On the perception of leaf morphology and visible light by Tetranychus urticae Koch (Acariformes, Tetranychidae)
Tsolakis, Haralabos  1
; Ragusa, Ernesto
1
; Ragusa, Ernesto  2
; Sinacori, Milko
2
; Sinacori, Milko  3
and Lombardo, Alberto
3
and Lombardo, Alberto  4
4
1✉ Department of Agricultural, Food and Forest Sciences, University of Palermo, Viale delle Scienze, 90128 Palermo, Italy.
2Department of Agricultural, Food and Forest Sciences, University of Palermo, Viale delle Scienze, 90128 Palermo, Italy.
3Department of Agricultural, Food and Forest Sciences, University of Palermo, Viale delle Scienze, 90128 Palermo, Italy.
4Department of Engineering, University of Palermo, Viale delle Scienze, 90128 Palermo, Italy.
2022 - Volume: 62 Issue: 2 pages: 404-417
https://doi.org/10.24349/62cg-0jj4Original research
Keywords
Abstract
Introduction
The behaviour of phytophagous mites on plants has important practical implications for the management of their populations in the field. Various authors have investigated the influence of environmental factors (temperature, relative humidity, photoperiod, UV wavelengths etc.) on these microarthropods (Force 1967; Rodriguez et al. 1967; Barcelo 1981; Nihoul 1993) and others have focused on the influence of plant chemistry (Juniper and Southwood 1986), of leaf surface morphology or of some features of the external foliar architecture on phytophagous mites (Grostal & O'Dowd 1994; Walter & O'Dowd 1995; Karban et al. 1995; Park & Lee 2002), as well as on the phytoseiid mites and predator–prey interactions (Duso 1992; Duso & Pasqualetto 1993; Norton et al. 2001; Seelmann et al. 2007; Schmidt 2014). Leaf architecture, including microarchitecture, provides microhabitats that strongly affect the presence and performance of mites on host plants (Walter 1992; Karban et al. 1995; Kreiter et al. 2002; Ferreira et al. 2011; Schausberger 2021). Foliar tomentum for example, positively influences the abundance of many species of mites in all trophic levels, both in natural and agricultural ecosystems, while glabrous leaves are less preferred by the mites (Duso & Pasqualetto 1993; Walter & O'Dowd 1995; Seelmann et al. 2007). Leaves represent for micro-arthropods not only a food source but also the microhabitats on which they live (Southwood 1986; Walter 1996). The adaxial (upper) and abaxial (under) leaf surfaces, have different microclimates due mainly to transpiration (Willmer 1986), and this can be decisive for the choice of living place for the tiny mites. Moreover, epicuticular wax deposits are more evident on the adaxial leaf surface (Baker et al. 1975; Eigenbrode & Espelie 1995) and this could be an insurmountable limit for various phytophagous mite species with very short and tiny stylets, leading them to prefer the abaxial leaf surface.
Tetranychus urticae Koch, for example, clearly prefers the abaxial leaf surface, although its stylet lengths allow it to penetrate foliar tissues and get food from both leaf surfaces (Sances et al. 1977; Park & Lee 2002), leading to the hypothesis that other factors may be important in abaxial/adaxial surface choice. Barcelo (1981) demonstrated the detrimental effects of UV-B on various biological parameters of T. urticae females and Ohtsuka and Osakabe (2009) argued that the preference for the lower leaf surfaces in T. urticae is a behavioural adaptation to enable avoidance of UV-B radiation, helped by their ability to perceive and avoid 320–340 nm wavelengths (Sakai & Osakabe 2010). However, new UV-blocking films used in greenhouses as protection from different pests and diseases (Espí & Salmerón 2002), are able to neutralize the UV negative effect, allowing the species to infest also the upper surfaces of the leaves as well. Using visible light source, Mori (1962) showed a positive photokinesis of T. urticae females at temperatures ranging from 15°C to 30°C, but no data are available on the perception of leaf architecture by T. urticae in relation to the effects of visible light.
In the present study we used leaves of two plant species, the common bean Phaseolus vulgaris L. and lemon, Citrus limon (L.) Osbeck, both being suitable hosts for T. urticae but showing strong differences in their leaf surface morphology. We aimed to investigate on the effects of visible light and leaf architecture on the selection of sites on which T. urticae females prefer to live and feed.
Material and methods
Mite cultures
The two-spotted spider mite T. urticae was collected from weeds and Solanum melongena L. in an organic farm in Balestrate (Palermo, Italy) (38° 1′41.49″N 13° 1′55.61″E) and reared on potted bean plants (P. vulgaris) in a conditioned greenhouse (22–32°C, 55–74% RH and natural photoperiod), for eight months before the beginning of the experiments. In order to adapt the population to the Citrus plants, about 700 females and males of T. urticae were transferred onto lemon fruits, kept in a conditioned room at 26±2°C and 75±5% RH, one month before the beginning of tests.
To obtain the young females for tests, about 50 females from each rearing, were transferred with a fine paintbrush (4/0) onto a clean bean leaf, or onto a clean lemon fruit, and removed after 24 hours. About 200 eggs were laid in this time interval. After completion of the postembryonic development, young females (max 24h old) were individually paired with a male on a leaf disc (bean or lemon leaf), for 24 hours to ensure fertilization and afterwards used for the experiments.
Experimental design
Light source and experimental conditions
The light source used in the experiments produces visible light, λ ≈ 550 nm, supplied by two NL-T8 36W/865 cool daylight fluorescent lamps with a luminous flux of 3,250 lm and 6,500 K of colour temperature. Light sources were placed about 70 cm above the experimental area (1 m2). Temperature and relative humidity measured in the experimental area did not differ from that registered at the centre of the conditioned room (25±1°C and 70±5% of R.H.). The photoperiod was of 16:8h (Light:Dark).
No-choice experiment
In order to verify the influence of food quality obtained by the mites from both the adaxial and abaxial surface of each type of leaves, we carried out a no-choice test using each leaf surface for both bean and lemon leaves. The outcomes for comparisons were the survival and the oviposition rate of mated young females. Bean or lemon leaf-discs (3cm diameter) were used for these tests. Discs were individually placed on wet cotton wool in a Petri dish (9cm diameter, 1.4cm height). For each leaf type, two sets of tests were carried out: i) Adaxial surface upward (AdSU); ii) Abaxial surface upward (AbSU). One fertilized young female was introduced on each leaf disc. Each test was replicated 30 times and lasted 5 days. Every 24 hours, the number of alive females and the number of eggs laid were recorded; eggs were removed daily.
Choice experiment
To verify the influence of the leaf surface morphology and visible light on mite distribution, whole bean or lemon leaves were used. Available leaf areas were 22.1 ± 3.11 cm2 and 28.5 ± 3.15 cm2 (mean ± SE per side) for bean and lemon leaves respectively. Females allowed to move between the adaxial and abaxial surfaces of natural- or reversed-orientated leaves of bean or lemon. Illumination was supplied at the top of leaves (Figure 1). The choice tests performed, are reported in table 1. Whole petiolate leaves were used in all choice tests. The petiole was wrapped with cotton wool and inserted into a plastic test tube (10 x 1.4 cm) filled with water, in order to keep the leaf turgid (Figure 1). Test tubes with leaves were placed in a 12-place tube-holder (5 tubes per each tube-holder). One young T. urticae female was put on each leaf and each test was replicated 30 times.
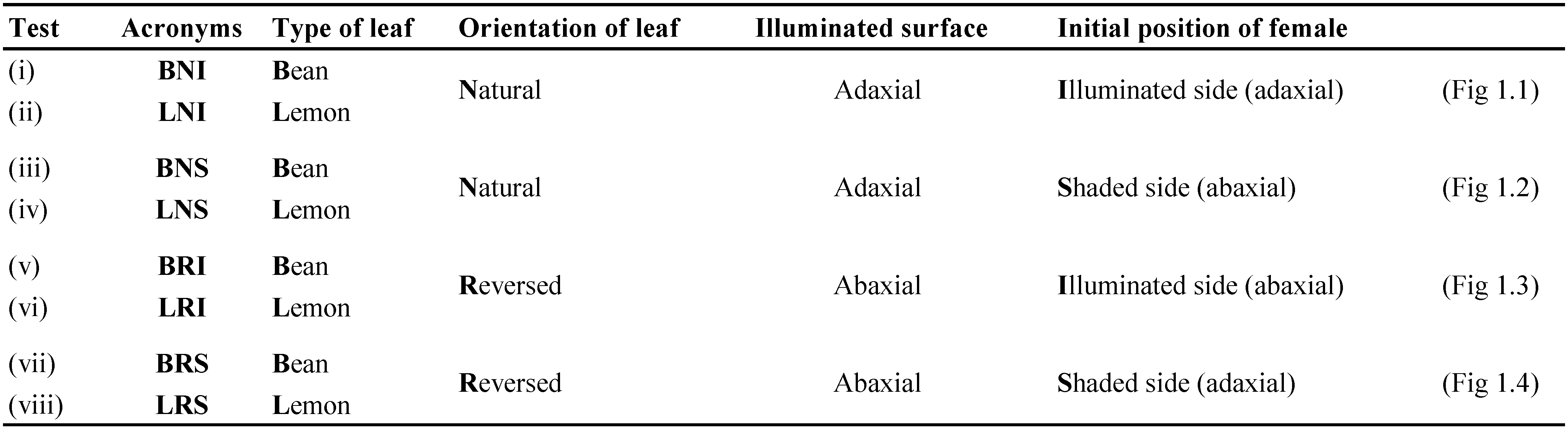





For each replicate, the position of the females was recorded at one-hour interval for the first five hours and afterwards at a 24-hour interval for five days. The female's survivorship and oviposition rate were recorded as mentioned above.
Statistical analysis
To analyse survivorship in the no-choice tests, as the response is live or death, the Binary Logistic Regression (BLR) was adopted, using the time (5 days) and the four treatments (adaxial and abaxial surface of bean and lemon leaves) as factors. For the oviposition rate, the General Linear Model (GLM) was adopted. The assumption of normality was verified on the basis of the analysis of residuals and the comparison between treatments was evaluated using the Bonferroni simultaneous tests for differences between means, with family-wise error rate equal to 5%.
In the choice tests, the response was the position of the female at the different time intervals: up (illuminated surface) and down (shaded surface). Being a binary response, a BLR analysis was used to check the significance of the following factors: i) type of leaf (bean and lemon), the initial placement of the female (on the illuminated or on the shaded surface), ii) the orientation of the leaf (''natural'' or ''reversed'') and iii) time. The Bonferroni simultaneous tests were used to test the hypothesis H0: P=0.5, i.e. the chance of finding the female on the illuminated (up) or on the shaded (down) surface of the leaf, is the same (family error equal to α=0.05 and k=8).
To analyse the oviposition rate, the general linear model (GLM) was used, as for the no-choice tests. The interactions between leaf type, initial placement of mite, and orientation of leaf were taken into account and were analysed conjointly, using the Bonferroni Pairwise Comparisons (family-wise error rate equal to 5%). Analyses were carried out with Minitab® 17.1.0 software.
Results
No-choice experiment
Statistical analysis showed that, regarding survivorship, both time (χ2 = 26.21; df = 4; P < 0.001) and leaf type (χ2 = 131.45; df = 3; P < 0.001) were highly significant. The survival rate of T. urticae at the end of the tests was high on bean leaves, up to 90%, and no significant differences were found between the two leaf surfaces (Odds ratio = 2.5684; 95% C.I.: 0.49–13.52). On the contrary, differences were registered between the two lemon leaf surfaces: 33.3% vs. 76.7% of survival was registered at the end of the tests for the adaxial and abaxial surfaces respectively (Odds ratio = 0.2842; 95% C.I.: 0.17–0.49) (Figure 2). Both of these survival rates were significantly lower than those on bean leaves. As regards the oviposition rate, no significant differences were noted between the adaxial and abaxial surfaces of either leaf type (t = -0.33; df = 509; P = 0.745), while the oviposition on both surfaces of bean leaves was significantly higher than that on both surfaces of lemon leaves (t = -40.88; df = 509; P < 0.001) (Figure 2): the mean oviposition rate in the test period was 10.5 and 10.4 eggs/female/day on the adaxial and abaxial surface of bean leaves respectively and 1.1 and 1.2 eggs/female/day on the adaxial and abaxial surface of lemon leaves respectively.



Choice experiment
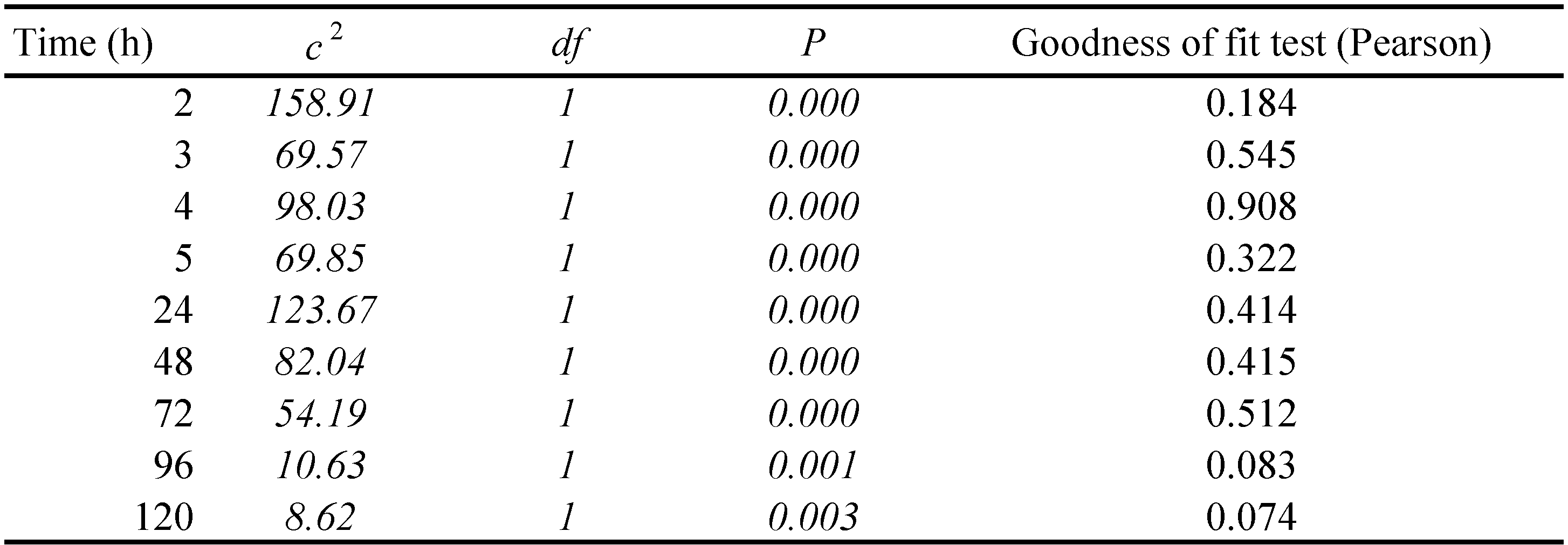
The position of the mites one hour after the start of the tests, was found to be related to the type of leaf, the orientation of the leaf and the initial position of mite (Table 2). The most influential factor was the leaf type (χ2 = 46.29; df = 1; P < 0.001), followed by the interaction between the type of leaf and its orientation (χ2 = 20.46; df = 1; P < 0.001), the orientation of the leaf (χ2 = 15.13; df = 1; P < 0.001) and finally by the initial position of the mite (χ2 = 13.53; df = 1; P < 0.001). This result led us to analyse each test separately because both factors and interactions were significant. To analyse the position of T. urticae females at the different time intervals, the position of the mite in the previous time interval was added into the model. In these new models, the type of leaf remained significant, but the position of the mite at the previous time interval (1st hour), became the most significant factor (χ2 = 158.91; df = 1; P < 0.001). Similar trend was registered in all the following time intervals (Table 3).
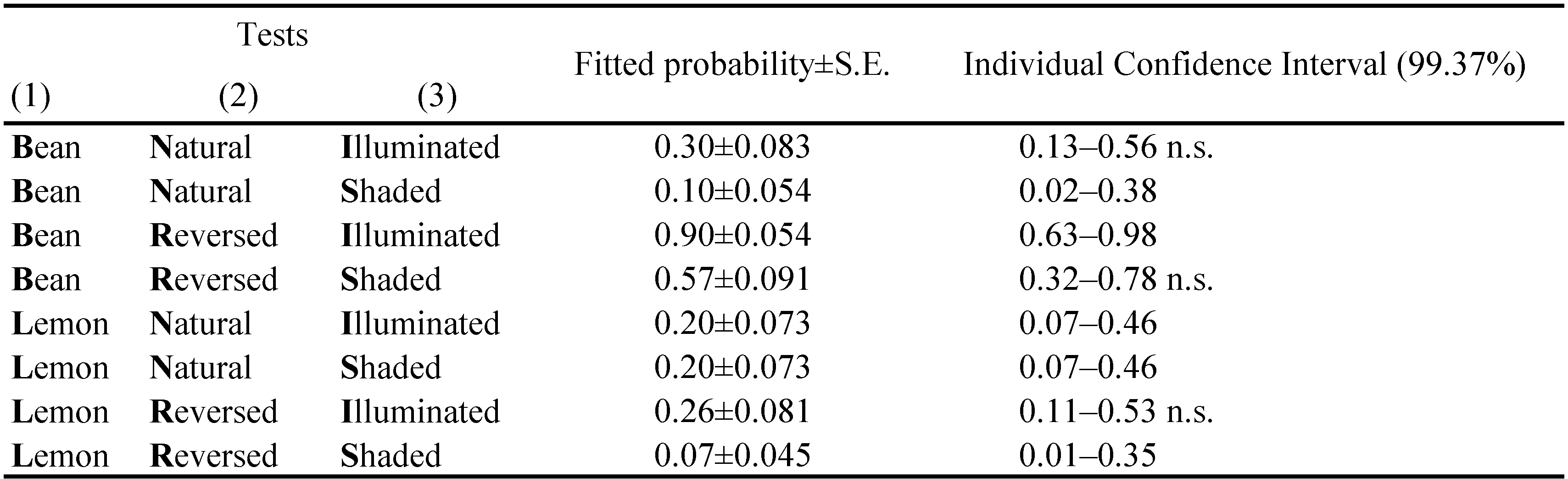




On natural oriented bean leaves (BNI) (Figure 3A1), T. urticae females showed a strong preference for the shaded surface of the leaf, but the initial position of the mite also slightly influenced their choice. Indeed, when tetranychid females were initially placed on the illuminated surface (adaxial), 30% of them (9 females) stayed there and 70% (21 females) migrated onto the abaxial one, remaining there until the end of the tests (p = 0.30, 95% C.I.: 0.13–0.56) (Table 2). However, the family error significance level of 0.05 was not reached in the first hour, because the individual level for the Bonferroni procedure has to be constrained at a very low level (0.00625). On the contrary, when the initial position of mites was on the shaded surface (abaxial) (Figure 3A2 and Table 2) (BNS tests), only 10% of females migrated onto the upper surface of the leaf (adaxial) (p = 0.10, 95% C.I.: 0.02–0.38). In both tests, the movements of females between the two leaf surfaces mostly occurred during the first hour. For example, in BNI tests 21 females moved towards the shaded surface of the leaf (abaxial) within the 1st hour, one female moved after 2 hours, two females after 3 hours, one female after 24 hours and one after 2 days. Only four females did not change their initial position, remaining on the adaxial illuminated surface. In BNS tests, only one female moved from the abaxial (shaded) surface to the adaxial one (illuminated) and stayed there for the entire test period. Another two females moved towards the illuminated surface during the first 1–3 hours, but afterwards returned to the shaded one, staying there until the end of the tests.



This situation was dramatically different in tests with ''reversed'' bean leaves. When T. urticae females were placed on the illuminated surface of the leaf (in this case the abaxial one) (Figure 3A3 - BRI tests and table 2), 87% of mites (26 females) stayed there and only 7% migrated towards the shaded surface (adaxial) maintaining their position until the end of the tests (p = 0.90, 95% C.I.: 0.63–0.98). On the other hand, when tetranychid females were initially placed on the shaded surface of the leaf (adaxial) (Figure 3A4 - BRS tests), more than 50% of them migrated to the illuminated surface (abaxial) after 1 hour (p = 0.57, 95% C.I.: 0.32–0.78) (Table 2).
In BRI tests, only two females changed their initial position moving towards the shaded adaxial surface, while 26 females maintained their initial position; two females changed more times their position moving between the illuminated and shaded surface of the leaf. On the contrary, BRS tests greatly upset the females' behaviour. Nine females remained in the initial position and 12 of them moved towards the illuminated abaxial surface within the first hour, remaining there until the end of the tests. The remaining 9 females changed their position between the two leaf surfaces several times during the test period. Behaviour of females in all abovementioned tests did not change significantly after 24 hours in comparison with that recorded after one hour, and also after the last position of females after 5 hours. Similarly, female behaviour was mainly conditioned by the position recorded at 24 hours, at all the following times: 48, 72, 96 and 120 hours (Table 3).
The behaviour of females on lemon leaves, differed significantly from that described above on bean leaves, considering the four tests overall, but some similarities were recorded in individual tests. For example, in both tests with ''natural'' orientation of lemon leaves (Figure 4A1–2 - LNI and LNS tests respectively), T. urticae females showed a clear preference for the shaded surface of the leaf after the first hour (p=0.20, C.I. 0.07–0.47, in both the LNI and LNS tests) (Table 2), but they were particularly agitated afterwards. In LNI tests two females remained in the initial position and 11 females moved towards the shaded abaxial surface within the first hour, remaining there until the end of the tests, but 17 females (56%) changed their position multiple times during the remaining test period. The number of females that maintained their initial position for the whole test period in LNS tests, was higher (18 females) in comparison to the previous tests, even if not significantly different, while 12 females changed their position multiple times. No one female remained on the illuminated adaxial surface for the entire test period.
In LRI tests (Figure 4A3 and Table 2), the females' behaviour was similar to that observed in the above mentioned tests, although the family error significance level of 0.05 was not reached (p=0.26, C.I. 0.11–0.53), but was very different to that observed in the same kind of test with bean leaves (P\textless0.001). As a matter of fact, only four females stayed on the illuminated abaxial surface and 11 moved towards the adaxial shaded surface, remaining in these positions until the end of the test or death. The other females changed their position multiple times (Figure 4A3). On the contrary, in LRS tests (Figure 4A4), about half of the females stayed in their initial position until the end of the tests and only one moved onto the illuminated surface staying there for the whole test period (p=0.06, C.I. 0.009–0.34) (Table 2). Fifteen females changed their position multiple times. This behaviour differed significantly from that in the corresponding tests on bean leaves (P\textless0.001).



The behaviour of females in all the abovementioned tests on lemon leaves, did not changed after 24 hours in comparison with that observed after 1 hour or after the last position of females observed at 5th hour. Similarly, female's behaviour was mainly conditioned by the position recorded in the previous time interval, for the entire test period.
The oviposition rate of T. urticae females was strongly influenced by the type of leaf (factor 1) (F = 1174.02; df = 1, 966; P < 0.001), but also by the orientation of the leaf surface (factor 2) (F = 14.57; df = 1, 966; P = 0.000), time (factor 3) (F = 17.09; df = 4, 966; P < 0.001) and the initial position of the female (factor 4) (F = 8.27; df = 1, 966; P = 0.004). It should be mentioned that interactions between factors 1-2-3 and factors 1-2-4 were also significant (F = 7.92; df = 4, 966; P < 0.001 and F = 4.95; df = 1; P = 0.026 respectively); therefore, these factors were analysed conjointly. No differences were found for the oviposition rate between the two leaf surfaces during the first 5 hours in all tests. As regards bean leaves (Figure 3B1–4), the oviposition rate recorded on the first day was significantly lower than at subsequent times (F=10.73; df = 4, 966; P < 0.001), while no differences were noted among the latter (F = 1.28; df = 1, 28; P = 0.267) (Table 4). On the other hand, no differences were noted for the oviposition rate among the set of tests on lemon leaves (Figure 4B1–4). With regards leaf orientation, T. urticae females laid more eggs on the ''naturally'' oriented bean leaves (9.15 and 9.18 eggs/female/day for BNI and BNS tests respectively) than on ''reversed'' oriented leaves (7.01 and 7,87 eggs/female/day for BRI and BRS tests respectively), regardless of initial position of the mite (F = 4.00; df = 1, 545; P = 0.046), but no differences were found among tests on lemon leaves (F = 2.25; df = 1, 400; P = 0.135). On the other hand, no differences were found in the daily oviposition rate between the illuminated and the shaded surface of leaves in any test (F = 0.53; df = 1, 545; P = 0.466 and F = 0.76; df = 1, 400; P = 0.552 for bean and lemon leaves respectively) except BRI. In the latter test, oviposition was higher during the first day on the illuminated abaxial surface (F = 9.6; df = 1, 28; P=0.004), while it was lower during the 72 and 96h intervals (F = 4.46; df = 1, 26; P = 0.044 and F = 8.23; df = 1, 25; P = 0.008 respectively) (Figure 3B3 and Table 4).



Discussion
No difference in the oviposition rate of T. urticae females was observed between the adaxial and abaxial surface of bean leaves, indicating the same food quality of both leaf surfaces. Consequently, selection of the abaxial or adaxial surface does not seem to be related to the search for better food quality or for an easier way to obtain food. Similar results were reported by Sakai et al. (2012), although the latter authors reported greater fecundity on the adaxial bean leaf surface facing downward. Some authors have speculated on differences in the epidermal thickness or the adaxial-biased palisade mesophyll (Tateishi 1987; Vasquez et al. 2008). However, the epicuticular wax deposits on bean leaves are distributed as amorphous films (Jeffree 1986) and do not differ significantly between the two leaf surfaces: 0.9 μg cm-2 and 0.6 μg cm-2, for the adaxial and abaxial surface respectively (Baker & Hunt 1981), allowing mites to feed easily on both surfaces. This lack of difference is also supported by the survivorship of females, which was high and similar on both surfaces (Figure 2A). On the contrary, the higher mortality on lemon leaves, in particular that recorded on the adaxial surface, and the lower oviposition rate on both surfaces, indicates a) the worse food quality of lemon leaves than of bean leaves, or at least shows a clear preference of mites for bean constituents and b) greater difficulty feeding on the adaxial side of lemon leaves. Besides the presence of secondary toxic metabolites on Citrus leaves (Hasegawa & Miyake 1996), there is also a greater amount of granular waxes (31 μg cm-2) (Baker et al. 1975), with a thicker layer on the adaxial surface (Jeffree 1986), making piercing of tissues by T. urticae females more difficult.
Both survival and oviposition rates recorded in our experiments were significantly higher on bean leaves, and similar to those obtained by other authors on the same host plant (Modarres Najafabadi et al. 2014). On the contrary, both the survivorship and oviposition rate were significantly lower on lemon leaves. This could seem paradoxical, because T. urticae is considered a dangerous pest on various Citrus species (McMurtry 1985; Bruessow et al. 2010; Jacas & Urbaneja 2010). Moreover, various authors have shown rapid adaptation of the species to a new host (Fry 1990; Agrawal 2000). However, some authors (Gotoh et al. 1993; Fytrou & Tsangarakou 2014) showed the presence of distinct host races with a preference for the plant of origin and Aguilar-Fenollosa et al. (2012) reported the weak performance of T. urticae populations originating from Festuca arundinacea Schreber (Fam. Poaceae) and reared on Citrus clementina Tanaka. It is possible that the one-month period on lemon fruits, adopted in our experiments, was not enough for complete adaptation of our populations to the new host.
The results of the choice tests confirm the above discussion and provide new data on the influence of light in choosing a leaf surface for residence and feeding. In particular, T. urticae females chose the shaded surface of leaves in naturally oriented leaves, regardless of their initial position, and they made this choice within the first hour of tests. On the other hand, also Li and Margolies (1991) found no influence of the initial placement of mites on their final choice in similar tests on corn leaves using Oligonychus pratensis (Banks). According to Barcelo (1981), T. urticae females did not avoid visible UV-A wavelengths (the same wavelengths used in our experiments), while being able to detect visible light but also the more dangerous UV-C radiation (McEnroe & Dronka 1969; Ohtsuka & Osakabe 2009). Naegele et al. (1966), using the visible spectrum on T. urticae females, showed phototaxis at 375 nm and photokinesis at 525 nm, but it is believed that this phototaxis is also influenced by environmental factors and the level of leaf damage (Suski & Naegele 1963). Therefore, the selection of the abaxial shaded bean leaf surface by the mites should be ascribed to factors other than light. Sakai et al. (2012) attributed this choice to gravity, but in our experiments T. urticae females remained on the illuminated abaxial surface of bean leaves when this was their initial position (BRI tests). This behaviour indicates that a) the mite can recognize differences between the two leaf surfaces and b) the leaf architecture of the abaxial bean leaf surface seems to be a priority factor. The arrangement of raised veins (in relief) on the abaxial bean leaf surface offers the mite the possibility of creating the complicated web needed for protection and egg laying more rapidly and more easily, and this seems to be a priority in comparison to the influence of visible light or gravity. Other mite species also showed the ability to differentiate the adaxial and abaxial sides of the leaf, i.e., O. pratensis can clearly distinguish the adaxial and abaxial corn leaf surface (Li & Margolies 1991), whereas two phytoseiid mites, Kampimodromus aberrans (Oudemans) and Typhlodromus pyri Scheuten, clearly prefer the tomentous abaxial grape leaves (Duso 1992; Duso & Pasqualetto 1993).
On the other hand, mites initially put on the adaxial shaded bean leaf surface seemed to be confused. About the half of the females moved towards the illuminated abaxial surface, preferring this to the shade. It is noteworthy that about a third of females changed their position multiple times during the test period, indicating the difficulty of choosing between the shade and the leaf surface most appropriate to their needs. This behaviour was not recorded on reversed lemon leaves, where T. urticae females clearly preferred the shaded leaf surface, regardless of their initial position, even though about half of the females changed their position multiple times during the test period. This restlessness indicates the reluctance of females to stay and feed on the smooth adaxial lemon leaf, confirming observations reported for the no-choice tests, but also the negative influence of visible light when associated with an inadequate leaf structure and bad food conditions, as also argued by Suski and Naegele (1963).
It is well known that foliar surfaces influence both microclimate and leaf chemistry (Juniper & Southwood 1986), affecting the behaviours of microarthropods (Ferro & Southwick 1984; Wilmer 1986). Various authors have also shown the negative effect of UV-B radiation on T. urticae, describing the abaxial-biased distribution, as a consequence of avoiding deleterious wavelengths (Ohtsuka & Osakabe 2009). It could be inferred, that in greenhouses covered with UV-blocking films, T. urticae could equally infest the adaxial or abaxial leaf surfaces. However, according to our results, T. urticae choice depends more on the leaf surface morphology, than on the various light wavelengths. The two spotted spider mite is one of the most polyphagous known species, being able to feed on more than 1,100 plant species (Migeon & Dorkeld 2021), which is why it is feared by growers. However, the species have geographic and plant-adapted demes (Aguilar-Fenollosa et al. 2012; Fytrou & Tsangarakou 2014) that should involve different control approaches.
Further genetic and behavioural investigations of various T. urticae demes, inhabiting different plant species in natural and agricultural ecosystems, could provide new data for the rational and ecofriendly management of this interesting as well as damaging species.
Acknowledgements
Authors are deeply indebted to Dr. Tetsuo Gotoh and to an anonymous reviewer for their precious advices and constructive comments. This work was supported by funding from ''Fondo di Finanziamento per la Ricerca 2018-2021'', University of Palermo (FFR-D13).
References
- Agrawal A.A. 2000. Host-range evolution: adaptation and trade-offs in fitness of mites on alternative hosts. Ecology, 81(2): 500-508. https://doi.org/10.1890/0012-9658(2000)081[0500:HREAAT]2.0.CO;2
- Aguilar-Fenollosa E., Pina T., Gómez-Martínez M.A., Hurtado M.A., Jacas J.A. 2012. Does host adaptation of Tetranychus urticae populations in clementine orchards with a Festuca arundinacea cover contribute to a better natural regulation of this pest mite? Entomologia Experimentalis et Applicata, 144: 181-190. DOI: 10.1111/j.1570-7458.2012.01276.x https://doi.org/10.1111/j.1570-7458.2012.01276.x
- Baker E.A., Hunt G.M. 1981. Developmental changes in leaf epicuticular waxes in relation to foliar penetration. The New Phytologist, 88: 731-747. https://doi.org/10.1111/j.1469-8137.1981.tb01750.x
- Baker E.A., Procopiou J., Hunt G.M. 1975. The cuticles of Citrus species. Composition of leaf and fruit waxes. Journal of the Science of Food and Agriculture, 26: 1903-1101. https://doi.org/10.1002/jsfa.2740260807
- Barcelo J.A. 1981. Photoeffects of visible and ultraviolet radiation on the two-spotted spider mite Tetranychus urticae. Photochemistry and Photobiology, 33: 703-706. https://doi.org/10.1111/j.1751-1097.1981.tb05477.x
- Bruessow F., Asins MJ., Jacas JA., Urbaneja A. 2010. Replacement of CTV-susceptible sour orange rootstock by CTV-tolerant ones may have triggered outbreaks of Tetranychus urticae in Spanish citrus. Agriculture, Ecosystems and Environment, 137: 93-98. https://doi.org/10.1016/j.agee.2010.01.005
- Duso C. 1992. Role of Amblyseius aberrans (Oud.), Typhlodromus pyri Scheuten and Amblyseius andersoni (Chant) (Acari, Phytoseiidae) in vineyards. Journal of Applied Entomology, 114:455-62. https://doi.org/10.1111/j.1439-0418.1992.tb01151.x
- Duso C., Pasqualetto C. 1993. Factors affecting the potential of phytoseiid mites (Acari: Phytoseiidae) as biocontrol agents in north-Italian vineyards. Experimental and Applied Acarology, 17: 241-258. https://doi.org/10.1007/BF02337274
- Espí E., Salmerón A. 2002. Anti-UV Ag films minimize crop diseases. Modern Plastics, 79(5): 36-37.
- Ferreira J.A.M., Cunha D.F.S., Pallini A., Sabelis M.W., Janssen A. 2011. Leaf domatia reduce intraguild predation among predatory mites. Ecological Entomology, 36: 435-441. https://doi.org/10.1111/j.1365-2311.2011.01286.x
- Ferro D.N., Southwick E.E. 1984. Microclimates of small arthropods: Estimating humidity within the leaf boundary layer. Environmental Entomology, 13: 926-929. https://doi.org/10.1093/ee/13.4.926
- Force D.C. 1967. Effect of temperature on biological control of two-spotted spider sites by Phytoseiulus persimilis. Journal of Economic Entomology, 60(5): 1308-1311. https://doi.org/10.1093/jee/60.5.1308
- Fry J.D. 1990. Trade-offs in fitness on different hosts: evidence from a selection experiment with a phytophagous mite. The American Naturalist, 136: 569-580. https://doi.org/10.1086/285116
- Gotoh T., Bruin J., Sabelis M.W., Menken S.B.J. 1993. Host race formation in Tetranychus urticae: genetic differentiation, host plant preference, and mate choice in a tomato and a cucumber strain. Entomologia Experimentalis et Applicata, 68: 171-178. https://doi.org/10.1111/j.1570-7458.1993.tb01700.x
- Grostal R., O'Dowd D.J. 1994. Plants, mites and mutualism: leaf domatia and the abundance and reproduction of mites on Viburnum tinus (Caprifoliaceae). Oecologia 97: 308-315. https://doi.org/10.1007/BF00317319
- Hasegawa S., Miyake M. 1996. Biochemistry and biological functions of citrus limonoids. Food Reviews International, 12(4): 413-435. https://doi.org/10.1080/87559129609541089
- Jacas J.A., Urbaneja A. 2010. Biological control in citrus in Spain: from classical to conservation biological control. In: Ciancio A., Mukerji K.G. (Eds). Integrated Management of Arthropod Pests and Insect Borne Diseases 5. Springer, Dordrecht, The Netherlands p. 57-68. https://doi.org/10.1007/978-90-481-8606-8_3
- Jeffree C.E. 1986. The cuticle, epicuticular waxes and trichomes of plants, with reference to their structure, functions and evolution. In: Juniper B., Southwood R. (Eds). Insects and the Plant Surface. Edward Arnold, London. p. 23-64.
- Juniper B., Southwood R. 1986. Insects and the plant surface. Edward Arnold, London. pp. 360.
- Karban R., English-Loeb G., Walker M.A., Thaler J. 1995. Abundance of phytoseiid mites on Vitis species: effects of leaf hairs, domatia, prey abundance and plant phylogeny. Experimental & Applied Acarology, 19: 189-197. https://doi.org/10.1007/BF00130822
- Kreiter S., Tixier M-S., Croft B.A., Auger P., Barret D. 2002. Plants and leaf characteristics influencing the predaceous mite Kampimodromus aberrans (Acari: Phytoseiidae) in habitats surrounding vineyards. Environmental Entomology, 31(4): 648-660. https://doi.org/10.1603/0046-225X-31.4.648
- Li J., Margolies D.C. 1991. Factors affecting location of Banks grass mite, Oligonychus pratensis (Acari: Tetranychidae), on corn leaves. Experimental & Applied Acarology, 12: 27-34. https://doi.org/10.1007/BF01204397
- McEnroe W.D., Dronka K. 1969. Eyes of the female two-spotted spider mite, Tetranychus urticae. II. Behavioral analysis of the photoreceptors. Annals of the Entomological Society of America, 62(3): 466-469. https://doi.org/10.1093/aesa/62.3.466
- McMurtry J.A. 1985. Citrus. In: Helle W., Sabelis M.W. (Eds). Spider mites - Their biology, natural enemies and control. World crop pests. Vol. 1B. Elsevier Science Publishers, Amsterdam, The Netherlands. p. 339-347.
- Migeon A., Dorkeld F. 2021. Spider mites web: a comprehensive database for the Tetranychidae. Available from http://www1.montpellier.inra.fr/CBGP/spmweb
 (Accessed 15/09/2021)
(Accessed 15/09/2021) - Modarres Najafabadi S.S., Vafaei Shoushtari R., Zamani A.A., Arbabi M., Farazmand H. 2014. Life parameters of Tetranychus urticae (Acari: Tetranychidae) on six common bean cultivars. Journal of Economic Entomology, 107(2): 614-622. https://doi.org/10.1603/EC11205
- Mori H. 1962. The effects of photo-stimulus on the thermal reaction in four species of spider mites (Acarina: Tetranychidae). Journal of the Faculty of Agriculture, Hokkaido University, 52(1): 10-19.
- Naegele J.A., McEnroe W.D., Soans A.B. 1966. Spectral sensitivity and orientation response of the two-spotted spider mite, Tetranychus urticae Koch, from 350 mμ to 700 mμ. Journal of Insect Physiology, 12: 1187-1195. https://doi.org/10.1016/0022-1910(66)90131-4
- Nihoul P. 1993. Do light intensity, temperature and photoperiod affect the entrapment of mites on glandular hairs of cultivated tomatoes? Experimental & Applied Acarology, 17:709-718. https://doi.org/10.1007/BF00058510
- Norton A.P., English-Loeb G., Belden E. 2001. Host plant manipulation of natural enemies: leaf domatia protect beneficial mites from insect predators. Oecologia, 126: 535-542. https://doi.org/10.1007/s004420000556
- Ohtsuka K., Osakabe MH. 2009. Deleterious effects of UV-B radiation on herbivorous spider mites: they can avoid it by remaining on lower leaf surfaces. Environmental Entomology, 38(3): 920-929. https://doi.org/10.1603/022.038.0346
- Park Y-L., Lee J-H. 2002. Leaf cell and tissue damage of cucumber caused by two-spotted spider mite (Acari: Tetranychidae). Journal of Economic Entomology, 95(5): 952-957. https://doi.org/10.1093/jee/95.5.952
- Rodriguez J.G., Singh P., Seay T.N., Walling M.V. 1967. Ingestion in the two-spotted spider mite, Tetranychus urticae Koch, as influenced by wavelength of light. Journal of Insect Physiology, 13: 925-932. https://doi.org/10.1016/0022-1910(67)90055-8
- Sakai Y., Osakabe MH. 2010. Spectrum-specific damage and solar ultraviolet radiation avoidance in the two-spotted spider mite. Photochemistry and Photobiology, 86: 925-932. https://doi.org/10.1111/j.1751-1097.2010.00739.x
- Sakai Y., Sudo M., Osakabe MH. 2012. A comparison of the effects of gravity and the nutritional advantage of leaf surfaces on fecundity in the two-spotted spider mite (Acari: Tetranychidae). Journal of the Acarological Society of Japan, 21(1): 1-6. https://doi.org/10.2300/acari.21.1
- Sances F.V., Wyman J.A., Ting I.P. 1979. Morphological responses of strawberry leaves to infestations of two-spotted spider mite. Journal of Economic Entomology, 72: 710-713. https://doi.org/10.1093/jee/72.5.710
- Schausberger P. 2021. Not seeing the mites for the hairs. Comment on Möth et al. Unexpected effects of local management and landscape composition on predatory mites and their food resources in vineyards. Insects, 12, 671. https://doi.org/10.3390/insects12080671
- Schmidt R.A. 2014. Leaf structures affect predatory mites (Acari: Phytoseiidae) and biological control: a review. Exp. Appl. Acarol., 62: 1-17. DOI 10.1007/s10493-013-9730-6 https://doi.org/10.1007/s10493-013-9730-6
- Seelmann L., Auer A., Hoffmann D., Schausberger P. 2007. Leaf pubescence mediates intraguild predation between predatory mites. Oikos 116: 807 817, 2007 doi: 10.1111/j.2007.0030-1299.15895.x, https://doi.org/10.1111/j.2007.0030-1299.15895.x
- Southwood R. 1986. Plant surface and insects - an overview. In: Juniper B., Southwood R. (Eds). Insects and the plant surface. Edward Arnold, London. p. 1-22.
- Suski Z.W., Naegele J.A. 1963. Light response in the two-spotted spider mite, Tetranychus urticae -I. Analysis of behavioral response; II. Behavior of the 'Sedentary' and 'Dispersal' phases. Recent Advances in Acarology, 1: 435-453.
- Tateishi S. 1987. The feeding activates of the mite Tetranychus urticae (Koch) and its effects on leaf burn in the pear Pyrus communis. Journal of the Faculty of Agriculture Shinshu University, 24: 1-79.
- Vàsquez C., Aponte O., Morales J., Sanabria M.E., García G. 2008. Biological studies of Olygonychus punicae (Acari: Tetranychidae) on grapevine cultivars. Experimental & Applied Acarology, 45: 59-69. https://doi.org/10.1007/s10493-008-9154-x
- Walter D.E. 1996. Living on leaves: Mites, tomenta, and leaf domatia. Annual Review of Entomology, 41: 101-114. https://doi.org/10.1146/annurev.en.41.010196.000533
- Walter D.E., O'Dowd D.J. 1995. Life on the forest phylloplane: hairs, little houses, and myriad mites. In: Lowman M., Nadkami N. (Eds). Forest canopies-a review of research on this biological frontier. New York Academic. p. 325-351.
- Walter DE. 1992. Leaf surface structure and the distribution of Phytoseius mites (Acarina: Phytoseiidae) in south-east Australian forests. Australian Journal of ZooIogy, 40(6): 593-603. https://doi.org/10.1071/ZO9920593
- Wilmer P. 1986. Microclimatic effects on insects at the plant surface. In: Juniper B., Southwood R. (Eds). Insects and the Plant Surface. Edward Arnold, London. p. 23-64.



2021-11-30
Date accepted:
2022-03-31
Date published:
2022-04-07
Edited by:
Migeon, Alain

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Tsolakis, Haralabos; Ragusa, Ernesto; Sinacori, Milko and Lombardo, Alberto
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




