New data on some Antennoseius Berlese species (Acari: Ascidae) from Russia
Joharchi, Omid  1
; Marchenko, Irina I.
1
; Marchenko, Irina I.  2
and Stanyukovich, Maria K.3
2
and Stanyukovich, Maria K.3
1✉ Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Semakova Str. 10, 625003 Tyumen, Russia.
2Institute of Systematics and Ecology of Animals, Novosibirsk, Russia.
3Zoological Institute of Russian Academy of Sciences, Saint Petersburg, Russia.
2022 - Volume: 62 Issue: 2 pages: 378-395
https://doi.org/10.24349/4vni-nuttOriginal research
Keywords
Abstract
Introduction
A remarkable diversity of phoretic and parasitic mites is found on ground beetles, representing three major groups: the Mesostigmata, Prostigmata, and Astigmatina (Olynyk and Freitag 1979; Hunter and Rosario 1988; Felska et al. 2018). Deutonymphs and adults of many families of Mesostigmata have established close phoretic relationships with ground beetles, and phoresy by adult females is common phenomenon among two genera of family Ascidae – Antennoseius Berlese and Anystipalpus Berlese (Lindquist et al. 2009; Trach 2013). Antennoseius is well known as a group of predatory mites generally found in soil, litter, moss in meadow, and forests, as well as in nests of rodents and birds but many species are also recorded on carabid beetles as phoretic (Ryke 1962; Costa 1969; Bregetova 1977; Karg 1993; Halliday et al. 1998; Eidelberg 2000; Beaulieu et al. 2008; Trach and Makarova 2008; Lindquist and Moraza 2009; Trach 2013; Moraza and Kazemi 2009; Kazemi and Moraza 2013; Faraji et al. 2017; Kazemi 2018). The genus comprises about 58 nominal species that are recorded worldwide (Moraes et al. 2016; Kazemi 2018; Bahrami and Kazemi 2019). Of these species, about 23 species of Antennoseius are phoretic on ground beetles (Faraji et al. 2017). Before this study, only five species regarded to belong to Antennoseius had been reported phoretic on ground beetles from Russia (Bregetova 1977; Faraji et al. 2017; Belova and Makarova 2017): A. (A.) bullitus Karg, 1969; A. (V.) multisetus Eidelberg, 2000; A. (V.) ovaliscutalis Eidelberg, 2000; A. (A.) pseudospinosus Eidelberg, 1990; A. (A.) sabulicola Bregetova, 1977. During a survey of ground beetle-associated gamasid mites in Russia, Antennoseius (Vitzthumia) bregetovae Chelebiev; Antennoseius (Antennoseius) bullitus Karg; A. (A.) pannonicus Willmann and A. (A.) ponticus Trach and Makarova were collected. The aim of this paper is to review the morphology of these species, and to add more details to their original descriptions. Moreover, we provides additional morphological information and new illustrations for A. (V.) bregetovae Chelebiev; A. (V.) hyperboreus Nikolsky and A. (V.) koroljevae Chelebiev, species known only from the type series to facilitate species delimitation.
Material and methods
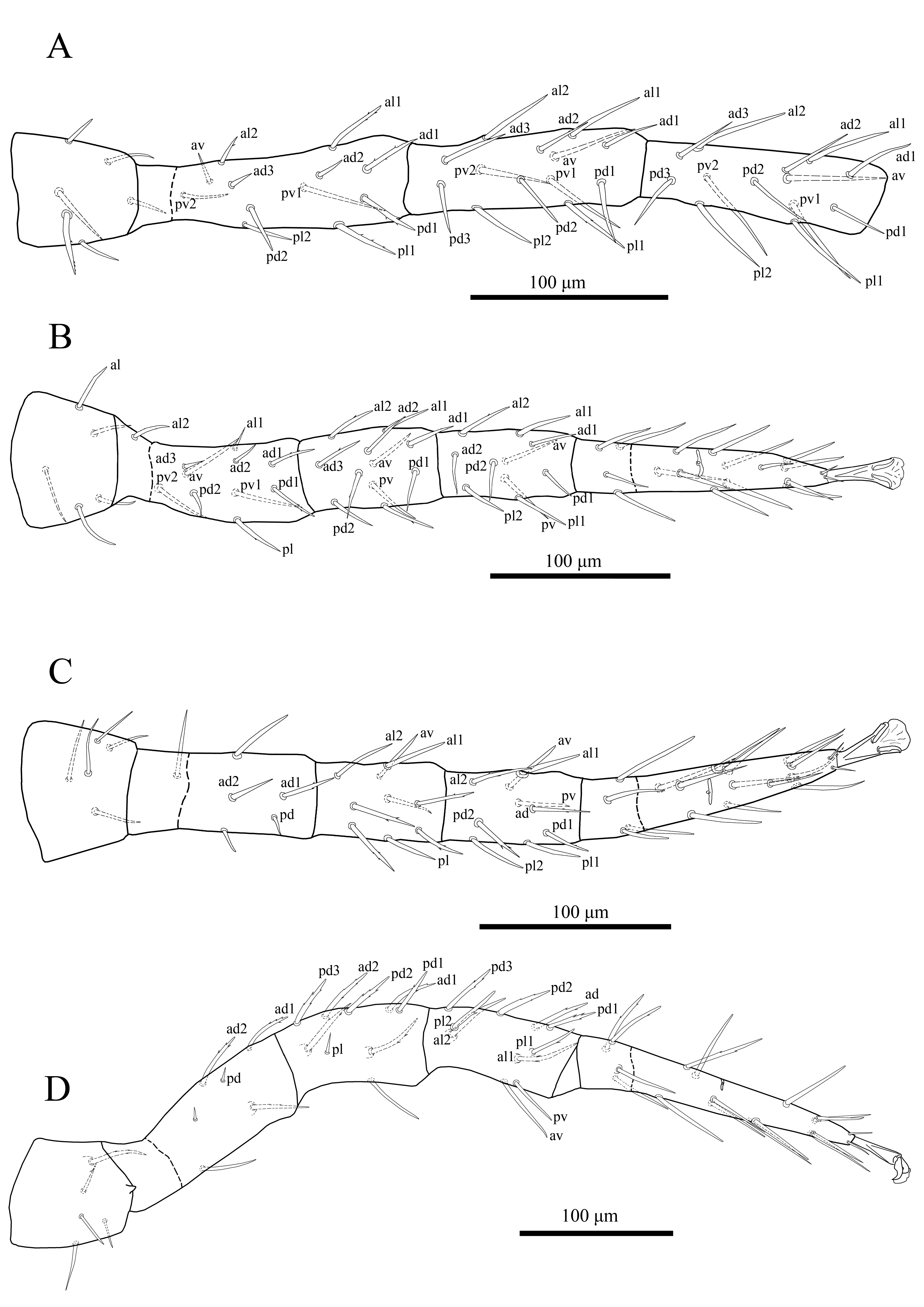
This study is based on phoretic Antennoseius species on ground beetles that were collected during several biological expeditions in Russia over a period of two years (2020–2021) and also on examination of type series of some specimens deposited in the collection of the Institute of Systematics and ecology of animals (ISEA, Novosibirsk), and collection of the Zoological Institute of the Russian Academy of Sciences (ZIN, Saint Petersburg). Host beetles were collected from different habitats by individual hand picking and placed individually in vials with 96% ethanol. Alcohol sediments from the vials were inspected for phoretic mites. Mites were removed from the beetles and alcohol sediments, cleared in lactic acid solution and mounted in Hoyer's medium (Walter and Krantz 2009). The line drawings and examinations of the specimens were performed with Zeiss Axio Imager A2 and Leica DM 2500 compound microscopes equipped with drawing tubes and differential interference contrast and phase contrast optical systems, attached to cameras AxioCam ICc 5 and ICC50 HD, respectively. Most images were captured in stacks (with focal depth manually controlled). Selected images were combined using Helicon Focus 7.6.4 Pro (Helicon Soft Ltd., 2000). Digital drawings were prepared using Adobe Photoshop CS2 software based on the original pencil line drawings. Images and morphological measurements were taken via ZEN 2012 software (version 8.0) and Leica Application Suite (LAS) software (version 4.2, Live and Interactive Measurements modules). Photomicrographs were taken with an AxioCam 506 camera (Carl Zeiss, Germany). Measurements of structures are expressed as ranges (minimum–maximum) in micrometres (μm). Podonotal and opisthonotal shields length were taken from anterior to posterior margins as midline and their width, respectively, from lateral margins at the level of dorsal setae r2 and at level of setae S1. Length and width of sternal shield measured at midline and level of st2, respectively. The length of the genital shield was measured along the midline from the anterior margin of the hyaline extension to the posterior margin of the shield, and its width where maximal (at level of setae st5). Anal shield was measured on midline length from anterior to posterior margins including cribrum and its width at the broadest point. Leg length was measured from the base of the coxa to the apex of the tarsus (excluding the pre-tarsus). The nomenclature used for the dorsal idiosomal chaetotaxy follows that of Lindquist and Evans (1965), the notations for leg and palp setae follow those of Evans (1963a, b), and other anatomical structures mostly follow Evans and Till (1979). Notations for idiosomal pore-like structures (gland pores and poroids/lyrifissures) and peritrematal shield follow mostly Athias-Henriot (1971, 1975). The notations for pore-like structures on the sternal shield and for the peritrematal shield region also follow modifications and additions by Johnston and Moraza (1991).
Taxonomy
Genus Antennoseius Berlese, 1916
Type species: Antennoseius delicatus Berlese, 1916, by original designation.Diagnosis: The diagnosis of Antennoseius used here is based on that of Moraes et al. (2016).
Antennoseius (Vitzthumia) bregetovae Chelebiev, 1984
Antennoseius (Vitzthumia) bregetovae Chelebiev, 1984: 1629.
Antennoseius (Vitzthumia) bregetovae Lindquist and Walter, 1989: 1293; Beaulieu et al., 2008: 54; Lindquist and Moraza, 2009: 33; Moraza and Kazemi, 2009: 64; Moraes et al., 2016: 67.
(Figures 1-2)






Specimens examined — Type material: slide number 1443 with one adult female (paratype), Saran, Karaganda province, Kazakhstan, 14.4.1973, K.A. Chelebiev coll., in nest of Lagurus lagurus (Pallas) (Rodentia: Cricetidae); deposited in the ZIN, Saint Petersburg. Other material: two adult females, Aktash, Ulagansky District, Altai Republic, Russia, 50°14′28.0″N 87°41′57.0″E, 30 July 2020, O. Joharchi coll., on Harpalus sp. (Coleoptera: Carabidae).
Redescription — Female — two specimens measured.
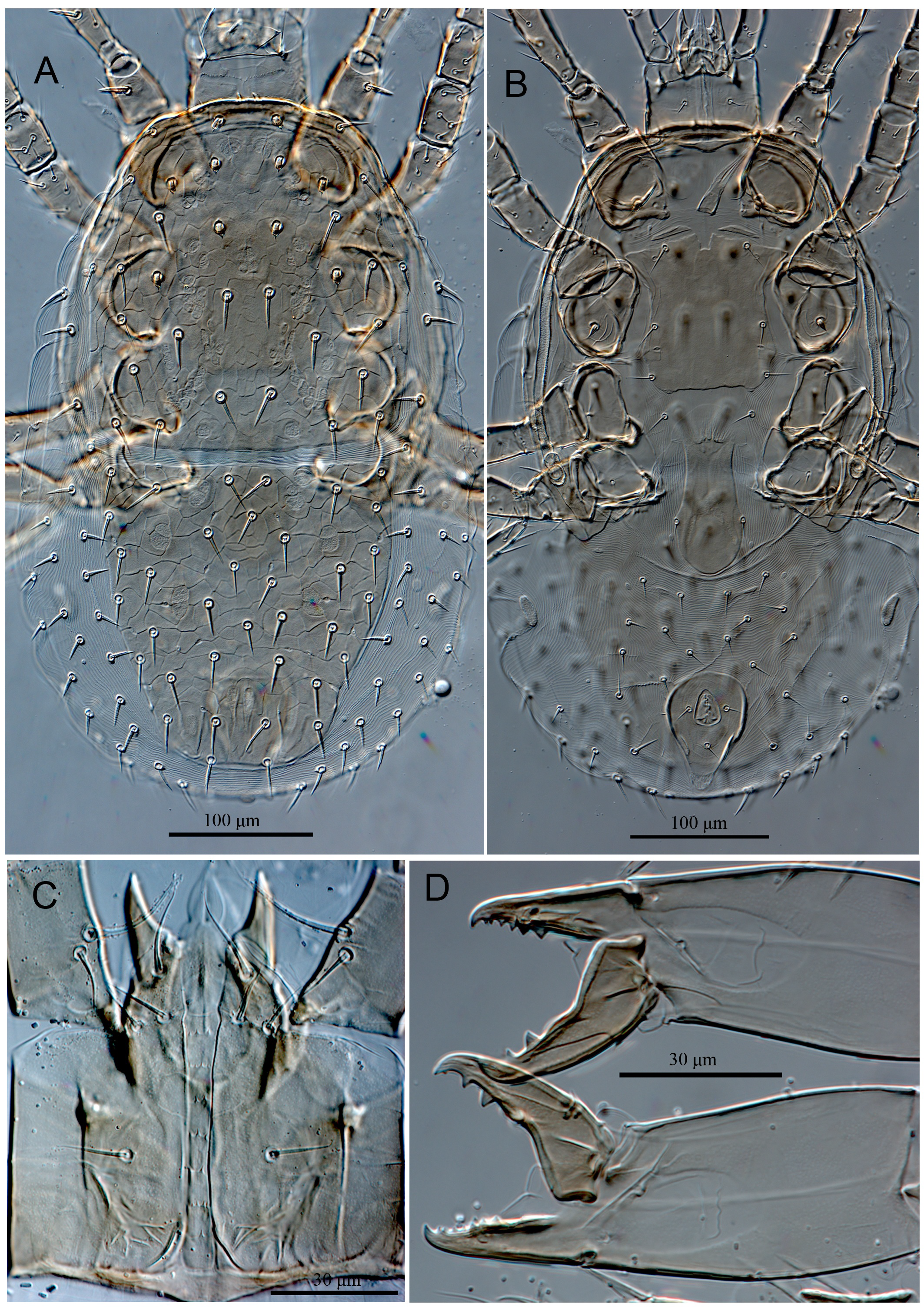
Dorsal idiosoma (Figures 1A & 2A) — Idiosoma oval (510–515 long × 305–328 wide); dorsal shield divided and lineate-reticulate over entire surface (Figures 1A & 2A). Podonotal shield (279–284 long × 262–275 wide) with 19 pairs of setae (j1–6, z1–6, s1–6, r2), r3–6 off shield, of which four pairs (j3–5 and z4) short (16–18) strongly thickened, spinelike, with a short thin tip (Figures 1A & 2A) and nine pairs of pore-like structures, including four pairs of gland openings (gd1, gd2, gd4, gd5) and five pairs of poroids (id1–2, id4–6). Setae j1 (11–13), j2 (14–16), z1 (7–9) smooth and short, j1 slightly spinelike, other podonotal shield setae sub-equal (20–28) and mostly smooth, occasionally with 1–2 small barbs in s and r series (Figures 1A & 2A). Opisthonotal shield (229–232 long × 262–275 wide), evenly rounded posteriorly, with 15 pairs of setae (19–25) (J1–5, Z1–5, S1–5), setae in Z and S series with 1–2 small barbs, Z5 (36–38) longest, and 13 pairs of pore-like structures, including three pairs of gland openings (gd6, gd8, gd9) and ten pairs of poroids (idm1–idm6, is1, idl1, idl3, idx) (Figures 1A & 2A); transverse interval between setae J5 about two times shorter than those between J4 and Z5. Lateral soft cuticle with one pair descramble poroid (idr6) and 21–22 pairs of setae, mostly sparsely barbed, including setae r3-r6 (18–21) and 17–18 pairs of setae R and UR (12–19). Muscle-marks (sigillae) visible mostly on podonotal shield (Figures 1A & 2A).
Ventral idiosoma (Figures 1B & 2B) — Tritosternum with paired sparsely pilose laciniae (90–94), fused basally (13–15), columnar base 20–22 × 13–16 wide; presternal area with pair of narrow indistinct platelets merged to anterior margin of sternal shield, sternal shield length 121–126, width 88–92, fused to endopodal elements between coxae I and II, with a median notch in its anterior margin, posterior margin more or less straight, lineate on lateral margins, with three pairs of smooth subequal setae st1–3 (28–32), and three pairs of poroids (Figures 1B & 2B). Metasternal setae st4 (29–31) smooth, inserted on soft cuticle. Endopodal platelets between coxae II-III and III-IV present, small and free. Genital shield drop-shaped length 127–133, width 55–57, faintly lineated laterally, anterior margin of shield convex, not overlapping posterior margin of sternal shield, posterior margin evenly rounded, setae st5 (23–25) on lateral margins of shield, paragenital poroids iv5 located on soft cuticle laterad to shield near setae st5 (Figures 1B & 2B). Anal shield obtriangular, length 100–103, width 75–77, with lineate-reticulate over entire surface, paraanal setae (18–20) shorter than post-anal seta (28–30), cribrum goatee-shaped, consisting of a terminal tuft with 3–4 irregular rows of spicules, restricted to area behind postanal seta (Figures 1B & 2B); anal gland pores (gv3) on anterolateral margin of anal shield, posterior to paranal setae, a pair of postanal sigillae present. Soft opisthogastric cuticle with pair of sub-oval metapodal plates (26–28 long × 9–11 wide), nine pairs of smooth setae (Jv1–5, Zv1–4) (15–26), three pairs of poroids (two ivo; ivp) and a gland pores gv2, laterad to coxae IV. Peritrematal shield fused with exopodal elements along coxae II to IV, anteriorly fused to podonotal shield, with longitudinal lines, evenly rounded posteriorly behind coxae IV, with two pairs of glandular pores and two pairs of poroids; gland pore gp1 located on shield's ventral edge near abutment of endopodal extension of sternal shield between coxae I and II, poroid ip1 and adjacent gland pore gp2 inserted at level between coxae II and III, and poroid ip2 next to posterior edge of stigma (Figures 1B & 2B). Peritremes long, extending from stigmata at mid-level of coxae IV to anterior margin of coxae I (Figures 1B & 2B).
Gnathosoma (Figures 1C, 1D & 1E) — Anterior margin of epistome convex, irregularly denticulate, mid surface of epistome with transverse line of denticles, slightly concave medially (Figure 1D). Hypostomal groove with seven transverse rows of denticles, with smooth anterior and posterior transverse lines, all connected laterally, rows 1–5 similar in width, each bearing one or two median denticles, 6th and 7th rows slightly widened and with four denticles (Figure 1C). Hypostome with four pairs of smooth setae, h1 (31–33) < h3 (25–27) < pc (18–20) < h2 (16–18). Corniculi robust and horn-like, slightly shorter than smooth internal malae. Supralabral process not distinguishable. Labrum blade-like, slightly shorter than internal malae. All palp setae smooth and setiform, palptarsal claw two-tined. Fixed digit of chelicera with an offset distal tooth (gabelzhan), followed by seven small sized teeth, a minute setiform pilus dentilis, and a hyaline flap at basal area, dorsal cheliceral seta thick, prostrate, movable digit with two large teeth medially and a minute tooth subapically, with a ventral projection, arthrodial membrane with a rounded flap, cheliceral lyrifissures distinct (Figure 1E).
Legs — Legs II (328–332) and III (335–342) short, legs I (430–438) and IV (418–423) longer.
Legs I to IV with chaetotactic formulae of femora, genua, tibiae as described for genus. Coxae II–IV lineate on posterior inner surfaces (Figure 1B); both setae of coxa I and posterior seta of coxa II modified as spines, pointed abruptly at tip, av on coxa II and setae of coxae III–IV normal, slender (Figure 1B). Leg I with setae pd of trochanter and pd2 of femur stout, pointed, spinelike, and with pd3 of genu stout, spinelike, blunt (Figure 1F); pd1 on femur slightly thickened, other setae normal, slender (Figure 1F). Legs II–IV without modified setae and smooth. Tibia III having eight setae (2–1/1–2/1–1).
Remarks — Antennoseius (V.) bregetovae was described from Kazakhstan (Chelebiev 1984). It has been found in nest of steppe vole, Lagurus lagurus (Pallas) (Rodentia: Cricetidae). The description of this species is brief and both the description and illustrations lack many important details. Since that time, there has been no other formal occurrence or recording of this species in the world, and is now recorded in Russia for the first time, on ground beetle Harpalus sp. (Coleoptera: Carabidae). Lindquist and Moraza (2009) suspected this species to be a senior synonym of Antennoseius (Vitzthumia) koroljevae Chelebiev due to both smooth-morph (phoretic) and granular-morph (free-living) occurred in the same locality and habitat, were discussed by Lindquist and Walter (1989). But according to Chelebiev (1984) and our observations on labels of type series of both species, these two species have been collected from two different nest of Rodents in two different regions and habitats (see provided information in specimens examined section for both species in the current study). In the meantime, A. (V.) koroljevae was collected about two years after A. (V.) bregetovae. Therefore, we believe these are two distinct species. The diagnosis given above is based primarily on specimens from Russia, but also in comparison with the type material. The species is easily recognized by the podonotal shield with four pairs of spur-like setae (j3–5 and z4), both setae of coxa I and posterior seta of coxa II modified as spines (pointed abruptly at tip), and leg I with setae pd of trochanter and pd2 of femur stout, pointed, spinelike, and with pd3 of genu stout, spinelike, blunt.
Antennoseius (Antennoseius) bullitus Karg, 1969
Antennoseius bullitus Karg, 1969: 398.
Antennoseius bullitus Karg, 1971: 301; 1977: 5; 1993: 306; Gwiazdowicz, 2007: 55; Kontschán, 2007: 101; Belova and Makarova, 2017: 978.
Antennoseius (Antennoseius) bullitus Bregetova, 1977: 251; Moraes et al., 2016: 67; Faraji et al., 2017: 338.
(Figure 3)



Specimens examined — Four females, vicinity of lake Kuchak, Tyumen Province, RUSSIA, 57°21′N, 66°03′E, 23 September 2021, O. Joharchi coll., on Harpalus sp. (Coleoptera: Carabidae).
Remarks — Antennoseius (A.) bullitus was described from Vogtland, Germany (Karg 1969) where it was found on grass. The species has been recorded as phoretic on different species of ground beetles in Europe (Hungary, Ukraine, Poland, Romania; see Faraji et al. 2017). Belova and Makarova (2017) collected the species on seven different carabid species from Southern Taiga Forest of Vologda, European Russia. Evidently, this species may be widely distributed in Central and Eastern Europe where associated with a diversity of ground beetles. It is now recorded in North Asia for the first time, on Harpalus sp. (Coleoptera: Carabidae). The species is easily recognized by podonotal shield with setiform setae, except seta j1 distally expanded (Figure 3A), sternal shield with a mid-longitudinal delineation (Figure 3B), coxal setae on legs I–IV slender and setiform (Figure 3B), genital shield drop-shaped, with lateral margins constricted anterior to genital setae and posterior margin more or less bluntly pointed (Figure 3B), anal shield with only circumanal setae (Figure 3B), hypostomal groove with seven transverse rows of denticles, rows 1–5 each with one or two median denticles, 6th and 7th rows bearing four and seven denticles, respectively (Figure 3C), anterior margin of epistome convex, sparsely denticulate, mid surface of epistome with transverse line of denticles, slightly concave medially (Figure 3D), fixed digit of chelicera with an offset distal tooth (gabelzhan), followed by 12-13 small sized teeth, movable digit with two large teeth medially and a minute tooth subapically (Figure 3E).
Antennoseius (Vitzthumia) hyperboreus Nikolsky, 1988
Antennoseius (Vitzthumia) hyperboreus Nikolsky, 1988: 30.
Antennoseius (Vitzthumia) hyperboreus Beaulieu et al., 2008: 55; Moraza and Kazemi, 2009: 65; Moraes et al., 2016: 69.
(Figures 4-6)








Specimens examined — Type material: slide number 75 with two adult females (holotype and paratype), Gydanskiy Peninsula, vicinity of Nosok village, Krasnoyarskii Krai, tundra, 24.07.1977, B.S. Yudin coll. on Microtus oeconomus (Pallas) (Rodentia: Cricetidae); deposited in ISEA, Novosibirsk.
Redescription — Female — Holotype and one paratype measured.
Dorsal idiosoma (Figures 4A, 5A & 5C) — Idiosoma oval (595–599 long × 341–345 wide); dorsal shield divided and harshly lineate-reticulate over entire surface (Figures 4A & 5A). Podonotal shield (320–324 long × 323–326 wide) with 20 pairs of setae (j1–6, z1–6, s1–6, r2, and one extra set of paired setae x between s3–s4), setae r2 and x off shield on the left side (in holotype), and nine pairs of pore-like structures, including four pairs of gland openings (gd1, gd2, gd4, gd5) and five pairs of poroids (id1–2, id4–6) (Figures 4A & 5A). Setae j1 (32–34) longest, slightly thicker, sparsely barbed, z1 (16–18) shortest, other podonotal shield setae sub-equal (27–35) and smooth (Figures 4A, 5A & 5C). Opisthonotal shield (273–275 long × 302–306 wide), evenly rounded posteriorly, with 15 pairs of setae (27–35) (J1–5, Z1–5, S1–5), R1 on the right side of anterolateral corner of the shield in holotype (Figures 4A & 5A), setae Z5 (40–42) longest, slightly thicker, sparsely barbed, and 13 pairs of pore-like structures, including three pairs of gland openings (gd6, gd8, gd9) and ten pairs of poroids (idm1–idm6, is1, idl1, idl3, idx) (Figures 4A & 5A). Lateral soft cuticle with 35–36 pairs of setae (including ventral setae), including r3–6 and 31–32 pairs of hypertrichous series R and UR, and two pairs of poroids. Muscle-marks (sigillae) visible mostly on podonotal shield (Figures 4A & 5A).
Ventral idiosoma (Figures 4B, 5B, 5D-E) — Tritosternum with paired pilose laciniae (100–103), fused basally (20–22), columnar base 24–26 × 20–23 wide; presternal area with a pair of narrow and faint platelets, sternal shield length 134–137, width 117–121, fused to endopodal elements between coxae I and II, with a median notch in its anterior margin, posterior margin irregularly straight, slightly lineate on lateral margins, region anterior to iv1 poorly sclerotized, with three pairs of smooth setae (st1 35–37, st2 33–35, st3 29–31) (st3 off shield on right side in holotype), and three pairs of poroids (iv3 absent on left side in holotype) (Figures 4B, 5B & 5E). Metasternal setae st4 (28–30) smooth, inserted on soft cuticle. Endopodal platelets between coxae II-III and III-IV present, small and free. Genital shield drop-shaped length 174–176, width 73–76, faintly lineated anteriorly, anterior margin of shield convex, not overlapping posterior margin of sternal shield, posterior margin evenly rounded, setae st5 (27–29) on lateral margins of shield, paragenital poroids iv5 located on soft cuticle laterad to shield near setae st5 (Figures 4B, 5B & 5D). Anal shield subtriangular, length 113–116, width 92–95, with lineate-reticulate over entire surface, para-anal setae and post-anal seta subequal in length (20–22), cribrum large, goatee-shaped, restricted to area behind postanal seta (Figures 4B, 5B & 5D); anal gland pores (gv3) on anterolateral margin of anal shield, posterior to paranal setae, a pair of postanal sigillae present. Soft opisthogastric cuticle with pair of sub-oval metapodal plates (20–22 long × 9–12 wide), ten pairs of setae, Jv1–3 and Zv1–4 smooth, Jv4–5, and Zv5 slightly ticker, with 1–2 small barbs, and four pairs of poroids (three ivo; ivp) and a gland pores gv2, laterad to coxae IV. Peritrematal shield fused with exopodal elements along coxae II to IV, anteriorly fused to podonotal shield, with longitudinal lines, evenly rounded posteriorly behind coxae IV, with one pair of glandular pore and two pairs of poroids; poroid ip1 and adjacent gland pore gp2 inserted at level between coxae II and III, and poroid ip2 next to posterior edge of stigma (Figures 4B & 5B). Peritremes long, extending from stigmata at mid-level of coxae IV to anterior margin of coxae I (Figures 4B & 5B).
Gnathosoma (Figures 4C-D & 5F-G) — Anterior margin of epistome convex, irregularly denticulate, mid surface of epistome with transverse line denticles, concave medially (Figure 4C & 5G). Hypostomal groove with seven transverse rows of denticles, each row with 4–9 small denticles, with smooth anterior and posterior transverse lines, all connected laterally, 6th and 7th rows slightly convex and concave, respectively (Figure 5F). Hypostome with four pairs of smooth setae, h1 (44–46) < pc (33–35) < h2 ≈ h3 (27–30). Corniculi robust and horn-like, slightly shorter than smooth internal malae. Supralabral process not distinguishable. Labrum blade-like, slightly shorter than internal malae. All palp setae smooth and needle-like except d1 on palpfemur with some barbs, palp-tarsal claw two-tined. Fixed digit of chelicera with an offset distal tooth (gabelzhan), followed by 19–20 small sized teeth, a minute setiform pilus dentilis, dorsal cheliceral seta thickened, thorn-like, movable digit with two large teeth medially, with a ventral projection, arthrodial membrane with a rounded flap, cheliceral lyrifissures distinct (Figure 4D).
Legs (Figure 6) — Legs II (506–512) and III (480–486) short, legs I (694–700) and IV (606–612) longer. Legs I to IV with chaetotactic formulae of femora, genua, tibiae as described for genus (Figure 6). Coxae II–IV lineate on posterior inner surfaces and setae of coxae I–IV normal, slender (Figure 4B). Legs I–IV setae unmodified, some setae sparsely barbed (more distinct on legs I and IV, Figure 6), not elongated as macrosetae, except four subapical setae on tarsus I elongated (ratio of subapical setae / tarsus I length ≈ 0.65). Tibia III having nine setae, pl2 present. Complement of setae on segments of legs I-II-III-IV: coxae 2–2–2–1; trochanters 6–5–5–5; femora 2–3/1–2/2–2; 2–3/1–2/2–1; 1–2/1–1/0–1; 1–2/0–1/1–1; genua 2–3/1–3/2–2; 2–3/1–2/1–2; 2–2/1–2/1–1; 2–2/0–3/1–1; tibiae 2–3/1–3/2–2; 2–2/1–2/1–2; 2–1/1–2/1–2; 2–1/1–3/1–2 and tarsi II-IV 3–3/2–1/1–3/2–3.
Remarks — Antennoseius (V.) hyperboreus was described from tundra in Northern Russia (Nikolsky 1988). It has been found on tundra vole, Microtus oeconomus (Pallas) (Rodentia: Cricetidae). Since that time, there has been no other formal occurrence or recording of A. (V.) hyperboreus in the world. The original description of the species is brief and its illustrations are incomplete, lacking many important details. We herein redescribe A. (V.) hyperboreus on the basis of type series to complement the original description which provided by Nikolsky (1988). The species is easily recognized by the podonotal shield with 20 pairs of setae, none of the setae enlarged (in distinction from others), setae j1 and Z5 sparsely barbed and slightly thicker than other setae, sternal shield with strong anterior medial notch (reaching to level of iv1), lateral soft cuticle with 35–36 pairs of setae (including ventral setae), coxal setae on legs I–IV slender and setiform, four long subapical setae on tarsus I (ratio of subapical setae / tarsus I length ≈ 0.65).
Antennoseius (Vitzthumia) koroljevae Chelebiev, 1984
Antennoseius (Vitzthumia) koroljevae Chelebiev, 1984: 1631.
Antennoseius (Vitzthumia) koroljevae Lindquist and Walter, 1989: 1293; Beaulieu et al., 2008: 55; Moraza and Kazemi, 2009: 63; Moraes et al., 2016: 70; Joharchi et al., 2020: 477.
Antennoseius (Vitzthumia) koreljevae (sic) Lindquist and Moraza, 2009: 3
(Figure 7)



Specimens examined — Type material: slide number 1792 with one adult female (paratype), vicinity of Nura River, Tokarevka, Karaganda province, Kazakhstan, 07.06.1975, K.A. Chelebiev coll., in nest of Microtus gregalis (Pallas) (Rodentia: Cricetidae); deposited in the ZIN, Saint Petersburg.
Remarks — Antennoseius (Vitzthumia) koroljevae is known only from its original description, being five females collected in nest of narrow-headed vole, Microtus gregalis (Pallas) (Rodentia: Cricetidae), in Kazakhstan (Chelebiev 1984). The description of the species is brief and both the description and illustrations lack many important details. We herein provide additional morphological characters to complement the previous description (Chelebiev 1984) of A. (V.) koroljevae on the basis of type series:
Dorsal shields densely granulate-tuberculate, lacking reticulate patterning (Figure 7A), podonotal shield with 21 pairs of setae including three pairs of sx setae and four unpaired seta x were present between setae j3–j5 (Figure 7A), setae r2, r3, r4 off the shield (Figure 7A), opisthonotal shield with 17 pairs of setae including two pairs of Sx setae and three unpaired seta X were present between Z and S series (Figure 7A), all dorsal setae moderately barbed (Figure 7A), setae j1 and Z5 longer and thinker than other setae; presternal area with pair of narrow distinct platelets (Figure 7B & 7C), sternal shield with strong anterior medial notch reaching half-way between st1 and st2 (Figure 7B & 7C); genital shield drop-shaped, its lateral margins widened posteriorly, posterior margin evenly rounded (Figure 7B & 7D); anal shield densely granulate-tuberculate, lacking reticulate patterning (Figure 7B & 7D); anterior margin of epistome convex, irregularly denticulate (Figure 7E), hypostomal groove with seven transverse rows of denticles, each row with 10–13 small denticles, with smooth anterior transverse line (Figure 7F); all palp setae smooth and needle-like, palp-tarsal claw two-tined, fixed digit of chelicera multidentate; almost all leg setae moderately barbed, setae pd on trochanter and pd2 on femur of leg I slightly thickened. Tibia III having eight setae (2–1/1–2/1–1).
Antennoseius (Antennoseius) pannonicus Willmann, 1951
Antennoseius pannonicus Willmann, 1951: 109.
Antennoseius pannonicus Athias-Henriot, 1961: 461; Ryke, 1962: 662; Karg, 1971: 298; 1977: 4; 1993: 305.
Antennoseius (Antennoseius) pannonicus Bregetova, 1977: 248; Beaulieu et al., 2008: 47; Lindquist and Moraza, 2009: 34; Moraes et al., 2016: 73; Faraji et al., 2017: 339; Bahrami and Kazemi, 2019: 357.
(Figure 8)



Specimens examined — Two females, vicinities of Uspenka, Tyumen Province, Russia, 57°04′N, 65°04′E, 18 October 2021, Vladimir A. Khaustov coll., on Harpalus sp. (Coleoptera: Carabidae).
Remarks — Antennoseius (A.) pannonicus was described from Austria (Willmann 1951) where it was found in meadow soil. The species has since been recorded as phoretic on different species of ground beetles in Europe (see Faraji et al. 2017) and Iran (see Bahrami and Kazemi, 2019). It is now recorded in Russia for the first time, on Harpalus sp. (Coleoptera: Carabidae).
Faraji et al. (2017) have redescribed this species based on newly collected specimens from the France. Our specimens agree very well with both the original description and redescription of the species given by Willmann (1951) and Faraji et al. (2017), respectively. This species can be readily recognized by the presence of four pairs of spur-shaped setae on podonotal shield (including j5) (Figure 8A), sternal shield with a prominent brownish crown-shape configuration between st1 setae (Figure 8B), setae st3 off sternal shield on soft cuticle (Figure 8B) and coxal setae on legs I-IV setiform (Figure 8B).
Antennoseius (Antennoseius) ponticus Trach and Makarova, 2008
Antennoseius ponticus Trach and Makarova, 2008: 181.
Antennoseius (Antennoseius) ponticus Lindquist and Moraza, 2009: 33; Moraes et al., 2016: 73; Faraji et al., 2017: 338.
(Figure 9)


Specimens examined — Three females, near the Moinakskoye Lake, Yevpatoria, Western Crimea, Russia, 45°11′31.0″N 33°19′59.0″E, 13 June 2021, O. Joharchi coll., on Harpalus sp. (Coleoptera: Carabidae).
Remarks — Antennoseius (A.) ponticus was originally described from southern Ukraine (Odessa and Mykolaiv Regions) where it was found on carabids (Harpalus serripes (Quensel), Amara sp.) and leaf beetle, Chrysolina gypsophilae (Küster) (Coleoptera: Chrysomelidae) (Trach and Makarova 2008), and afterward has been recorded from Eastern Ukrain (Trach 2013). It is now recorded in Russia for the first time, on Harpalus sp. (Coleoptera: Carabidae). Our specimens agree very well with the description given by Trach and Makarova 2008. The species may be recognized primarily by the podonotal shield with five pairs of spinelike (with a short thin tip) setae (including z4) (Figure 9A), presternal region with one pair of oblique narrow distinct platelets (Figure 9B), ventral posterior seta on coxae I and II modified as spines, pointed abruptly at tip (Figure 9B), fixed digit of chelicera with six teeth and movable digit with two large teeth medially and a minute tooth subapically (Figure 9D). The illustration and descriptions of the hypostomal groove in Trach and Makarova's (2008) shows six transverse rows of denticles, each row with 6–7 small denticles. Based on the specimens collected in Crimea, we found hypostomal groove with seven transverse rows of denticles, with smooth anterior and posterior transverse lines, rows 1–6 each bearing 2–4 small denticles, 7th row with only one median denticle (Figure 9C).
Acknowledgements
The present research was supported by the grant from the Russian Science Foundation, project number 20–64–47015. We cordially thank Dr. Alexander Khaustov, Mr. Vladimir A. Khaustov and Mr. Roman Latyntsev (Tyumen State University, Russia) for their help in collecting samples, and in logistics, respectively. We are deeply grateful to Zoological Institute of the Russian Academy of Sciences (ZIN, Saint Petersburg), and Institute of Systematics and ecology of animals (ISEA, Novosibirsk) for their hospitality of the first author during his visit for examining the mite collections.
References
- Athias-Henriot C. 1961. Mesostigmates (Urop. Excl.) édaphiques meditérranéens (Acaromorpha, Anactinotrichida) (collect. Prof. H. Franz et C. Athias-Henriot). Pemière Série. Acarologia, 3(4): 381-509. [in French].
- Athias-Henriot C. 1971. La divergence néotaxique des Gamasides (Arachnides). Bulletin Sc Bourgogne, 28: 93-106. [in French].
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. 2. Le relevé organotaxique de la face dorsale adulte (gamasides, protoadéniques, Phytoseiidae). Acarologia, 17: 20-29. [in French].
- Bahrami F., Kazemi S. 2019. First record of Antennoseius pannonicus Willmann (Mesostigmata: Ascidae) from Iran. Persian J. Acarol., 8(4): 357-359. https://doi.org/10.22073/pja.v8i4.53561
- Belova Yu.N., Makarova O.L. 2017. Mites (Acari) phoretic on ground beetles (Coleoptera, Carabidae) in a southern taiga forest in the environs of Vologda. Entomol. Rev., 97 (7): 975-983. https://doi.org/10.1134/S0013873817070120
- Berlese A. 1911. Alcuni Acari entomofili nuovi. Redia, 7: 183-186.
- Berlese A. 1916. Centuria terza di Acari nuovi. Redia, 12: 289-338.
- Beaulieu F., Déchêne A.D., Walter D.E. 2008. Phase morphs and phoresy: new species of Antennoseius (Vitzthumia) mites (Acari: Mesostigmata: Ascidae) associated with pyrophilous carabids (Carabidae: Sericoda spp.) in Alberta, Canada. Zootaxa, 1961: 37-57. https://doi.org/10.11646/zootaxa.1961.1.4
- Bregetova N.G. 1977. Family Antennoseiidae Karg, 1965. In: Ghilyarov, M.S., Bregetova, N.G. (Eds). Key to the soil-inhabiting mites, Mesostigmata. Leningrad: Nauka. p. 244-253. [in Russian].
- Chelebiev K.A. 1984. Mites of the genus Antennoseius (Parasitiformes: Mesostigmata) from central Kazakhstan. Zool. Zh., 63, 1629-1633. [in Russian].
- Costa M. 1969. Antennoseius bytinskii sp. nov., with notes on the genus Antennoseius Berlese (Acari: Mesostigmata) in Israel. Israel J. Entomol., 4: 217-226.
- Eidelberg M.M. 2000. Three new mite species of the family Antennoseiidae (Parasitiformes, Gamasina). Zool. Zh., 79(12): 1396-1401. [in Ukrainian].
- Evans G.O. 1963a. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata). Bull. Br. Mus. (Nat. Hist.), Zool., 10: 277-303. https://doi.org/10.5962/bhl.part.20528
- Evans, G.O. 1963b. Some observations on the chaetotaxy of the pedipalps in the Mesostigmata (Acari). Ann. Mag. Nat. Hist., Ser. 13, 6: 513-527. https://doi.org/10.1080/00222936308651393
- Evans G.O., Till W.M. 1979. Mesostigmatic mites of Britain and Ireland (Chelicerata: Acari Parasitiformes). An introduction to their external morphology and classification. Transactions of the Zoological Society of London, 35: 145-270. https://doi.org/10.1111/j.1096-3642.1979.tb00059.x
- Faraji F., Dehelean S.B., Vuyk S.M., Bakker, F. 2017. Four new species records of Antennoseius and Anystipalpus (Acari: Mesostigmata: Ascidae) phoretic on Carabidae beetles from France. Acarologia, 57(2): 337-353. https://doi.org/10.1051/acarologia/20174159
- Felska M., Wohltmann A., Mąkol J. 2018. A synopsis of host-parasite associations between Trombidioidea (Trombidiformes: Prostigmata, Parasitengona) and arthropod hosts. Syst. Appl. Acarol., 23: 1375-1479. https://doi.org/10.11158/saa.23.7.14
- Halliday R.B., Walter D.E., Lindquist E.E. 1998. Revision of the Australian Ascidae (Acarina: Mesostigmata). Invertebr. Taxon., 12: 1-54. https://doi.org/10.1071/IT96029
- Hunter P.E., Rosario R.M.T. 1988. Associations of Mesostigmata with other arthropods. Annu. Rev. Entomol., 33: 393-417. https://doi.org/10.1146/annurev.en.33.010188.002141
- Johnston D.E., Moraza M.L. 1991. The idiosomal adenotaxy and poroidotaxy of Zerconidae (Mesostigmata: Zerconina). In: Dusbábek F., Bukva V. (Eds). Modern Acarology. Vol. 2. Academia: Prague. p. 349-356.
- Karg W. 1969. Untersuchungen zur Kenntnis der Ascaoidea Karg, 1965 (Acarina: Parasitiformes) mit der Beschreibung von acht neuen Arten. Zool. Anzeiger, 182: 393-406.
- Karg W. 1971. Acari (Acarina), Milben, Unterordnung Anactinochaeta (Parasitiformes): Die freilebenden Gamasina (Gamasides), Raubmilben. In: Die Tierwelt Deutschlands und der Angrenzenden Meeresteile, 59. Teil. Gustav Fischer Verlag, Jena. 475p.
- Karg W. 1977. Die Milbengattung Antennoseius Berlese, 1916 (Acarina, Parasitiformes) mit einer neuen Art aus dem Leutratal bei Jena (DDR). Abhandlungen und Berichte des Naturkundemuseums Görlitz, 50(4): 1-7.
- Karg W. 1993. Acari (Acarina), Milben Parasitiformes (Anactinochaeta) Cohors Gamasina Leach, Raubmilben. In: Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und ihrer Lebensweise. 59. Teil. 2 überarbeitete Auflage, Gustav Fischer Verlag, Jena, 523 pp.
- Kazemi S. 2018. Redescription of Antennoseius (Antennoseius) avius Karg (Acari: Mesostigmata: Ascidae), a senior synonym of A. (A.) alexandrovi Bregetova and A. (A.) arvensis Kalúz, with additional information for Antennoseius Berlese, and a key to the Iranian species of the genus. Internat. J. Acarol., 44: 171-179. https://doi.org/10.1080/01647954.2018.1471101
- Kazemi S., Moraza M.L. 2013. Mites of the genus Antennoseius Berlese (Acari: Mesostigmata: Ascidae) from Iran. Persian J. Acarol., 2(2): 217-234.
- Kontschán J., 2007. New and rare mesostigmatid mites to the fauna of Hungary. Folia Historico Naturalia Musei Matraensis, 31: 99-106.
- Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entomol. Soc. Can. 47: 1-64. https://doi.org/10.4039/entm9747fv
- Lindquist E.E., Moraza M.L. 2009. Anystipalpus, Antennoseius and Vitzthumia: a taxonomic and nomenclatural conundrum of genera (Acari: Mesostigmata: Dermanyssina), with description of four species of Anystipalpus. Zootaxa, 2243: 1-39. https://doi.org/10.11646/zootaxa.2243.1.1
- Lindquist E.E., Walter D.E. 1989. Antennoseius (Vitzthumia) janus n.sp. (Acari: Ascidae), a mesostigmatic mite exhibiting adult female dimorphism. Can. J. Zool., 67: 1291-1310. https://doi.org/10.1139/z89-184
- Lindquist E.E., Krantz G.W., Walter D.E. 2009. Order Mesostigmata. In: Krantz G.W., Walter, D.E. (Eds). A Manual of Acarology. 3rd Edition. Texas: Texas Tech University Press, Lubbock. p. 1-807.
- Moraes G.J., Britto E.P.J., Mineiro J.L. de C., Halliday B. 2016. Catalogue of the mite families Ascidae Voigts & Oudemans, Blattisociidae Garman and Melicharidae Hirschmann (Acari: Mesostigmata). Zootaxa, 4112 (1): 1-299. https://doi.org/10.11646/zootaxa.4112.1.1
- Moraza M.L., Kazemi S. 2009. A new species of Antennoseius (Vitzthumia) Thor (Acari: Mesostigmata, Ascidae), associated with carabid beetles in Iran and a key to species. Internat. J. Acarol., 35(1): 59-65. https://doi.org/10.1080/01647950902884538
- Nikolsky V.V. 1988. New species of gamasid mite of the genus Antennoseius Berlese, 1916 (Parasitiformes, Ctamasina (sic)) from Siberia. In: Zolotarenko G.S. (Eds). Taksonomiya Zhivotnykh Sibiri. Novie i Maloizvestnye Vidy Fauny Sibiri. Akademiya Nauk SSSR: Sibirskoe Otdelenie. Biologicheskiy Institut. 20: p. 30-32. [in Russian].
- Olynyk J.E., Freitag R. 1979. Some phoretic associations of ground beetles (Coleoptera: Carabidae) and mites (Acarina). Can. Entomol., 111: 333-335. https://doi.org/10.4039/Ent111333-3
- Ryke P.A.J. 1962. The genus Antennoseius Berlese (Acarina: Rhodacaridae). Ann. Mag. Nat. Hist., 13(4): 657-663. https://doi.org/10.1080/00222936108651190
- Trach V.A., Makarova O.L. 2008. A new gamasid mite species of the genus Antennoseius (Parasitiformes, Ascidae) from the southwest of Ukraine. Vest. Zool., 42(2): 181-184.
- Trach V.A. 2012. A new species of mites of the genus Anystipalpus (Mesostigmata, Ascidae) from the Eastern Ukraine. Vest. Zool., 46(1): 73-77. https://doi.org/10.2478/v10058-012-0005-1
- Trach V.A. 2013. On the fauna of gamasid mites of the genera Anystipalpus and Antennoseius (Mesostigmata, Ascidae) of the Eastern Ukraine. Vest. Zool., 47(5): 387-393. https://doi.org/10.2478/vzoo-2013-0037
- Walter D.E., Krantz G.W. 2009. Collecting, rearing and preparing specimens. In: Krantz G.W., Walter D.E. (Eds). A Manual of Acarology. Third Edition, Texas Tech University Press, Lubbuck, Texas, p. 83-95.
- Willmann C. 1951. Untersuchugen über die terrestrische Milbenfauna im pannonischen Klimagebiet Österreichs. Sitzungsberichte der Österreichischen Akademie der Wissenschaften, Mathematisch. Naturwissenschaftliche Abteilung I, 160: 91-176.



2022-02-23
Date accepted:
2022-03-24
Date published:
2022-03-28
Edited by:
Faraji, Farid

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Joharchi, Omid; Marchenko, Irina I. and Stanyukovich, Maria K.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




