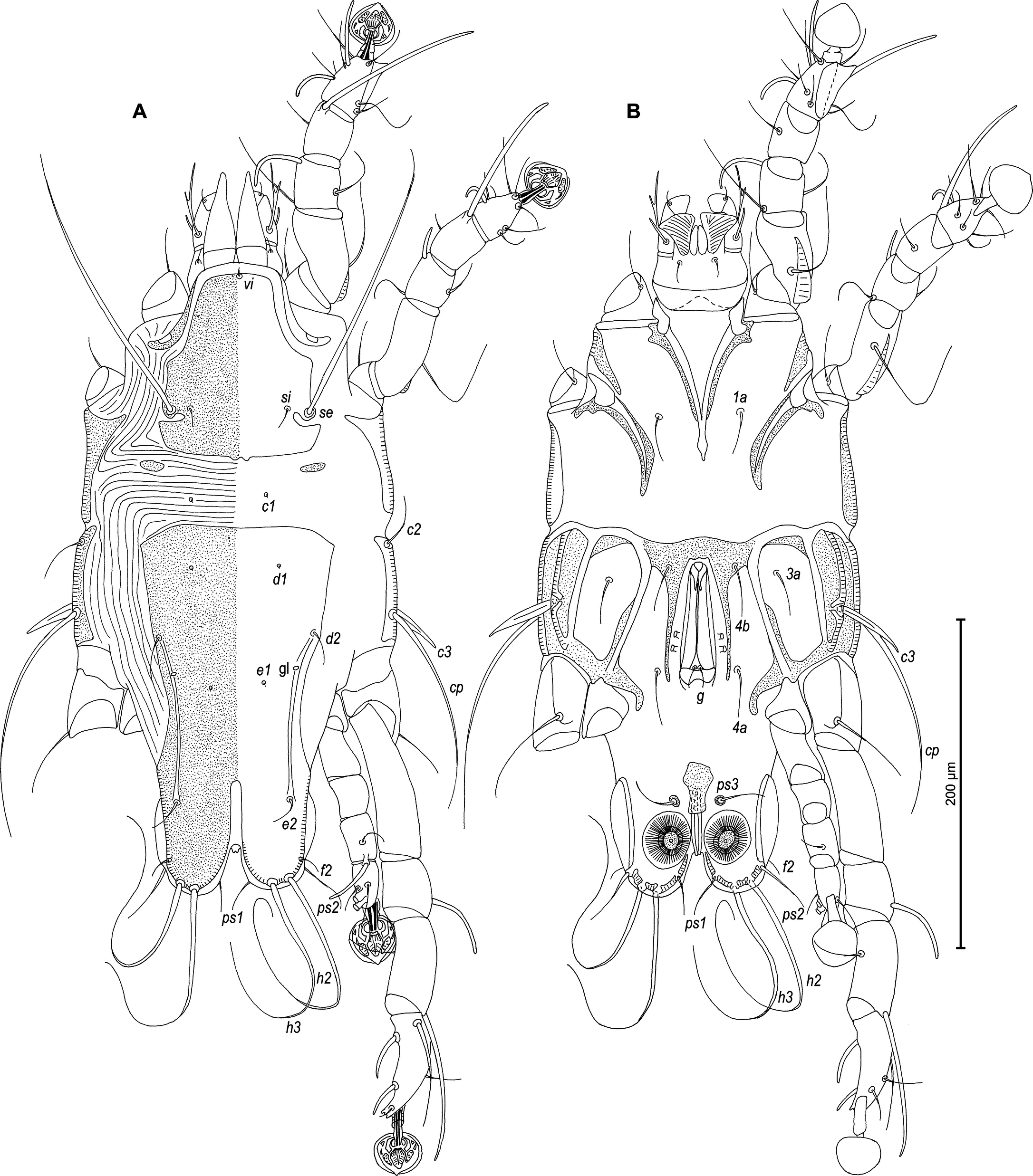
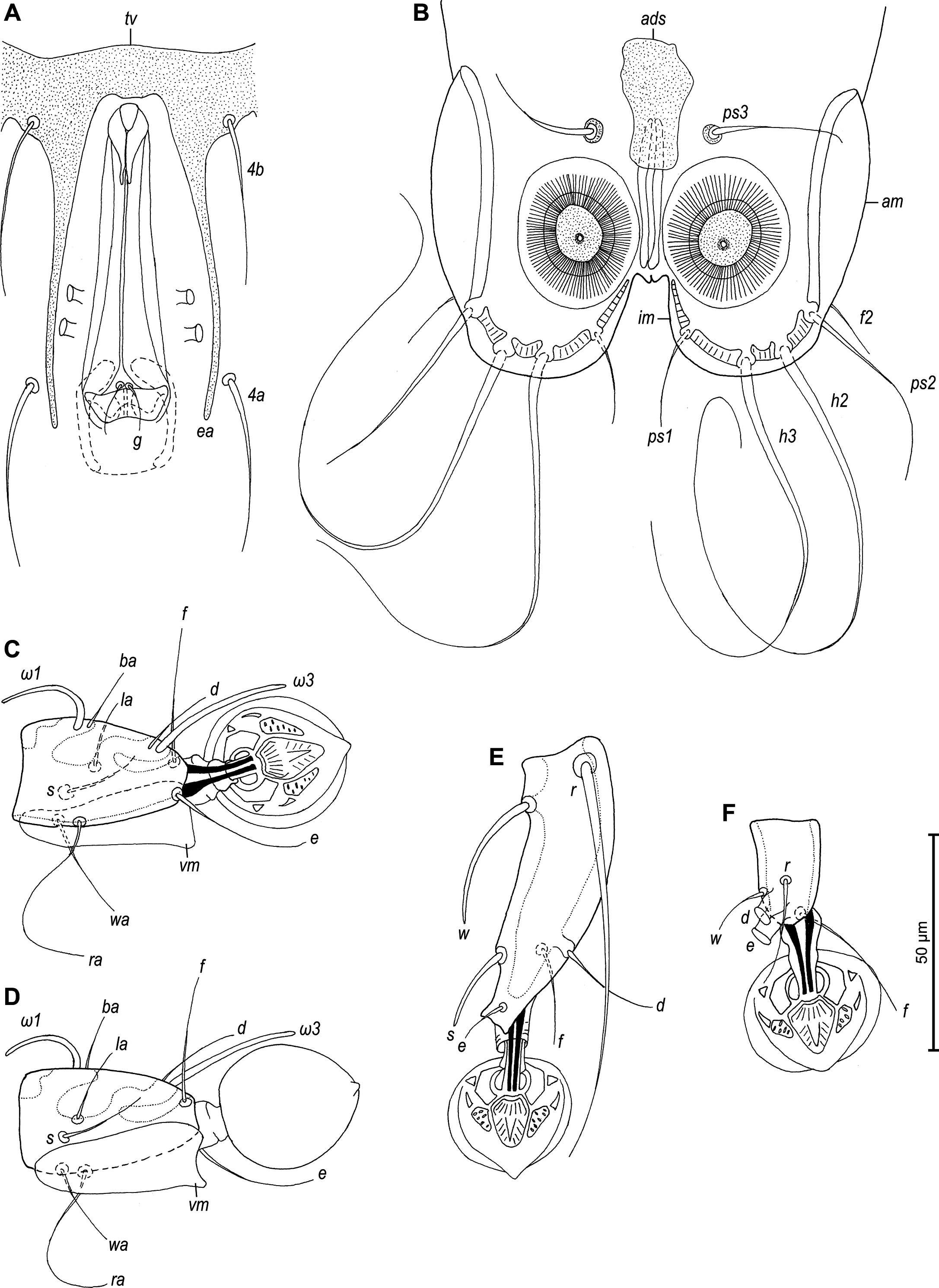
Male — (holotype, range for 5 paratypes in parentheses) (Figures 1, 2, 5 and 6). Length of idiosoma from anterior end to bases of setae h3 415 (400–430), greatest width at level of humeral shields 185 (180–200). Length of hysterosoma from sejugal furrow to bases of setae h3 260 (262–280). Prodorsal shield length along midline 145 (140–148), greatest width in posterior part 150 (140–150), lateral margins not fused with scapular shields, with anterolateral extensions produced laterally between bases of legs I, II, surface without ornamentation (Figure 1A). Internal scapular setae si filiform, 23 (17–27) long, separated by 75 (73–80); external scapular setae se situated near lateral margins of prodorsal shield, separated by 100 (89–106). External vertical setae ve minute. Humeral shield with setae c2 lanceolate, 39 (33–43) long. Setae c3 narrowly lanceolate, with acute apex, 23 (22–25) long. Dorsal hysterosoma with prohysteronotal and lobar shields completely separated. Prohysteronotal shield length 195 (183–200), width at anterior margin 133 (130–138), lateral margins with shallow incisions at level of trochanters III, margins of these incisions without sclerotized patch. Length of lobar shield excluding lamellae 78 (75–84). Apical parts of opisthosomal lobes separated by narrow terminal cleft, length of this cleft from anterior end to apices of lamellae 30 (30–33), width in anterior part 8 (8–12). Lamellae ovate in general shape, their margins with 11–12 rounded festoons, length from bases of setae h3 to lamellar apices 15 (17–18). Setae h1 anterior to setae h2. Distance between dorsal setae: c2-e1 90 (93–99), d2-e2 90 (90–92), e2-h2 63 (67–77), h2-h3 17 (15–18), h2-h2 37 (38–43), h3-h3 32 (30–32).
Epimerites I free. Rudimentary sclerites rEpIIa present, with irregular form. Genital apparatus situated between levels of trochanters III and IV, length including epiandrum and basal sclerite 42 (42–47), greatest width 20 (20–25) (Figure 1B). Epiandrum small ovate. Setae g filiform and relatively short, not touching at bases, distant from base of genital apparatus; genital shield and postgenital plaque absent. A pair of minutes paragenital sclerites (derivatives of epimerites IVa) at level of basal sclerite. Aedeagus long, extending to ends of parameres, parameres without denticles and other processes on distal ends (Figure 2E). Anterior and posterior genital papillae similar in size and at the same distance from midline. Adanal apodemes heavily sclerotized, with wide lateral membrane, without apophyses. Translobar apodeme present. Adanal shields absent, setae ps3 inserted on soft tegument anterior to adanal suckers. Adanal suckers 12 (12–13) in diameter. Anterior ends of epimerites IIIa reaching level of setae 4b. Epimerites IVa present, wide, anterior ends slightly exceeding level of setae 4a, with narrow extension of irregular form on anterior end. Setae 4b situated slightly anterior to level of setae 3a, setae g and 4a situated approximately at same transverse level. Distance between ventral setae: 4b-3a 35 (33–37), 4b-g 78 (77–80), g-ps3 73 (68–72), ps3-h3 73 (70–75).
Setae sR of trochanters III long filiform, 28 (22–25), setae cG of genua I, II long filiform. Tibia IV with apical thorn-like process at base of solenidia φ, tarsus IV 40 (40–43) long, modified setae d and e barrel-shaped, both with discoid cap, situated subapically (Figures 2D and 6C, D). Legs IV with ambulacral disc extending to level of posterior margin of terminal lamellae.



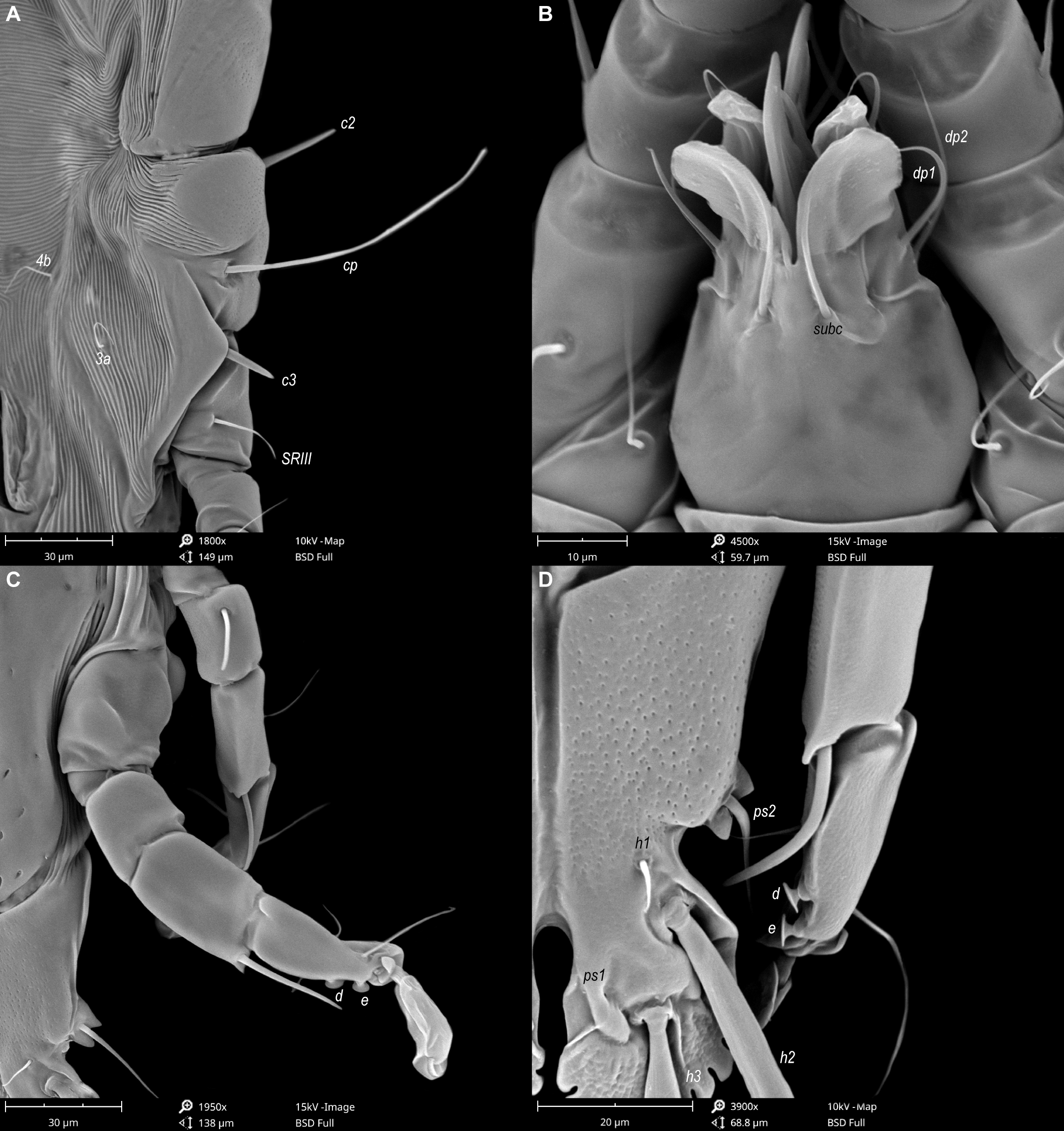




Female — (range for 5 paratypes). (Figures 3, 4 and 7). Length of idiosoma from anterior end to apices of lamellar lobar processes 490–510, greatest width 190–205. Length of hysterosoma from sejugal furrow to apices of lamellar lobar processes 340–355. Prodorsal shield shaped as in male, 143–150 in length, 150–163 in width, surface without ornamentation. Setae si filiform, 20–25 long, separated by 84–87, setae se situated near lateral margins of prodorsal shield, separated by 107–110. Humeral shields with setae c2 spiculiform, 37–42 long. Setae c3 narrowly lanceolate, 23–28 in length. Hysteronotal shield: length from anterior margin to bases of setae h3 300–310, width at anterior margin 140–150; lateral margins with shallow incisions at level of trochanters III, margins of these incisions without sclerotized patch; surface between levels of setae e1 and d1 with small ovate lacunae in median part levels, and between levels of setae e1 and e2 with big ovate lacunae in median part and with a few small ovate lacunae in lateral parts (Figures 3A and 7A, B). Setae h1 filiform, 7–8 long, situated anteromesal to bases of setae h2, 17–20 from each lateral margin of hysteronotal shield. Setae ps1, equidistant from outer and inner margins of opisthosomal lobes, closer to bases of setae h3 than to bases of setae h2. Width of opisthosoma at level of the setae h2 84–87. Distance from bases of setae h3 to membranous apices of lobes 33–42. Setae f2 present. Supranal concavity open. Terminal cleft as an inverted U, length 125–134, width of cleft at level of setae h3 17–22. Interlobar membrane occupying anterior 1/5 of terminal cleft, distance from free margin of membrane to membranous lobar apices 80–84. External copulatory tube represented by small conical extension on free margin of interlobar membrane, 2–3 long, primary spermaduct guide and basal guide of external copulatory tube absent. Spermatheca with secondary spermaducts 28–32 long (Figure 4E). Distance between dorsal setae: c2-d2 85–97, d2-e2 122–150, d1-d2 43-50, e2-h2 33–38, h2-h3 53–58, h2-h2 67–72, h3-h3 35–42, e1-e2 84–87, h1-h2 15–22, h1-h1 38–47, ps1-h3 8–10.
Epimerites I free. Epigynum 33–37 in length, 85–97 in width (Figure 3B). Epimerites IVa present, short. Setae sR of trochanters III filiform, 22–35 long. Legs IV with ambulacral discs extending to level of setae h3.
Etymology
The name of the species is derived from the common English name of the host family (Pycnonotidae).
Differential diagnosis
The new species described herein cannot be referred to any of 11 species groups previously established in the genus Trouessartia (Mironov 2023), because of having a unique combination of characters. The new species of Trouessartia is closest to Trouessartia latisetata Gaud, 1952 from host Ixocincla madagascariensis (Pycnonotidae). Both species of these species have dorsal shields with similar ornamentation; males have the terminal lamellae denticulate, and tibia IV with apical thorn-like process at base of solenidion φ; and females have setae h1 filiform. Trouessartia bulbuli differ from T. latisetata in having the following characters: in males, the hysteronotal shield is completely separated into the prohysteronotal and lobar shields, anterior ends of epimerites IVa are separated in small sclerites at base of basal sclerite of genital apparatus; in females, the external copulatory tube shaped as a short projection extending into terminal cleft. In males of T. latisetata, the hysteronotal shield is entire and its prohysteronotal and lobar parts are delimited from each other by small lateral incisions posterior to setae e2, epimerites IVa are entire and extended to the level of basal sclerite, and in females, the external copulatory tube is absent.
Family Pteronyssidae Oudemans, 1941
Genus Pteroherpus Gaud, 1981
Pteroherpus guangdongensis Constantinescu, sp. nov.
ZOOBANK: 99E03741-C90F-4B8C-A42A-D6F02170A484 ![]()
(Figures 8–13)
Type material
From Hemixos castanonotus Swinhoe (Passeriformes: Pycnonotidae): male holotype (ANA1877), 4 male (ANA1874–ANA1876, ANA1878) and 5 female (ANA1879–ANA1883) paratypes, in MGAB collection; 5 male (GD-ACARI-49–53) and 5 female (GD-ACARI-54–58) paratypes, in IZGAS collection; CHINA, Guangdong Province, Dinghu District, Dinghushan National Nature Reserve, 22°09′34″N, 112°32′17″E, 17 March 2019; coll. C. Adam.
Description







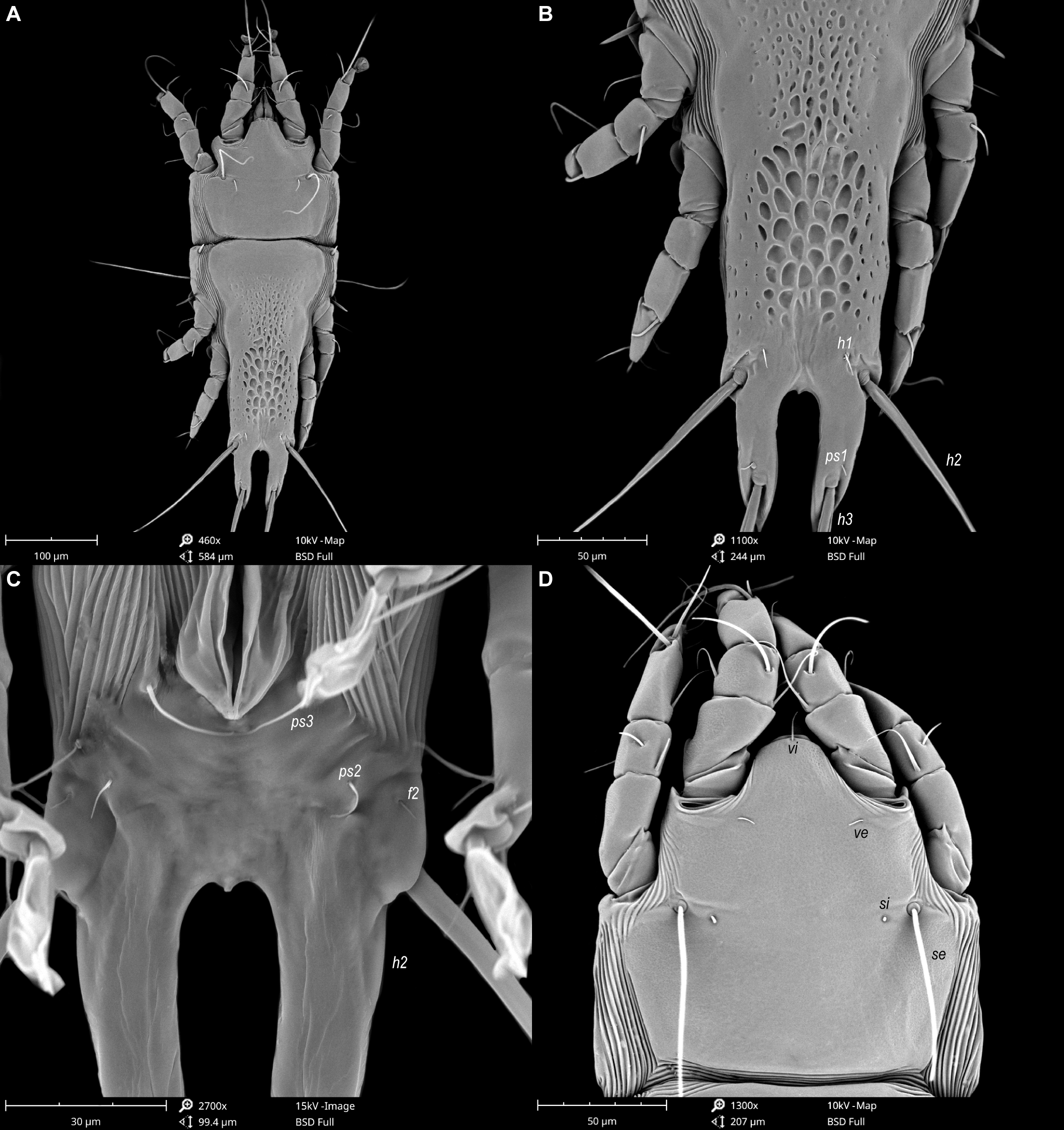
Male — (holotype, range measurements for 4 paratypes in parentheses) (Figures 8, 9, 11 and 12). Idiosoma 390 long (375–405), 195 wide (185–205). Prodorsal shield with posterolateral angles not expressed, lateral margins with small incisions posterior to bases of setae se, posterior margin almost straight, except central part with two small convexities, 100 long (112–125) and 92 (95–105) wide, surface with granular ornamentation, distance between setae se–se 84 (80–88). Setae c2 filiform 30 (23–30) long, setae c3 lanceolate 37 (37–47) long. Prodorsal and hysteronotal shields separated by large area with transverse striae, distance between this shields along midline 44 (38–50). A pair of small additional sclerites posterior to prodorsal shield present. Hysteronotal shield entire, with convex anterior margin, not encompassing bases of setae c1, with blunt-angular angles, sharp anterior angles, greatest length 208 (215–225), width at anterior margin 120 (113–125). Opisthosomal lobes short and rounded, terminal cleft small U–shaped, 25 (20–23) long. Supranal concavity opened posteriorly, anterior end extending beyond level of setae e2. Posterior and inner margins of lobes with narrow entire membrane, width of opisthosoma at level of setae f2 88 (79–85). Dorsal setae e1 situated posterior to openings gl. Lengths of dorsal setae: d2 18 (22–27), e2 17 (22–25). Dorsal measurements: c2-d2 80 (75–84), d2-e2 100 (100–104), d2-gl 28 (30–32), gl-e1 18 (18–25), e2-h3 52 (48–57), e2-e2 68 (65–75), h2-h2 65 (60–68), h3-h3 49 (42–50), ps2-ps2 82 (73–85). Transventral sclerite with anterior margins slightly convex in median part, and fused with epiandrum, length along midline 20 (21–23). Branches of epiandrum long, completely encompassing genital apparatus laterally; tips of branches thin, extending beyond level of setae 4a. Genital apparatus very long, 77 (75–87) in length, 28 (20–25) in width, aedeagus 19 (17–23) in length. Setae 4a and g approximately at same level. Diameter of anal suckers 25 (20–22). Adanal shield as longitudinal plate of irregular shape. Adanal membranes well developed. Ventral measurements: 4b-4a 63 (62–72), 4b-g 70 (72–80), g-ps3 77 (72–78), ps3-ps3 48 (32–47), ps3-h3 55 (55–62). Tarsus III 68 (70–78) long, with bidentate apex, seta r slightly longer than segment (Figures 9E and 12C). Tarsus IV shorter than tibia IV, setae d and e with apical caps, close to each other, situated near apex of segment (Figures 9F and 12D).






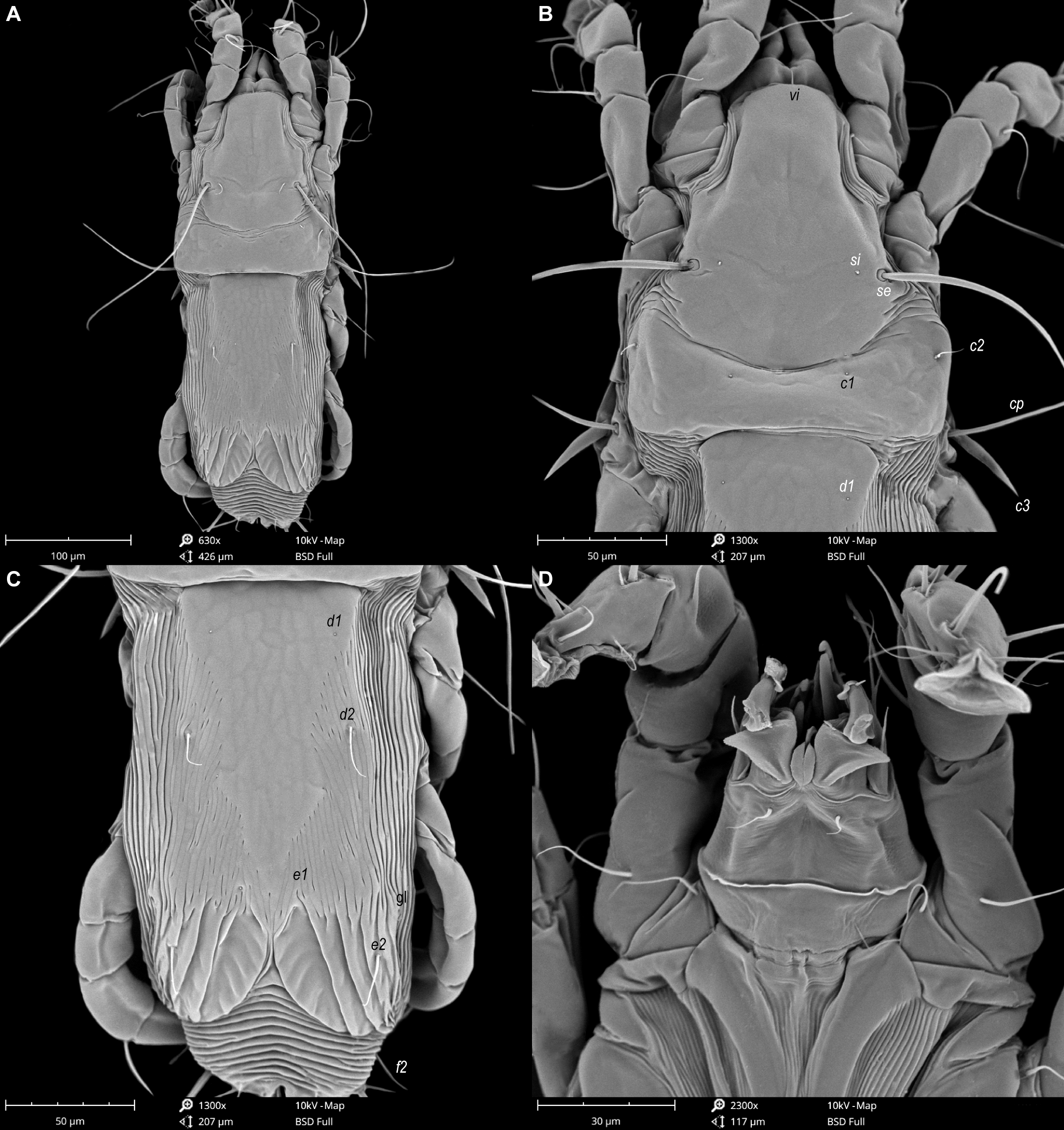


Female — (range measurements for 5 paratypes) (Figures 10 and 13). Idiosoma 420–440 long, 183–210 wide. Prodorsal shield as in male, except posterior margin being strongly convex, 125–138 long, 103–113 wide, distance se:se 80–90. Setae c2 filiform, 23–32 long; subhumeral seta c3 lanceolate, slightly curved, longer than trochanter III. Arrangement of hysteronotal shields: unpaired anterior hysteronotal sclerite, central sclerite, a pair of lateral opisthosomal sclerites and a pair of pygidial sclerites. Anterior hysteronotal sclerite represented by transverse plate narrowed in median part, with all margins irregular, with anterior margin strongly concave, encompassing bases of setae c1, c2. Anterior hysteronotal sclerite and central sclerite separated by relatively narrow band of soft tegument with five striae. Central sclerite of roughly triangular form (Figures 10A and 13C), anterior margin straight, lateral unevenly sinuous, length along midline 120–133 and width at anterior margin 70–83; setae d1 on anterior part of this sclerite; setae d2 and e1 off this sclerite, at level of its midlength and at posterior tip, respectively. Lateral opisthosomal sclerites represented by plates of irregular form, bearing setae e2, their surface with 5 oblique striae. Hysteronotal gland openings gl situated on striated tegument near anterolateral margins of opisthosomal sclerites. Pygidial sclerites small, roughly triangular in shape, encompassing bases of setae h2, h3 and ps1; seta f2 inserted laterally, on soft tegument. Dorsal measurements: c2-d2 113–125, d2-e2 100–110, e2-h3 65–78, d2-gl 78–90, e1-gl 45–53, h2-ps1 20–22, h2-h2 75–80, h3-h3 53–67.
Etymology
The specific name guangdongensis refers to the Guangdong Province, where the mite was collected.
Differential diagnosis
The new species, Pteroherpus guangdongensis sp. nov., belongs to the hoplophorus species group (Mironov and Wauthy 2008) and is closest to P. meghalayensis Constantinescu, 2014 described from the ashy bulbul, Hemixos flavala Blyth (Passeriformes: Pycnonotidae), in India. Males of both species have a strongly elongated genital apparatus (3-4 times longer than wide) and the epiandrum with long branches, and in females obtain the same arrangement of the hysterosomal shield sclerites (unpaired anterior hysteronotal sclerite as transverse plate, central sclerite, lateral opisthosomal sclerites and pygidial sclerites). The male of Pteroherpus guangdongensis is distinguished from P. meghalayensis in the following features: small additional dorsal sclerites in the sejugal area are present and the adanal shield is represented by a longitudinal plate of irregular shape. In the male of P. meghalayensis dorsal additional sclerites are absent and the adanal shield is T-shaped. In females of Pteroherpus guangdongensis, the opening gl are off the lateral opisthosomal sclerites, setae ps3 extending to posterior margin of opisthosoma, and setae 4a very short, shorter than trochanters III or IV. In female of P. meghalayensis opening gl are on margins of opisthosomal sclerites, setae ps3 extending far beyond the posterior margin of opisthosoma, setae 4a are long, longer than trochanters III or IV.
Acknowledgements
The present study was carried out in strict accordance with the Regulation for the Administration of Laboratory Animals (Decree No. 2, State Science and Technology Commission of the People's Republic of China, 14 November 1988). We obtained approval for the study from the Guangdong Institute of Zoology's (formerly Guangdong Institute of Applied Biological Resources) Administrative panel on Laboratory Animal Care. Moreover, we obtained permission from the Forestry Bureau of the Dinghu District, Guangdong Province, and the administrative staff of the Nature Reserve for catching birds within Dinghu National Nature Reserve. This study was supported by grant GIABR-GJRC201701 from the Introduction of Full-Time High-Level Talent Fund of the Institute of Zoology, Guangdong Academy of Sciences, grant 2019QN01N968 from the Pearl River Talent Recruitment Program of Guangdong Province, grant QN20200130012 from the Foreign Young Talent Plan, and grant 31961123003 from the National Natural Science Foundation of China. Gabriel Bogdan Chișamera was supported by project no. RO1567-IBB04/2023 from the Institute of Biology Bucharest of Romanian Academy. We thank the anonymous reviewers for their helpful comments on this manuscript.
References
- Arlott N. 2017. Birds of South-East Asia. London: William Collins. pp. 448.
- Carleton R.E., Proctor H.C. 2010. Feather mites associated with eastern bluebirds (Sialia sialis L.) in Georgia, including the description of a new species of Trouessartia (Analgoidea: Trouessartiidae). Southeast. Nat., 9: 605-623. https://doi.org/10.1656/058.009.0317
- Černý V. 1979. Feather mites (Sarcoptiformes, Analgoidea) of some warblers from Czechoslovakia. Folia Parasitol., 26: 81-84.
- Černý V., Lukoschus F.S. 1975. Parasitic mites of Surinam. XXXIII. Feather mites (Analgoidea). Stud. Fauna Suri. and other Guy., 15: 184-203. https://doi.org/10.1007/978-94-017-7106-1_3
- Clements J.F., Schulenberg T.S., Iliff M.J., Fredericks T.A., Gerbracht J.A., Lepage D., Billerman S.M., Sullivan B.L., Wood C.L. 2022. The eBird/Clements checklist of Birds of the World. Available from: https://www.birds.cornell.edu/clementschecklist/download/.
- Constantinescu I.C., Chişamera G.B., Pocora V., Stanciu C., Adam C. 2013. Two new species of feather mites (Acarina: Analgoidea) from the moustached warbler, Acrocephalus melanopogon (Passeriformes, Acrocephalidae), in Romania. Zootaxa, 3709: 267-276. https://doi.org/10.11646/zootaxa.3709.3.5
- Constantinescu I.C., Cobzaru I., Mukhim D.K.B., Adam C. 2016a. Two new species of the genus Trouessartia (Acari, Trouessartiidae) from laughingthrushes (Passeriformes, Leiothrichidae). ZooKeys, 571: 59-79. https://doi.org/10.3897/zookeys.571.7724
- Constantinescu I.C., Cobzaru I., Mukhim D.K.B., Adam C. 2016b. Two new species of the feather mite genus Trouessartia (Acari: Trouessartiidae) in Asia. Zootaxa, 4137: 357-374. https://doi.org/10.11646/zootaxa.4137.3.4
- Constantinescu I.C., Cobzaru I., Geamana N.A., Mukhim D.K.B., Adam C. 2017. Two new species of feather mites (Acarina: Psoroptidia) from the blue-throated blue flycatcher, Cyornis rubeculoides (Passeriformes: Muscicapidae). J. Nat. Hist., 5(5-6): 277-297. https://doi.org/10.1080/00222933.2017.1280194
- Constantinescu I.C., Chişamera G., Petrescu A., Adam C. 2018a. Two new species of feather mites (Acarina: Psoroptidia) from the Oriental Magpie Robin, Copsychus saularis (Passeriformes: Muscicapidae). Acarologia, 58: 313-331. https://doi.org/10.24349/acarologia/20184244
- Constantinescu I.C., Popa O.P., Popa L.O., Cobzaru I., Mukhim D.K.B., Adam C. 2018b. A new feather mite species of the genus Trouessartia Canestrini, 1899 (Acarina, Trouessartiidae) - an integrative description (morphology and DNA barcoding data). ZooKeys, 789: 19-35. https://doi.org/10.3897/zookeys.789.27829
- Constantinescu I.C., Chişamera G., Motoc R., Gustafsson D.R., Zou F., Chu X.-Z., Adam C. 2021. Two new species of feather mites (Acarina: Psoroptidia) from the Huet's fulvetta, Alcippe hueti (Passeriformes: Leiothrichidae), in China. Syst. Appl. Acarol., 26 (1): 146-165. https://doi.org/10.11158/saa.26.1.9
- Constantinescu I.C., Chișamera G.B., Mukhim D.K.B., Adam C. 2014. Two new feather mite species of the family Pteronyssidae (Acarina: Analgoidea) from Meghalaya (Northeast India). Zootaxa, 3774(4): 351-366. https://doi.org/10.11646/zootaxa.3774.4.4
- Constantinescu I.C., Chişamera G.B., Gustafsson D.R., Zou F., Chu X.-Z., Adam C. 2019. Two new species of feather mites genus Pteroherpus Gaud, 1918 (Analgoidea, Pteronyssidae) from China. Syst. Appl. Acarol., 24(10): 1851-1867. https://doi.org/10.11158/saa.24.10.5
- Faccini J.L., Atyeo W.T. 1981. Generic Revision of the Pteronyssinae and Hyonyssinae (Analgoidea: Avenzoariidae). Proc. Acad. Nat. Sci. Philadelphia, 133: 20-72.
- Gaud J. 1977. La faune terrestre de l′Ile de Sainte-Hélène. Acariens Sarcoptiformes plumicoles parasites d'oiseaux. Ann. Mus. Roy. Afr. Centr., 220: 260-269.
- Gaud J., Atyeo W. T. 1986. Les Trouessartia (Analgoidea, Trouessartiidae) parasites des hirondelles de 1′Ancien Monde. I. Le groupe appendiculata. Acarologia, 27: 263-274.
- Gaud J., Atyeo W. T. 1987. Les Trouessartia (Analgoidea, Trouessartiidae) parasites des hirondelles de 1′Ancien Monde. II. Le groupe minutipes. Acarologia, 28: 367-379.
- Gaud J., Atyeo W.T. 1996. Feather mites of the world (Acarina, Astigmata): the supraspecific taxa. Ann. Mus. Roy. Afr. Centr., 277: 1-193 (Part 1, text), 1-436 (Part 2, illustrations).
- Griffiths D.A., Atyeo W.T., Norton R.A., Lynch C.A. 1990. The idiosomal chaetotaxy of astigmatid mites. J. Zool., 220: 1-32. https://doi.org/10.1111/j.1469-7998.1990.tb04291.x
- Han Y.D., Mironov S.V., Min G.S. 2019a. Two new feather mites (Acari: Analgoidea) isolated from the grey-headed woodpecker, Picus canus (Piciformes: Picidae) in Korea. Syst. Appl. Acarol., 24: 2167-2183. https://doi.org/10.11158/saa.24.11.9
- Han Y.D., Min G.S. 2019b. Three feather mites (Acari: Sarcoptiformes: Astigmata) isolated from Tringa glareola in South Korea. J. Species Res., 8(2): 215-224.
- Hernandes F.A. 2014. Five new species of the feather mite genus Trouessartia Canestrini from South America (Acari: Trouessartiidae). Zootaxa, 3856 (1): 50-72. https://doi.org/10.11646/zootaxa.3856.1.2
- Hernandes F.A. 2017. Two new species of Trouessartia Canestrini 1899 (Astigmata: Trouessartiidae) from passeriform birds in Brazil. Syst. Parasitol., 94: 1019-1032. https://doi.org/10.1007/s11230-017-9755-z
- Hernandes F.A. 2022. Three new feather mite species (Acariformes: Proctophyllodidae, Trouessartiidae) from tyrant flycatchers (Passeriformes: Tyrannidae) in Brazil. Syst. Parasitol., 99 (2): 139-140. https://doi.org/10.1007/s11230-022-10026-8
- Hernandes F.A. 2023. Feather mites (Acariformes: Astigmata) from the yellow-rumped cacique, Cacicus cela (Linnaeus, 1758) (Passeriformes: Icteridae) in Brazil, with description of four new species. J. Nat. Hist., 57(1-4): 257-284. https://doi.org/10.1080/00222933.2023.2174459
- Hernandes F.A., OConnor B.M. 2017. Out of Africa: the mite community (Arachnida: Acariformes) of the Common Waxbill, Estrilda astrild (Linnaeus, 1758) (Passeriformes: Estrildidae) in Brazil. Parasit. Vectors, 10: 299 (1-19). https://doi.org/10.1186/s13071-017-2230-5
- Hernandes F.A., Valim M.P. 2015. A new species of the genus Trouessartia Canestrini (Acari: Trouessartiidae) from Neotropical passerines (Aves: Tyrannidae). Internat. J. Acarol., 41: 382-388. https://doi.org/10.1080/01647954.2015.1046921
- Hernandes F.A., Barbosa B.B., Ubaid F.K. 2022. A new feather mite of the genus Trouessartia Canestrini, 1899 (Acariformes: Trouessartiidae) from the bran-colored flycatcher, Myiophobus fasciatus (Muller PLS, 1776) (Passeriformes: Tyrannidae), in Brazil. Internat. J. Acarol., 48 (4-5): 382-386. https://doi.org/10.1080/01647954.2022.2080259
- Mauri R., de Alzuet A.B. 1968. Una nueva especie de ''Trouessartia Canestrini» 1899 (''Acarina: Proctophyllodidae″). Rev. Mus. La Plata, Secc. Zool., 10 (85): 169-172.
- Mironov S.V. 1983. Feather mites of the genus Trouessartia of the USSR fauna and descriptions of new species (Analgoidea). Parazitologiya, 17: 361-369. [In Russian with English summary]
- Mironov S.V. 1992. Five new species of the feather mite genus Pteroherpus Gaud (Analgoidea: Avenzoariidae) from passerine birds of Vietnam. Internat. J. Acarol., 18: 257-268. https://doi.org/10.1080/01647959208683958
- Mironov S.V. 2003. On some problems in the systematics of feather mites. Acarina, 11: 3-29.
- Mironov S.V. 2011. Pteroherpus surmachi sp. n., first record of the feather mite family Pteronyssidae (Acari: Analgoidea) from nuthatches (Passeriformes: Sittidae). Proc. Zool. Inst. Russ. Acad. Sci., 315: 452-460. https://doi.org/10.31610/trudyzin/2011.315.4.452
- Mironov S.V. 2021a. A new species of the feather mite genus Trouessartia (Acariformes: Trouessartiidae) from the Tristram's bunting Emberiza tristrami (Passeriformes: Emberezidae) in the Russian Far East. Acarina, 29(1): 35-42. https://doi.org/10.21684/0132-8077-2021-29-1-35-42
- Mironov S.V. 2021b. Two new species of the feather mite genus Trouessartia (Acariformes: Trouessartiidae) from robins and chats (Passeriformes: Muscicapidae) in the Russian Far East. Acarina, 29 (2): 155-167. https://doi.org/10.21684/0132-8077-2021-29-2-155-167
- Mironov S.V. 2022. Notes on systematics of the feather mite genus Trouessartia Canestrini, 1899 (Acariformes: Trouessartiidae) with an updated world checklist. Acarina, 30(2): 157-180. https://doi.org/10.21684/0132-8077-2022-30-2-157-180
- Mironov S. V., Bermúdez S. 2017. Feather mites (Acari: Analgoidea) associated with the Hairy Woodpecker Leuconotopicus villosus (Piciformes: Picidae) in Panama. Acarologia, 57 (4): 941-955. https://doi.org/10.24349/acarologia/20174218
- Mironov S.V., Chandler C.R. 2020. Feather mites of the genus Trouessartia (Acariformes: Trouessartiidae) from passerines (Aves: Passeriformes) in Georgia, USA. Zootaxa, 4860 (1): 1-54. https://doi.org/10.11646/zootaxa.4860.1.1
- Mironov S.V., Galloway T.D. 2002. New feather mite taxa (Acari: Analgoidea) and mites collected from native and introduced birds of New Zealand. Acarologia, 42: 185-201.
- Mironov S.V., Galloway T.D. 2019. Feather mites of the genus Trouessartia Canestrini (Acariformes: Trouessartiidae) from swallows (Passeriformes: Hirundinidae) in Canada. Zootaxa, 4568 (1): 1-39. https://doi.org/10.11646/zootaxa.4568.1.1
- Mironov S.V., González-Acuña D.A. 2013. Anew feather mite species of the genus Trouessartia Canestrini, 1899 (Acariformes: Trouessartiidae) from the White-crested Elaenia Elaenia albiceps (Passeriformes: Tyrannidae) in Chile. Acarina, 21(2): 123-132.
- Mironov S.V., Kopij G. 1996. New feather mite species (Acarina: Analgoidea) from some starlings (Passeriformes: Sturnidae) of South Africa. J. Afr. Zool., 110 (4): 257-269.
- Mironov S.V., Kopij G. 2000a. New feather mites species of the genus Trouessartia (Analgoidea: Trouessartiidae) from South African passerines (Aves: Passeriformes). Mitt. Hambg. Zool. Mus. Inst., 97: 99-115.
- Mironov S.V., Kopij G. 2000b. New feather mite species of the family Pteronyssidae (Astigmata: Analgoidea) from South African passerines (Aves: Passeriformes). Folia Parasitol., 47: 319-329. https://doi.org/10.14411/fp.2000.056
- Mironov S.V., Overstreet R.M. 2016. A new feather mite species of the genus Trouessartia Canestrini (Acariformes: Trouessartiidae) from the Northern rough-winged swallow Stelgidopteryx serripennis (Passeriformes: Hirundinidae) in Pennsylvania. Acarina, 24 (2): 3-9. https://doi.org/10.21684/0132-8077-2016-24-2-89-95
- Mironov S.V., Palma R.L. 2016. A new feather mite of the genus Trouessartia Canestrini, 1899 (Acariformes: Trouessartiidae) from the Seychelles magpie-robin, Copsychus sechellarum (Passeriformes: Muscicapidae). Acta Parasitol., 61 (3): 629-635. https://doi.org/10.1515/ap-2016-0084
- Mironov S.V., Proctor H.C. 2011. Four new feather mite species of the family Pteronyssidae (Astigmata: Analgoidea) from Laughing-Thrushes (Passeriformes: Timaliidae) in China. Acarina, 19: 35-51.
- Mironov S.V., Santillán M.A., Liébana M.S. 2021. Two new feather mites of the genus Trouessartia Canestrini, 1899 (Acariformes: Trouessartiidae) from tyrant flycatchers (Passeriformes: Tyrannidae) in Argentina. Syst. Appl. Acarol., 26 (9): 1735-1750. https://doi.org/10.11158/saa.26.9.8
- Mironov S.V., Wauthy G. 2006. Three new species of the feather mite genus Pteroherpus Gaud, 1981 (Astigmata, Pteronyssidae) from the bulbuls (Passeriformes, Pycnonotidae) in Africa. Acta Parasitol., 51: 65-72. https://doi.org/10.2478/s11686-006-0009-5
- Mironov S.V., Wauthy G. 2008. A systematic review of the feather mite genus Pteroherpus Gaud, 1981 (Astigmata: Pteronyssidae). Bull. Inst. R. Sci. Nat. Belg. Entomol., 78: 155-200.
- Mironov S.V., Zabashta A.V. 2022. A new species of the feather mite genus Trouessartia (Acari: Acariformes: Trouessartiidae) from the Cetti's Warbler Cettia cetti (Passeriformes: Cettiidae) in European Russia. Acarina, 30 (1): 13-22. https://doi.org/10.21684/0132-8077-2022-30-1-13-22
- Norton A.R. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Exp. Appl. Acarol., 22: 559-594.
- OConnor B.M. 2009. Cohort Astigmatina. In: Krantz G.W., Walter D.E. (Eds.) A manual of Acarology. 3rd Edition. Lubbock (TX): Texas Tech University Press. p. 565-657.
- OConnor B.M., Foufopoulos J., Lipton D., Lindström K. 2005. Mites associated with the small ground finch, Geospiza fuliginosa (Passeriformes: Emberizidae), from the Galapagos Islands. J. Parasitol., 91: 1304-1313. https://doi.org/10.1645/GE-581R.1
- Santana F.J. 1976. Areview of the genus Trouessartia (Analgoidea: Alloptidae). J. Med. Entomol., Suppl. 1: 1-128. https://doi.org/10.1093/jmedent/13.Suppl1.1
- Wang Z.Y., Fan Q.H. 2010. Psoroptidia (Acari: Astigmatina) of China: a review of research progress. Zoosymposia, 4: 260-271. https://doi.org/10.11646/zoosymposia.4.1.16



2023-02-06
Date accepted:
2023-05-12
Date published:
2023-05-31
Edited by:
Akashi Hernandes, Fabio

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Constantinescu, Ioana Cristina; Chișamera, Gabriel Bogdan; Motoc, Rozalia; Gustafsson, Daniel R.; Zou, Fasheng; Chu, Xingzhi and Costică, Adam
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



