A new species of Lasioseius (Endopodalius) (Acari: Mesostigmata: Blattisociidae) coexistent with the invasive agave weevil (Coleoptera: Dryophthoridae) in the southeast of the Iberian Peninsula
Moraza, Maria L.  1
and Balanzategui, Iñaki
1
and Balanzategui, Iñaki  2
2
1✉ Universidad de Navarra, Facultad de Ciencias, Departamento de Biología Ambiental, Campus Universitario, 31080, Pamplona España.
2Estación Experimental de Zonas Áridas (EEZA-CSIC), Departamento de Ecología Funcional y Evolutiva, 04120, Almería España.
2023 - Volume: 63 Issue: 2 pages: 605-614
https://doi.org/10.24349/vfv7-8tutZooBank LSID: 9267A4D1-3BC9-4138-B203-593CE2314521
Original research
Keywords
Abstract
Introduction
Scyphophorus acupunctatus Gyllenhal, 1838 (Coleoptera: Dryophthoridae) is the most important pest affecting the Agave species. Popularly known as the agave weevil, it is a beetle native to the southwestern United States, Mexico and Central America. It was introduced into the Iberian Peninsula, being first detected in 2007 (Riba i Flinch and Alonso-Zarazaga 2007) and is considered an invasive species (Molina 2013). This beetle feeds exclusively on plants of the Agavaceae and Dracaenaceae families (Ruíz-Montiel et al. 2009), although occasionally on Pachycereus pringlei (Maya et al. 2011).
The larval feeding on the plant tissues, together with its association with different species of bacteria, kills the agave plants (Rubio 2007). This species is present in various countries in Asia, Africa, North and South America, Europe, and Australia. In the Mediterranean region of the Iberian Peninsula, this beetle species has been detected in several locations (Barcelona, Murcia and Alicante) (Molina 2013), and its distribution is currently expanding into the southeast of Spain.
In the first survey of the mites associated with S. acupunctatus, the most abundant and frequent species was a new species of the subgenus Lasioseius (Endopodalius) Christian & Karg (2006), which is described here.
Lasioseius (Endopodalius) Christian & Karg, 2006 currently includes ten species: L. (E.) alter (Vitzthum, 1925), L. (E.) araucariae Hirschmann, 1972, L. (E.) convexus Krantz, 1962, L. (E.) gabrielae Santos & Argolo, 2018, L. (E.) hirschmanni Christian & Karg, 2006, L. (E.) humberti (Athias-Henriot, 1959), L. (E.) prorsoperitrematus Abo-Shnaf, Sánchez & Moraes, 2016, L. (E.) scutalis (Banks, 1914), L. (E.) tectus Hyatt, 1964 and L. (E.) vitzthumi Westerboer, 1963 (Christian & Karg, 2006; Moraes et al. 2016; Abo-Shnaf et al. 2016; Argolo et al. 2018), although several other species described under Lasioseius may actually belong to Endopodalius (Moraza and Lindquist 2018). Several species have been reported associated with Coleoptera: L. (E.) tectus on Hololepta humilis Paykull, 1811 (Histeridae) in Venezuela (Hyatt 1964); L. (E.) prorsoperitrematus Abo-Shnaf et al., 2016 associated with Sphenophorus levis Vaurie, 1978 (Curculionidae) in Brasil (Esteca et al. 2020), and L. (E.) scutalis (Banks, 1914) with scarabs (Scarabaeidae) in Brasil (Argolo et al. 2018).
The present paper describes a new species of L. (Endopodalius) phoretic on Scyphophorus acupunctatus.
Material and methods
All mite specimens were adult females, some were removed directly from the beetles using a brush and subsequently preserved in 70% ethanol and other females were found in the ethanol conserving several specimens of S. acupunctatus. Studied specimens were mounted in Hoyer's medium on microscope slides and sealed with GLPT insulating varnish.
Morphological observations, measurements, and illustrations were made using compound microscopes equipped with differential interference contrast and phase contrast optical systems, drawing tubes and stage-calibrated eyepiece micrometers. Setal notation for the idiosoma follows that of Lindquist and Evans (1965) as modified slightly by Lindquist (1994) and adapted for superfamilies of mesostigmatic mites in general by Lindquist et al. (2009). Measurements of structures are given in micrometers (µm) and indicate the ranges among specimens measured. Dorsal shield lengths were taken as midline length from the anterior margin anterior to the bases of vertex setae j1 to the caudal margin posterior to the bases of clunal setae J5. Ventrianal or anal shield lengths were taken midline, from the anterior margin to the posterior edge of the cribrum. Notation for leg and palpal setation follows that of Evans (1963, 1964, 1969); the siglas v-1 and v-2 indicate or encapsulate pseudosymmetrical pairs av-1, pv-1 and av-2, pv-2, respectively. Leg lengths were taken from the base of the coxa to the apex of the tarsus, excluding the pretarsus. Distinction of pore-like structures on the idiosomal integument as either poroids (lyrifissures) or glandular openings (solenostomes), as distinguished morphologically by Athias-Henriot (1969) and physiologically by Krantz and Redmond (1987), was applied. The holotype of L. (Endopodalius) tectus (BMNH(E)1963.10.3.11) was examined given the similarity between it and the specimens described. Images taken at different levels of focus were combined using CombineZ5© program.
Identification of the beetles associated with the mites was made by I. Balanzategui and M.A. Gómez de Dios.
The holotype and some paratypes of the new species are deposited in the Mesofauna Collection in the Estación Experimental de Zonas Áridas (EEZA-CSIC), Departamento de Ecología Funcional y Evolutiva, Almería, Spain; four paratypes are in the Museum of Zoology, University of Navarra (MZUNAV), Pamplona, Spain, and four paratypes females are in the Canadian National Collection of Insects and Arachnids (CNCI), Science & Technology Branch, Agriculture & Agri-Food Canada, Ottawa (Canada).
Taxonomy
Lasioseius Berlese, 1916
Lasioseius Berlese, 1916: 33; Lindquist & Evans, 1965: 46; Christian & Karg, 2006: 105; Moraes et al. 2016: 159.
Diagnosis (adult female). The genus diagnosis of Moraes et al. (2016) was followed.
Lasioseius (Endopodalius) ibericus n. sp.
ZOOBANK: 62A111C3-6F58-4E66-B035-777B0AD04192 ![]()
(Figures 1-5)
Diagnosis
Adult female: idiosomal shielding and gnathosomal elements with dense, fine puncta; anterolateral area of dorsal shield with delineation lines. Dorsal shield usually with a prominent transverse line and groove across entire face behind setae J4 and Z4. Dorsal setae heterogeneous in length and shape: setae j1-j6 and z2-z6 pointed and usually straight; setae z1, s1-s6, r-serie and all opisthonotal setae longer and filiform half their length; j5 and z6 half the length of j6; J4 slightly barbed; setae Z4 more than 2 times as long as J1 with penicillate distal region, Z5 smooth and J5 minute and basally spiculated; seven pairs of R setae and 3-4 pairs of UR setae. Sternal shield densely punctate together with large rounded clear puncta, about 0.7 longer along midline than the narrowest width between coxae II; second pair of presternal plates subtriangular in form, sometimes divided in two.
Description
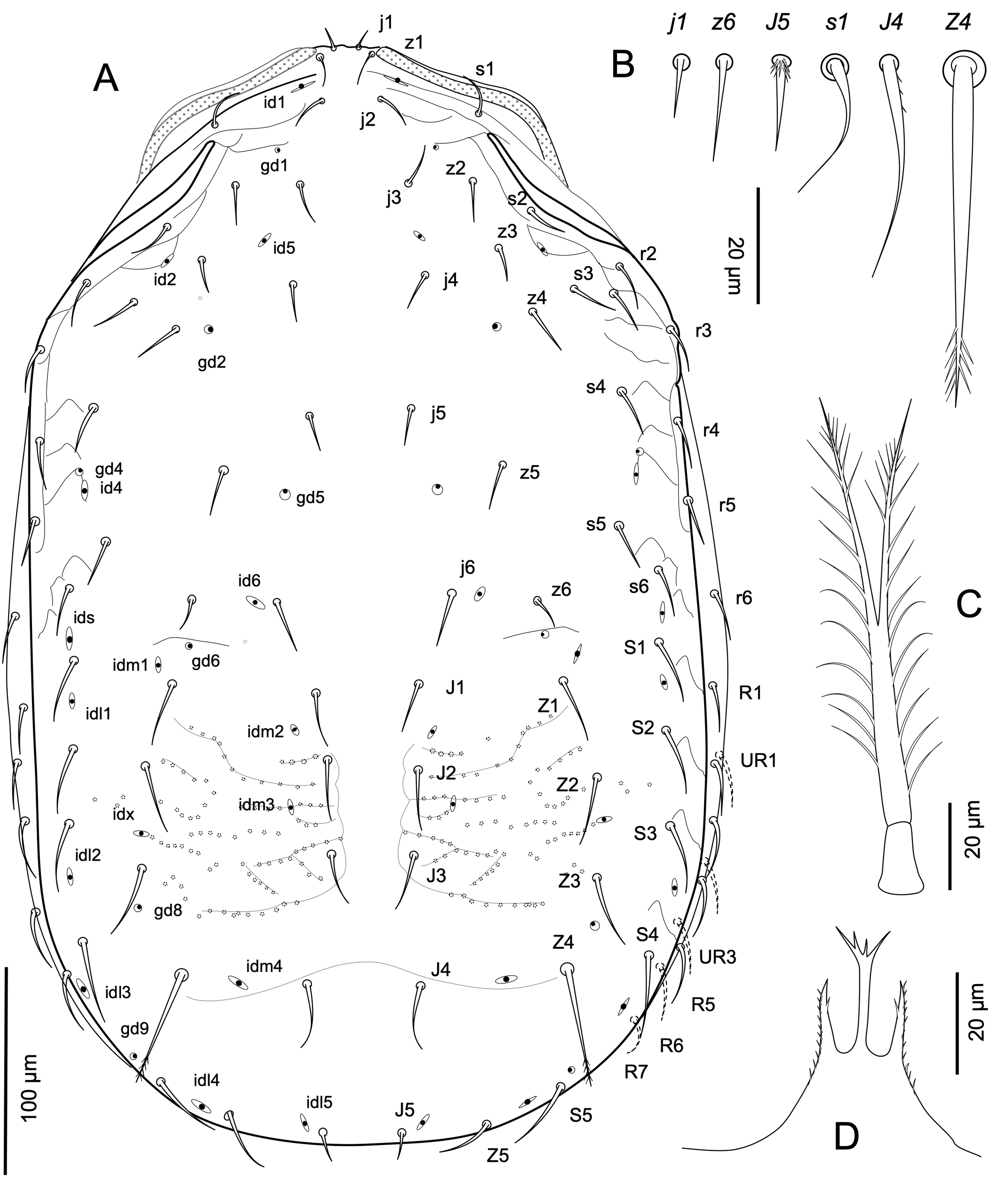


Idiosomatic dorsum — Adult female (Figure 1A). Dorsal shield length 500-573 long, 317-345 wide at its widest at level of setae S2 (n = 7). Dorsal shield with smooth margins, ornamented with dense small puncta over the entire surface, lineated anterolaterad setae j2 to s4, and variably transversely lineate laterally from setae s4 nearly to S4; with ornamental lines anterior to setae j3; shield with a pair of transverse costulae in area of consolidation of podonotal and opisthonotal shields behind setae z6; a light reticula of large puncta in the opisthonotal region between setae J1-J3 and Z1-Z3 and with a more or less prominent transverse line and groove across the entire face behind setae J4 and Z4 posterior to pygidial region of the dorsal plate with dense small puncta. Dorsal shield with 34 pairs of setae, r2 - r5 on shield (r5 sometimes asymmetrically off shield); setae smooth, except J4 scarcely barbed, Z4 distally penicillate, and J5 basally barbed, of dissimilar length, setae z1, s1-s6, r2-r5 and all opisthonotal setae with more finely attenuated tips, and setae j1-j6, z2-z6 without attenuated tips (Figure 1B): j1 (8-11), j2 (17-20), j3-j5 (16-18), j6 (24-30), z2, z3 (16-21), z4, z5 (24-27), z1, z6, s1 (16-19), s2, s3, r2-r5 (20-23), s4, s6 (23-25), s5 second longest (27-31) ; J1 (24-27), J2-J4 (27-30), J5 (10-12); Z1-Z3 (34-39), Z4 longest (54-60) about 1.5-1.6 longer than Z3, Z5 (27-28), S1-S3, S5 (34-37), S4 (41-45). Lateral soft cuticle usually with setae R1-R7, and three or four pairs of UR-setae (21-27). Peritrematal shields reduced to a narrow strip dorsal to peritreme, broadly united with dorsal shield anteriorly at level between setae s1 and z2; peritremes long, sharply angled subapically, and reaching anteriorly to level of setae z1 (Figure 1A). Dorsal shield with complement of 23 pairs of discernible pore-like structures (9 podonotal, 14 opisthonotal), of which seven pairs (four podonotal, three opisthonotal) superficially appear secretory (gland pores) and 16 pairs (five podonotal, 11 opisthonotal) non-secretory (poroids).
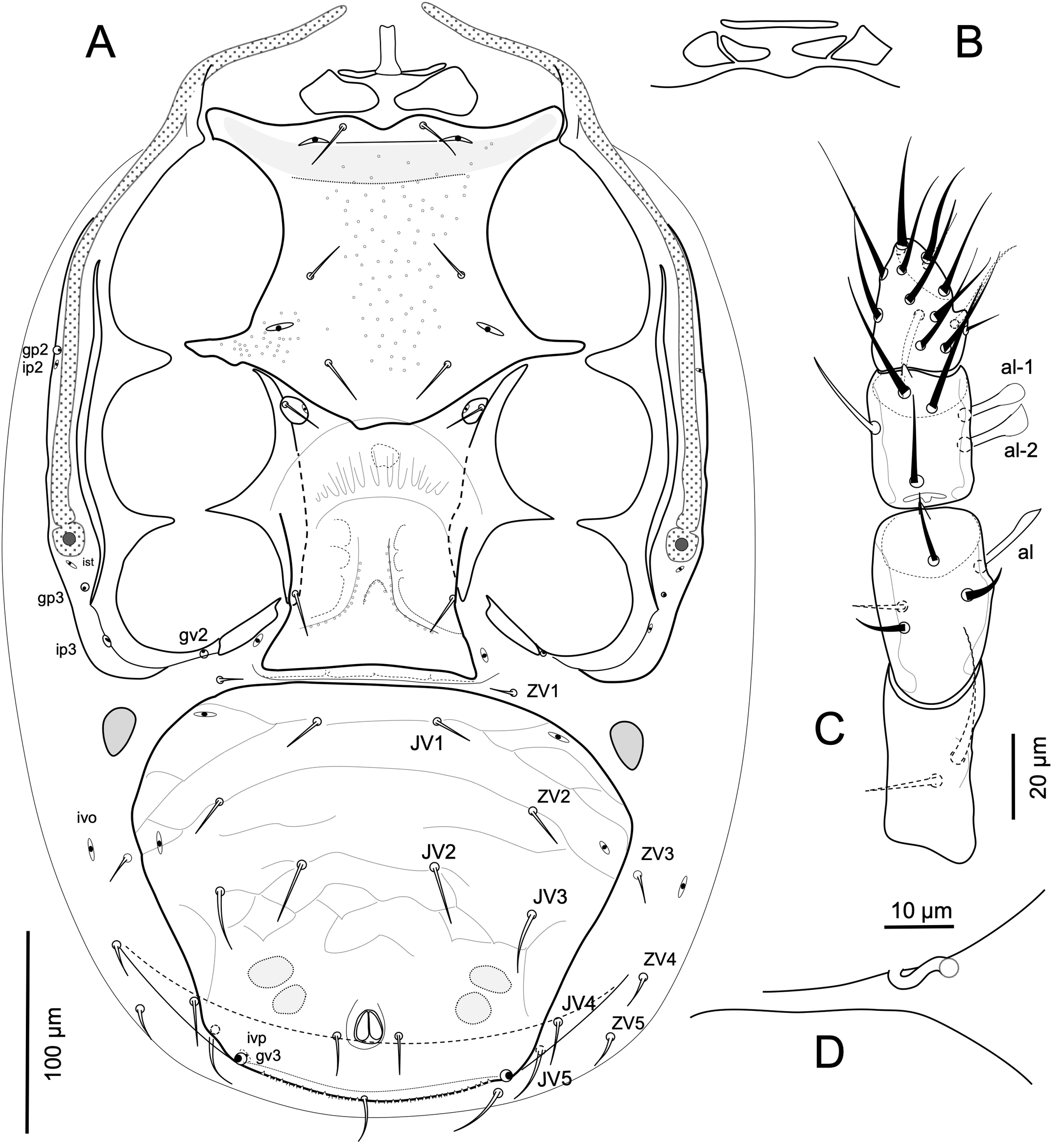


Idiosomatic venter — (Figure 2A). Tritosternum with base about twice as long (16- 18) as basal width (ca. 10), with laciniae fused for about 55 percent of total length (97-101 excluding base), their fused length with long, sparse, lateral setules, and free lengths more finely setulose distally (Figure 1C). Presternal area with a small platelet strip at base of tritosternum, sometimes interrupted medially, and one pair of large subtriangular platelets, 23-27 long 41-48 wide (Figure 2A), sometimes divided into two contiguous platelets (Figure 2B). Sternal shield entire, with strongly developed endopodal extensions between coxae I-II and II-III, and lacking gland pore (gbv); shield moderately elongate, length from anterior margin at level between setae st1 to posterior margin 147-154, narrowest width between legs II 101-110, densely punctate over nearly entire surface, with conspicuous rounded clear puncta in the medial area, and poorly lineate along lateral margins; with a line connecting poroids iv2 and a dark cuticular band behind this line continuing into broad strong endopodal extensions between coxae I and II; shield with anterior margin lobulate between setae st1, and with convex posterior margin extending medially to level between metasternal platelets; shield with strong endopodal extensions between coxae I-II and III; sternal shield with setae st1-st3 similar in their short length (20-23). Setae st4 (20-22) together with poroids iv3 on small metasternal plates. Large endopodal strips alongside coxae III and IV, their anterior ends sometimes touching but not uniting with posterolateral margins of sternal shieldand extending slightly under metasternal platelets. Epigynal shield 124-130 long, 107-111 at its widest at its posterolateral corners, ornamented with small puncta arranged along lateral lines, and with truncate posterior margin; setae st5 subequal in length to st4. Paragenital poroids iv5 on soft cuticle well removed from posterolateral margins of epigynal shield. Two pairs of linear postgenital sclerites present in cuticular fold between epigynal and ventrianal shields. Ventrianal shield broader than long, densely punctate, variably transversely lineated anterolaterally, with large puncta restricted to edges of sparse reticula, and laterad pigydial region with large dense puncta; shield with broadly rounded posterior margin; shield's greatest width (246-265) at level of setae ZV2 about 1.2 its mid-length (209-215); shield with four pairs of opisthogastric setae (JV1-JV3, ZV2) of dissimilar length; JV1, ZV2 (17-19), JV2 (27-31) slightly shorter than ZV3 (31-34); shield with two pairs of poroids, gland pores gv3 on margin posterior to level of anal opening, and a pair of weak preanal depressions; postanal seta (20-24) slightly longer than parianal setae (17-19); posterior shield margin with cribrum formed as a widely extended but narrow strip embedded in postanal fold of cuticle. Soft cuticle with six pairs of opisthogastric setae, ZV1 (9-11) anteriorly, JV4 (19-22), JV5 (27-29), ZV3 (15-18) ZV4-ZV5 (19-24) laterally, flanked variably by R4-R6 and one or two pairs of UR-setae. Peritrematal shield broadly consolidated with exopodal strip curving behind coxa IV, and bearing poroids ip2, gp2 at level of exopodal II-III, ip3 and gp3 behind stigma, and ist next to the stigma; shield free from exopodal elements from middle of coxae II to middle of coxae IV; adcoxal gv2 present. One pair of divided or undivided subtriangular metapodal plates, length 29-38, width 18-23. Spermathecal apparatus with sinuous tubular major duct (c.a. 3 diameter) opening between bases of coxae III and IV (Figure 2D).
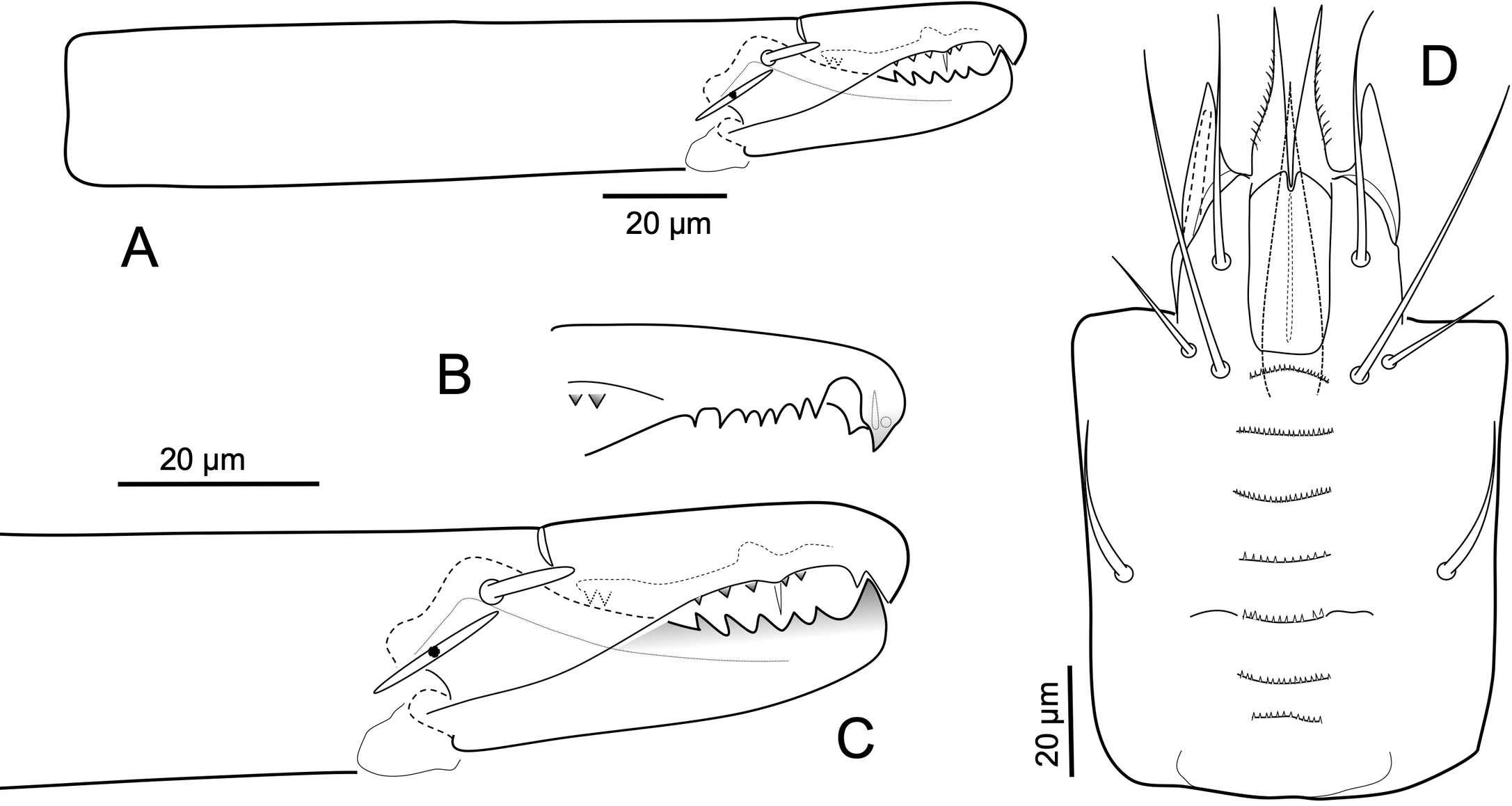


Gnathosoma — Gnathotectum (Figure 1D) with dorsal surface convex, densely punctate, with anterior margin projecting into three elongate, pilose/ciliated tines, medial tine (25-30) longer than lateral ones (15-18). Cheliceral shaft, excluding basal section, 153-159 long, with moderately small digits (Figure 3A); dorsal face of fixed digit with short blunt dorsal seta; fixed digit with minute (3-5) pilus dentilis, with bifid apical hook, with ridge of five to eight and two basal teeth (Figure 3B); movable digit (48-52) with five large teeth (Figure 3C). Corniculi moderately long (33-36), slender (10-12 where inserted), parallel; internal malae tapered (30-38), extending well beyond tips of corniculi and with lateral margins slightly fimbriated or ciliated. Subcapitulum (Figure 3D) with seven finely multidenticulate, similarly wide rows of deutosternal denticles, anteriormost row slightly convex, fifth with short lateral costulae, rows not connected by lateral margins. Subcapitulum with smooth setae; hp1 shorter (44-46) than hp3 (61-68); setae hp2 (22-29), pc (30-35). Palpus (160-170) nearly straight (Figure 2C); palptrochanter with inner seta inserted on protuberance, longer (33-40) and more attenuated than outer seta (17-20); palpfemoral seta al (17-18) with somewhat blunt, somewhat spatulate tip; palpgenual setae al-1 and al-2 spatulate, al-2 with more rounded tip; palptarsal claw with two tines spatulate with rounded tips.
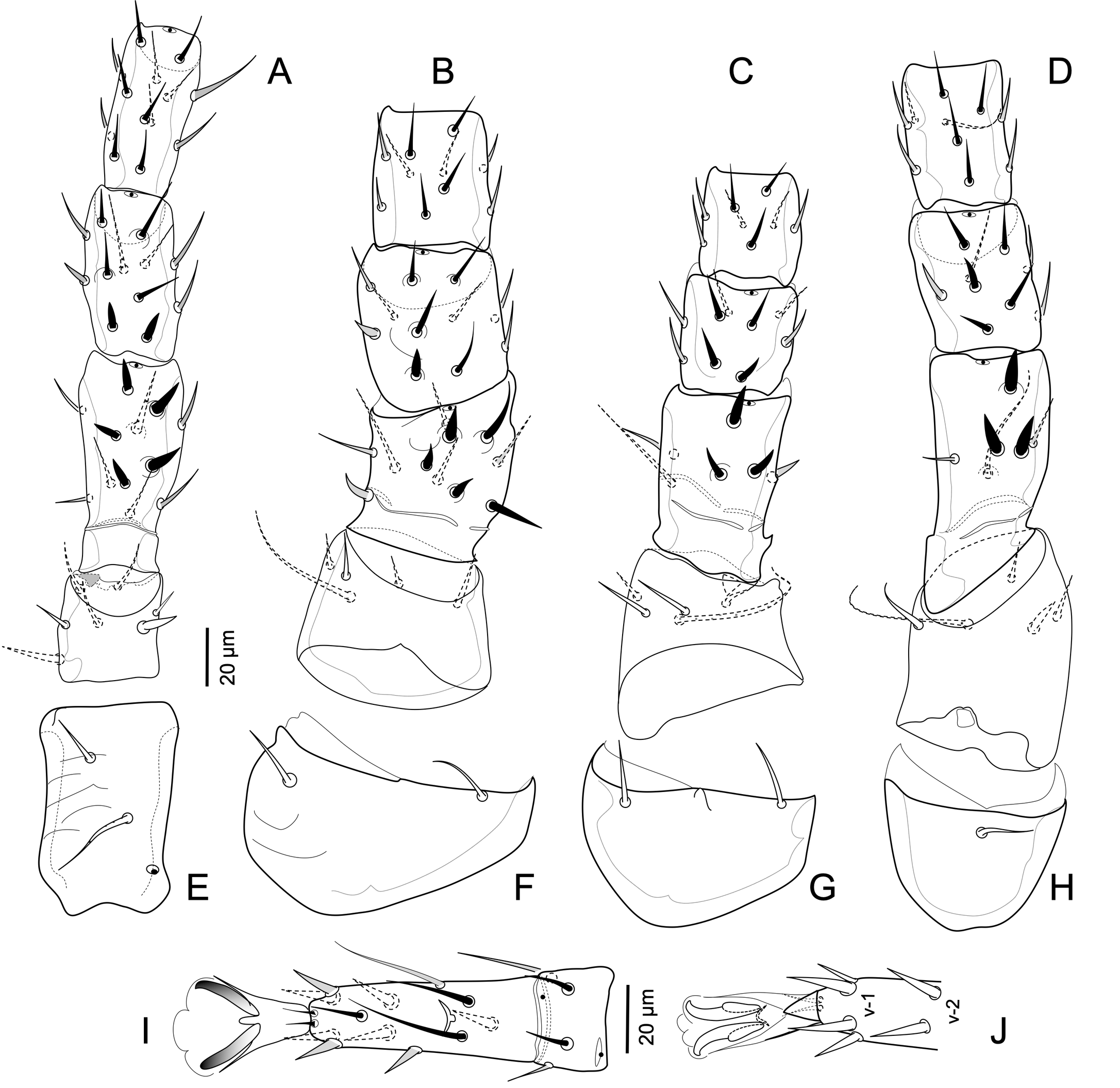


Legs — (Figure 4) Legs of moderate length, legs I and IV no longer than dorsal shield. Legs I slightly thinner, and legs II slightly thicker, than legs III and IV. Setation on segments of legs I to IV slightly deficient from full complement of Ascidae (as presented by Lindquist & Evans 1965 for Ascini), coxae, 2-2-2-1; trochanters, 6-5-5-5; femora, 12 (2 3/1 2/2 2) - 11 (2 2/1 3/2 1) - 6 (1 2/1 1/0 1) - 6 (1 2/1 1/0 1); genua, 13 (2 3/2 3/1 2) – 11 (2 3/1 2/1 2) – 9 (2 2/1 2/1 1) – 9 (2 2/1 3/0 1); tibiae, 13 (2 3/2 3/1 2) – 10 (2 2/1 2/1 2) – 8 (2 1/1 2/1 1) – 10 (2 1/1 3/1 2); genua IV lacking pv-1. Tarsi II-IV with ventral setae v-1 and v-2 somewhat spine-like and claws strong, vm spine-like but slenderer than v-1 (Figures 4I-J); other leg setae simple, not strongly modified. At level of genu and tibia, respectively, legs I slightly slenderer (width ca 31, 26), and legs II thicker (ca 49, 41) than legs III (ca 37, 34) and IV (ca 40, 37); legs I to IV (Figure 4A-H) each clearly shorter than dorsal shield length; leg lengths, excluding pretarsi: I 387-402, II 359-381, III 367-388, IV similar in length to leg I, 384-402. Leg I tarsus (105-11) about 1.4-1.8 longer than each of the similarly long femur (74-80), genu (58-63), and tibia (60-66). Tarsus I with well-developed pedicel bearing pretarsus (c.a. 20 to base of claws); tarsus I without markedly elongated setae apically, seta s imperceptively lanceolate subapically. Legs II to IV with tarsus about 1.8-2.2 times as long as tibia. Tarsi II-IV with pair of apical setal processes ad-1, pd-1 (11-12) inconspicuous, shorter than length of pretarsus to base of claws (14-16); with ventroapical process short (5-8), pointed apically, about half the length of pretarsus to base of claws (as in Figure 4J); pretarsi with paradactyli inconspicuous as narrow sclerotized shafts flanking, and slightly shorter than claws. Coxae I-II lineate on ventral surfaces; coxal setae simple, short (21-24), except v2 on coxae I long, flageliform (31-33) (Figures 4E-H). Leg segments with modified spine-like setae (Figures 4A-D), including pl on trochanters I and IV. Seta pv on trochanters I-IV and femora II-IV (Figures 4A-D) thin, markedly elongate with filiform end.
Type material
HOLOTYPE: adult female, from a specimen of S. acupunctatus Rodalquilar (Almería), Spain, 18 Nov. 2017, M.D. Alcázar-Alba colr. PARATYPES: 22 females with same data as holotype; two females from a specimen of S. acupunctatus, Algarrobo-Costa (Málaga), Spain, 27 Feb. 2018, D. Serrano colr.; fifty females from a specimen of S. acupunctatus, Torregarcía (Almería), Spain, 19 May 2019, J. Moya colr.; thirteen females from a specimen of S. acupunctatus, La Cañada de San Urbano (Almería), Spain, 18 Oct. 2019, E. de Mas colr.; eight females from a flying specimen of S. acupunctatus, La Cañada de San Urbano (Almería), 28 Nov. 2019, I. Balanzategui colr. Nine females 30 Jan. 2016 and two females 19 Apr. 2016, Nijar (Almería), and one female Cuevas del Almanzora (Almería) 29 Aug. 2016, found in the ethanol conserving several specimens of S. acupunctatus collected by M.A. Gómez de Dios. Adult males and immature instars were not available.
Etymology
The specific epithet refers to the geographical name of the Iberian Peninsula of southwestern Europe, occupied by Spain and Portugal, the region from where this species material was collected.
Differential diagnosis
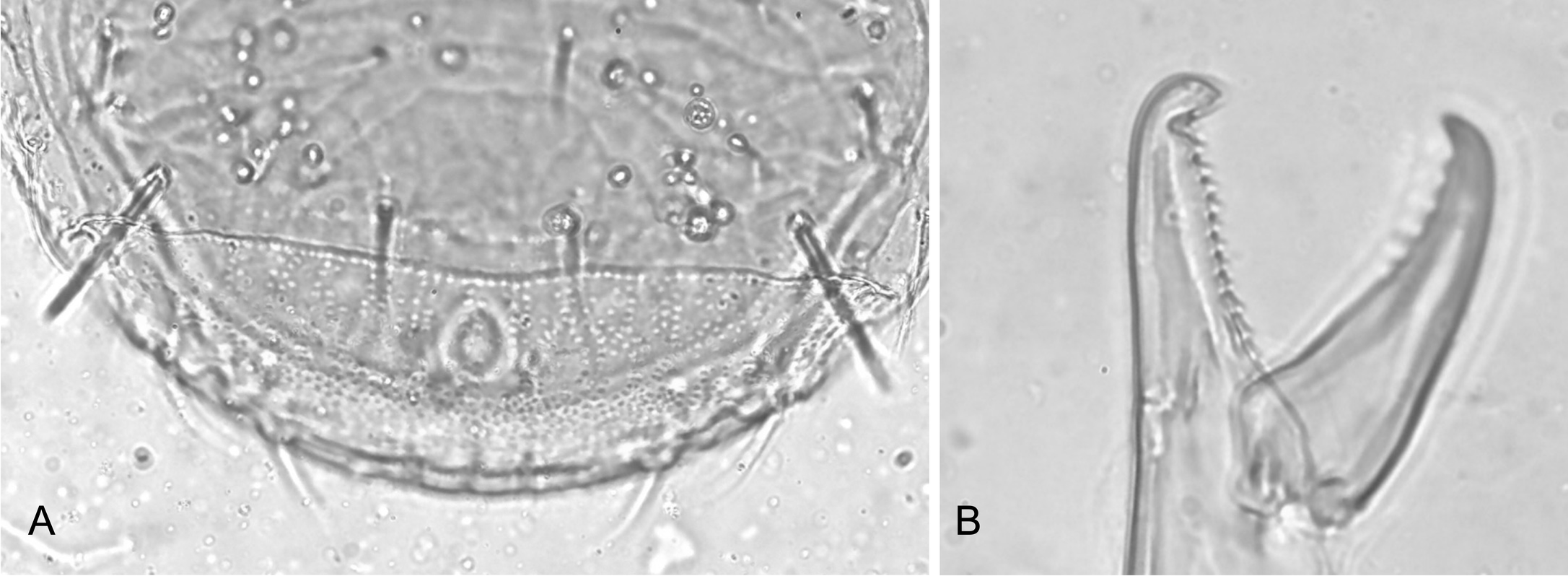


In the incomplete original description of L. (E.) tectus, Hyatt indicated the presence of dense large puncta on the concave (groove) area behind level of setae J4-Z4, which was clearly present in the studied holotype (Figure 5A), and a similar ornamentation on the pigydial region of the ventrianal shield. Specimens of L. (E.) ibericus n. sp. collected in Spain show a similar ventral ornamentation on several specimens. However, the dense dorsal area of large clear puncta is not obvious, although the line delimiting this region behind setae J4-Z4 is clearly observed. In the holotype of L. (E.) tectus, setae Z4 are clearly longer than J3, such as in the specimens studied now (the length of these two setae does not match the illustration given in Figure 1 of the original description).
Regarding the teeth on the fixed cheliceral digit, Hyatt indicated and illustrated in Figure 4, a row of 13 teeth (Figure 5B). Our specimens have a row of 7 or 8 teeth plus offset basal, excluding the two apical teeth of the bifid apical hook.
Lasioseius (E.) tectus and L. (E.) ibericus share with L. (E.) vitzthumi Westerboer, 1963 dorsal setae Z4 untapered, stout, blunt-tipped, penicillate distally, and Z5 simple. However, L. (E.) vitzthumi lacks the prominent transverse line across the entire face behind setae J4 and Z4, the presternal region with two pairs of platelets and the ventrianal shield bears 5 pairs of opisthogastric setae.
Discussion
Scyphophorus acupunctatus, the phoront of the new species of Lasioseius (Endopodalius) is distributed across five continents, and various website images of these beetles show a phoretic mite or two on them, some of which may be this type of mite species. In any case, a worldwide distribution of this mite is likely.
Acknowledgments
We thank all the agave weevil collectors, specially to M.A. Gómez de Dios (Migüi), who kindly lent us his personal collection. We are particularly grateful to Ms. Jan Beccaloni, Senior Curator at The Natural History Museum, London, for her kindness in every moment.
References
- Abo-Shnaf R.I., Sánchez L., de Moraes G.J. 2016. Plant inhabiting Gamasina mites (Acari: Mesostigmata) from the Dominican Republic, with descriptions of four new species of Lasioseius (Blattisociidae) and complementary descriptions of other species. Syst. Appl. Acarol., 21(5): 607-646. https://doi.org/10.11158/saa.21.5.5
- Argolo P.S., Santos J.C., Oliveira A.R., de Moraes G.J. 2018. Two new species of Lasioseius Berlese (Acari: Blattisociidae) from Brazil, and a key for separation of the Brazilian species of the genus. Syst. Appl. Acarol., 23(8): 1567-1577. https://doi.org/10.11158/saa.23.8.7
- Athias-Henriot C. 1969. Les organes cuticulaires sensoriels et glandulaires des Gamasides. Poroïdotaxie et adénotaxie. Bull. Soc. Zool. France, 94: 485-492.
- Berlese A. 1916. Centuria prima di Acari nuovi. Redia, 12: 19-67.
- Christian A., Karg W. 2006. The predatory mite genus Lasioseius Berlese, 1916 (Acari, Gamasina). Abh. Ber. Naturkundemus. Görlitz, 77: 99-250.
- Esteca F.C.N., Borges V., Santos J.C., Neves L. da S., de Moraes J.C. 2020. Report of the mite Lasioseius prorsoperitrematus Abo-Shnaf, Sánchez & Moraes, 2016 (Acari: Blattisociidae) in Brazil associated with the insect Sphenophorus levis Vaurie, 1978 (Coleoptera: Curculionidae). Entomological Communications, 2, 2020: ec02028. https://doi.org/10.37486/2675-1305.ec02028
- Evans G.O. 1963. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata). Bull. br. Mus. nat. Hist. Zool., 10: 275-303. https://doi.org/10.5962/bhl.part.20528
- Evans G.O. 1964. Some observations on the chaetotaxy of the pedipalps in the Mesostigmata (Acari). Ann. Mag. Nat. Hist. Series 13, 6: 513-527. https://doi.org/10.1080/00222936308651393
- Evans G.O. 1969. Observations on the ontogenetic development of the chaetotaxy of the tarsi of legs II-IV in the Mesostigmata (Acari). In: Evans G.O. (Ed.) Proceedings of the 2nd International Congress of Acarology, 1967. Akadémiai Kiadó, Budapest, pp. 195-200.
- Hyatt K.H 1964. A collection of Mesostigmata (Acari) associated with Coleoptera and Hemiptera in Venezuela. Bull. Br. Mus. Nat. Hist., Zool., 11: 467- 509. https://doi.org/10.5962/bhl.part.4723
- Krantz G.W., Redmond B.L. 1987. Identification of glandular and poroidal idiosomal systems in Macrocheles perglaber F. & P. (Acari: Macrochelidae). Exp. Appl. Acarol., 3: 243-253. https://doi.org/10.1007/BF01270460
- Lindquist E.E. 1994. Some observations on the chaetotaxy of the caudal body region of gamasine mites (Acari: Mesostigmata), with a modified notation for some ventrolateral body setae. Acarologia, 35: 323-326.
- Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem Entomol. Soc. Can. 47: 1-64. https://doi.org/10.4039/entm9747fv
- Lindquist E.E., Walter D.E., Krantz G.W. 2009. Order Mesostigmata. In: Krantz GW, Walter DE. (Eds.) A Manual of Acarology, Third Edition, Texas Tech University Press, Lubbock, p. 124-232.
- Maya Y., Palacios-Cardiel C., Jiménez M.L. 2011. El cardón Pachycereus pringley, nuevo hospedero para Scyphophorus acupunctatus (Coleoptera: Curculionidae) en Baja California Sur, México. Rev. Mex. Biodivers, 82: 1041-1045.
- Molina D. 2013. Contribución al conocimiento de la distribución actual de la especie invasora Scyphophorus acupunctatus Gyllenhal, 1838 (Coleoptera: Dryophthoridae) en la Península Ibérica. Rev. gadit. entomol., volumen IV núm. 1 (2013): 11-16. ISSN 2172-2595
- Moraes G.J., de Britto E.P.J., Mineiro J.L. de C., Halliday B. 2016. Catalogue of the mite families Ascidae Voigts & Oudemans, Blattisociidae Garman and Melicharidae Hirschmann (Acaria: Mesostigmata). Zootaxa, 4112 (1): 1-299. https://doi.org/10.11646/zootaxa.4112.1.1
- Moraza M.L., Lindquist E.E. 2018. A new species-group with new species of the genus Lasioseius (Acari: Mesostigmata: Blattisociidae) associated with neotropical hispine beetles in furled leaves of Heliconia. Acarologia, 58 (1): 62-98. https://doi.org/10.24349/acarologia/20184227
- Riba i Flinch J. M., Alonso-Zarazaga, M.A., 2007. El picudo negro de la pita o agave, o max del henequén, Scyphophorus acupunctatus Gyllenhal, 1838 (Coleoptera: Dryophthoridae): primera cita para la Península Ibérica. Bol. Soc. Ent. Aragonesa 41: 419- 422.
- Rubio C. 2007. Enfermedades del cultivo del agave. Pp 169-195. In: Rulfo V. et al. (ed). Conocimiento y prácticas agronómicas para la producción de Agave tequilana Weber en la zona de denominación de origen del tequila. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Centro de Investigación Regional del Pacífico Centro. Libro técnico nº 4. Tepatitlán de Morelos, Jal.
- Ruíz-Montiel C., Rojas J.C., Cruz-López L., González-Hernández H. 2009. Factors Affecting Pheromone Release by Scyphophorus acupunctatus (Coleoptera: Curculionidae). Environmental Entomology, 38(5): 1423-1428. https://doi.org/10.1603/022.038.0510



2023-03-21
Date accepted:
2023-05-08
Date published:
2023-05-23
Edited by:
Faraji, Farid

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Moraza, Maria L. and Balanzategui, Iñaki
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



