Mites occurring in farm buildings as allergic agents and indicators in forensic analyses
Solarz, Krzysztof  1
; Pająk, Celina2
; Pawełczyk, Olga
1
; Pająk, Celina2
; Pawełczyk, Olga  3
; Bobiński, Rafał4
; Ciechacka, Maria5
; Dutka, Mieczysław6
and Ulman-Włodarz, Izabela7
3
; Bobiński, Rafał4
; Ciechacka, Maria5
; Dutka, Mieczysław6
and Ulman-Włodarz, Izabela7
1✉ Department of Parasitology, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia in Katowice, Jedności 8, 41-218 Sosnowiec, Poland.
2Department of Biochemistry and Molecular Biology, Faculty of Health Sciences, University of Bielsko-Biała, Willowa 2, 43-300 Bielsko-Biała, Poland.
3✉ Department of Parasitology, Faculty of Pharmaceutical Sciences in Sosnowiec, Medical University of Silesia in Katowice, Jedności 8, 41-218 Sosnowiec, Poland.
4Department of Biochemistry and Molecular Biology, Faculty of Health Sciences, University of Bielsko-Biała, Willowa 2, 43-300 Bielsko-Biała, Poland.
5Department of Nursing, Faculty of Health Sciences, University of Bielsko-Biała, Willowa 2, 43-300 Bielsko-Biała, Poland.
6Department of Biochemistry and Molecular Biology, Faculty of Health Sciences, University of Bielsko-Biała, Willowa 2, 43-300 Bielsko-Biała, Poland.
7Department of Biochemistry and Molecular Biology, Faculty of Health Sciences, University of Bielsko-Biała, Willowa 2, 43-300 Bielsko-Biała, Poland.
2022 - Volume: 62 Issue: 1 pages: 36-47
https://doi.org/10.24349/gsxm-p9jjOriginal research
Keywords
Abstract
Introduction
It is impossible to draw a line between allochthonic and autochthonic fauna of mites inhabiting farm buildings (van Bronswijk 1981; Solarz 2009). Storage mites (Acari: Sarcoptiformes: Astigmatina), especially several species in the families Acaridae (Acarus siro, A. farris, Tyrophagus longior, and T. putrescentiae), Glycyphagidae (Glycyphagus domesticus and Lepidoglyphus destructor), and Chortoglyphidae (Chortoglyphus arcuatus), are commonly found in hay, straw, stored products, barns and other farming and occupational environments, as well as in dust from freight wagons and house dust (Boström et al. 1997; Franz et al. 1997; Mehl 1998; Sánchez-Ramos et al. 2004; Pike and Wickens 2008; Henszel et al. 2011; Solarz 2009; Solarz and Pająk 2019). The listed mite species were identified as a source of clinically important allergens, causing occupational allergies, commonly known as storage mite allergies. Asthma, rhinitis, and conjunctivitis commonly occur among farmers, grain storage workers, and other agricultural workers (Dutkiewicz et al. 1988; Revsbech and Dueholm 1990; Fain et al. 1990; van Hage-Hamsten and Johansson 1998). These mites cause IgE-mediated sensitisation in workers from these occupational categories who are exposed to organic dust containing these mites' allergens (Sánchez-Ramos et al. 2004; Arlian 2002; Berger et al. 2005; Cichecka et al. 2006; Solarz 2012).
Storage mites have not yet been explored widely in connection with forensic examinations, but there are good reasons for further investigations (Perotti et al. 2009). Indoor mites occur globally in farm buildings, however, the species' composition may vary between places, seasons, and even between sites within the indoor environment of a single farm. Subtle differences in the composition of the storage mite acarofauna between particular sites and types of farm buildings may provide valuable information, such as an indicator of time and circumstances of death (Solarz 2009).
This study aimed to investigate the possible occurrence and abundance of allergenic mites in farms. Moreover, the mite species compositions in material samples collected from farming environments were analysed in relation to forensic medicine.
Material and methods
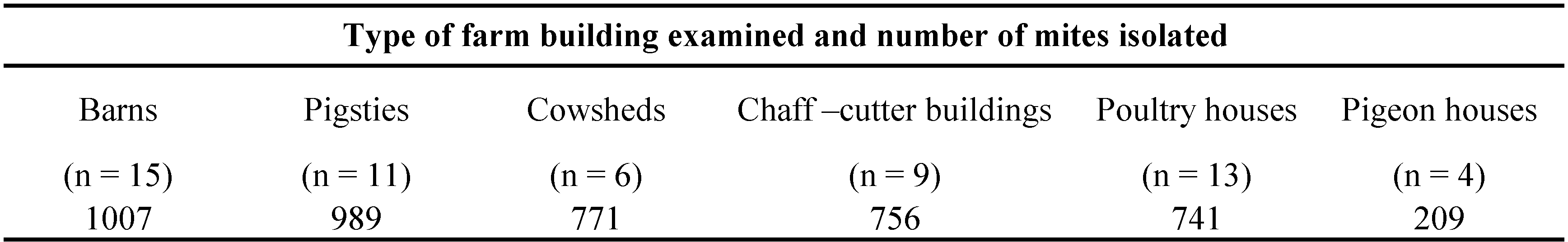
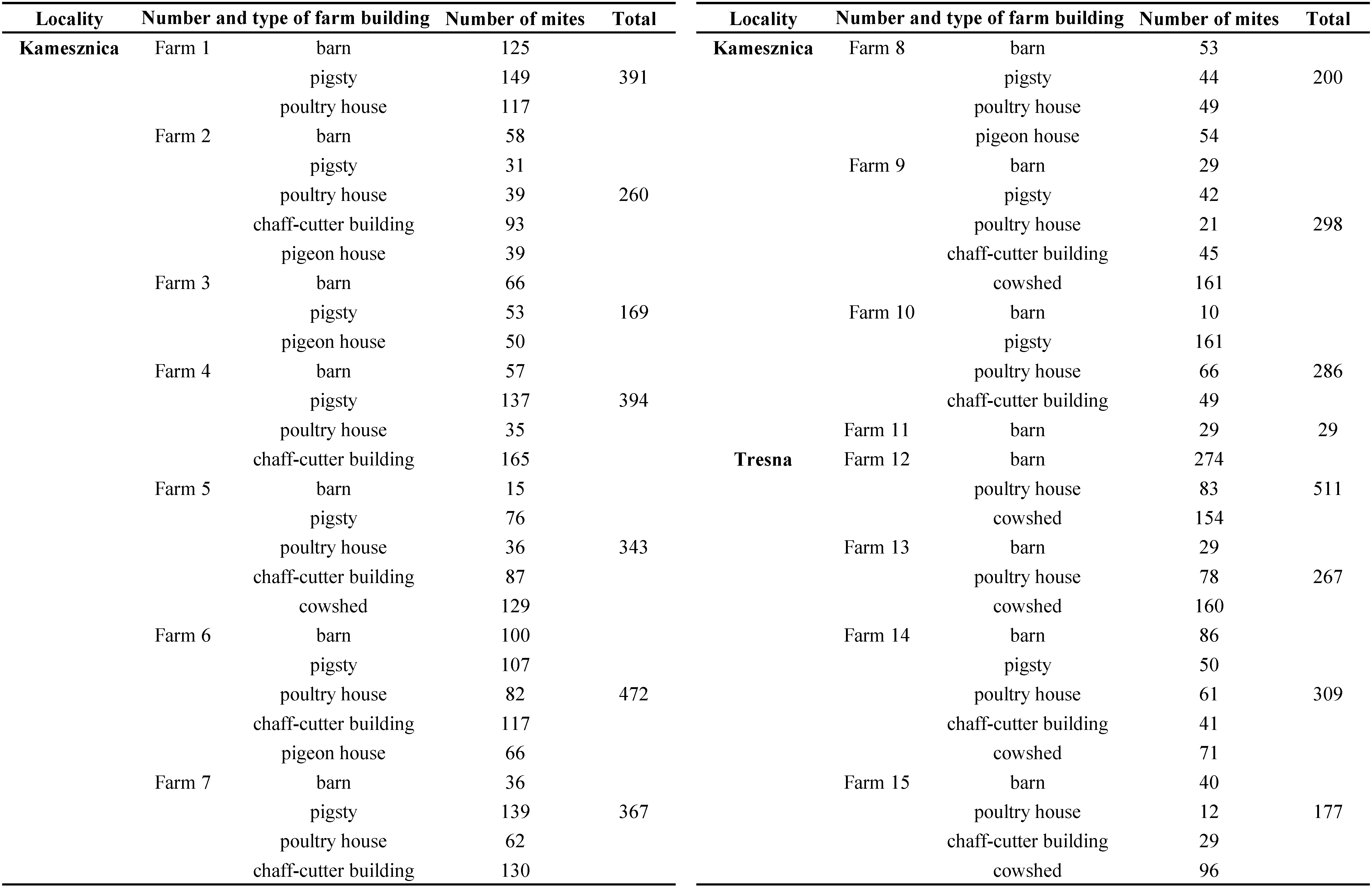
The study was carried out from July to September 2015. A total of 58 samples of materials collected from 15 farms in 2 localities (Kamesznica and Tresna) in the Żywiecki district (Silesian Province, south-western Poland) were examined as potential sources of allergenic mites in farm buildings with a special reference to forensic acarology (Table 1). All samples were collected in one-litre plastic bags. These samples were sweepings containing organic dust, litter, debris, residues, and other materials from certain farming environments such as barns (n=15), pigsties (n=11), cowsheds (n=6), chaff-cutters (n=9), poultry houses (n=13), and pigeon-houses (n=4) (Table 1). The mites were extracted using the Berlese–Tullgren funnel method described by Berlese (1905) and Tullgren (1918), with some modifications. This method creates a desiccation gradient over the examined samples, so that mobile arthropods will move away from the dry environment and fall into a vessel with 75% ethanol. Faure's medium was used to mount the mites on microscope slides and they were identified with the aid of a stereomicroscope Olympus Europe Highlight 2100. All mite specimens collected were examined using differential interference contrast (Nomarski DIC) (under a light microscope Zeiss Axioskop 2 plus) and phase contrast optics (under a light microscope Olympus CH 40). The mites were identified using descriptions of taxa and the following keys or publications: Hughes (1976), Zhang and Fan (2005), Fan and Zhang (2007a, b), Krantz and Walter (2009), and Solarz (2011a, 2012). The results were expressed as the number of mites per one sample.
The relative abundance and occurrence of the species of mite collected, as well as their categories of dominance and frequency, were calculated according to publications: Rajski (1961), Solarz and Senczuk (2003). The following levels of dominance (D) and frequency (F) were adopted:
- D
- Eudominant – species forming more than 10% of the total mite population.
- Dominant – species forming between 5.1–10% of the total mite population.
- Subdominant – species forming between 2.1–5% of the total mite population.
- Recedent – species forming between 1.1–2% of the total mite population.
- Subrecedent – species forming less than 1.1% of the total mite population.
- F
- Euconstant – species occurring in more than 25% of samples.
- Constant – species occurring in 11–25% of samples.
- Accessory – species occurring in 1–10% of samples.
- Accidental – species occurring in less than 1% of samples examined.
The average dominance [%] of collected mite species/taxa in the particular types of farm buildings examined was performed using CSS-Statistica for Windows version 12.
Results
Overall results
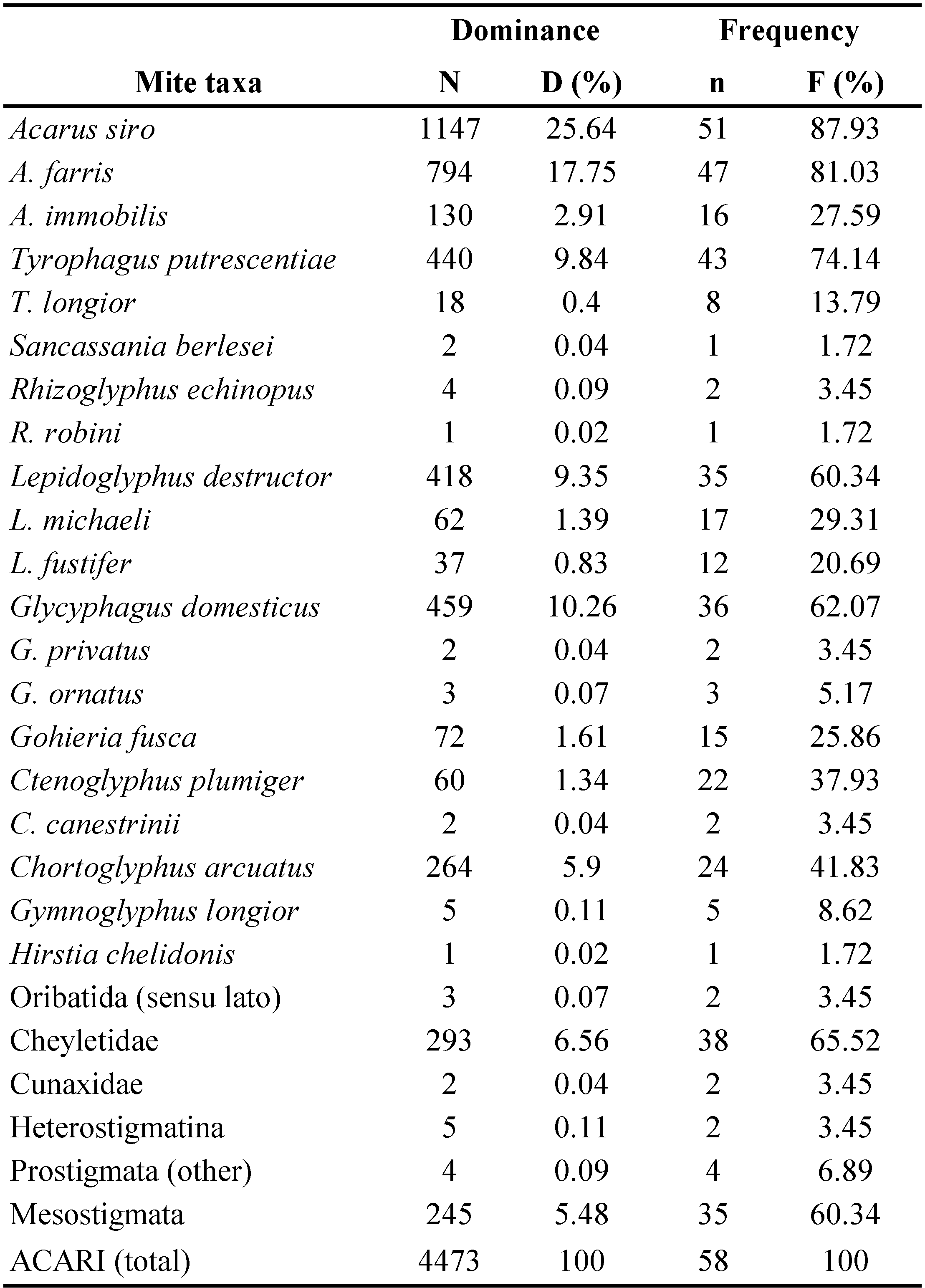
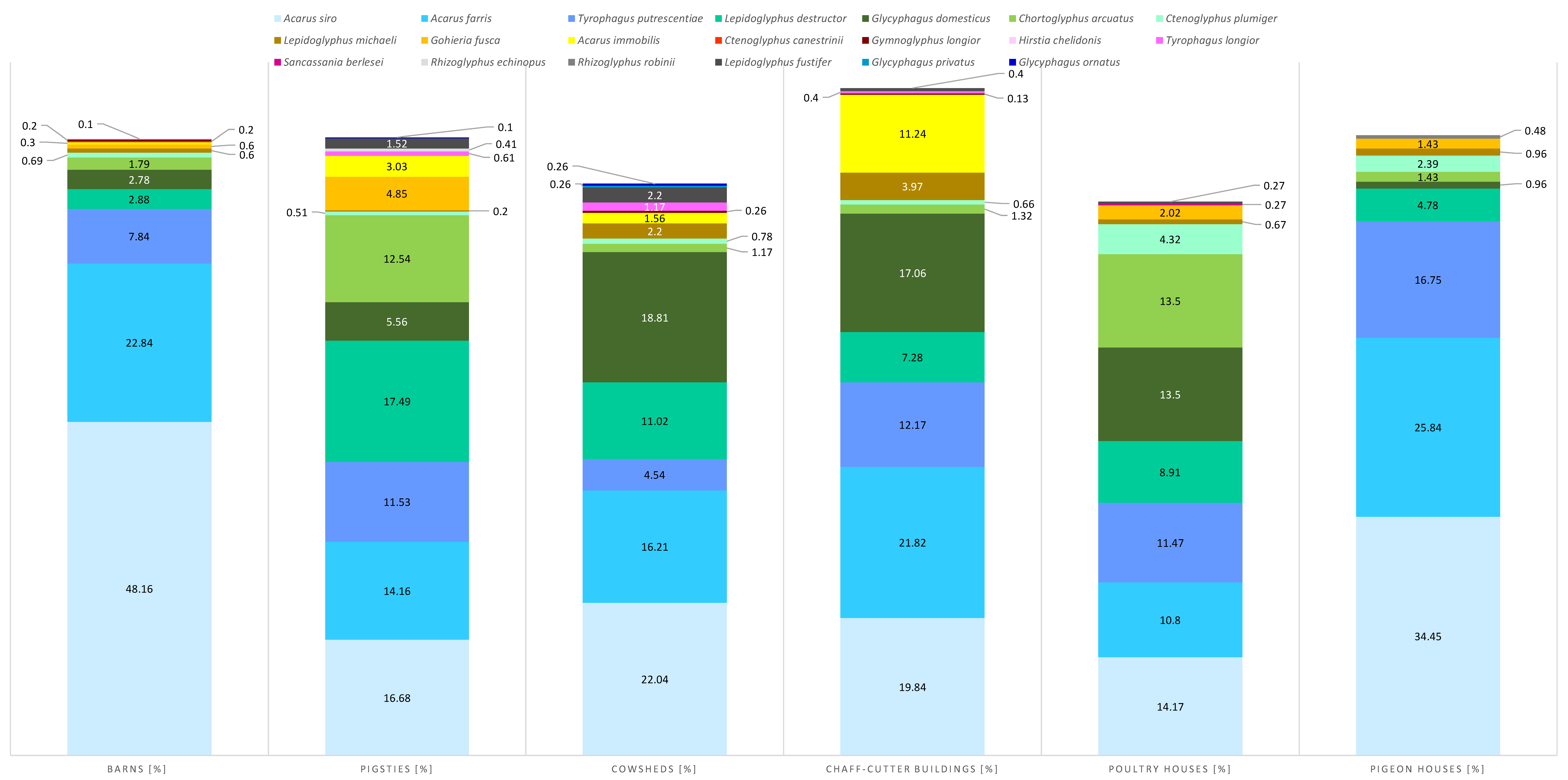
Mites were found in all the samples examined. In total 4,473 mites were isolated (Tables 1 and 2) and 20 species were identified from the different farm buildings (Table 3). Most of them were found in barns (n=1007, approx. 22.5% of the total count) and pigsties (n=989, 22.1%). The remaining mites were found in cowsheds (17.2%), chaff-cutters (16.9%), poultry houses (16.6%), and pigeon houses (4.7%) (Tables 1 and 2). The material was collected from 15 farms, situated in two localities – Kamesznica (11 farms) and Tresna (4 farms) (Table 2). The highest number of mites was collected in farm no. 12 from Tresna (n=511) and no. 6 from Kamesznica (n=472) (Table 2). The samples were dominated by Acaridae, Glycyphagoidea, and Cheyletidae. The species composition of the acarofauna varied between different types of examined farm buildings (Table 4; Figures 1-2). Generally, eudominant species belonged to the family Acaridae (Astigmatina) – Acarus siro (25.6% of all mites) and A. farris (17.75%), as well as Glycyphagus domesticus (10.3%) of the family Glycyphagidae (Astigmatina). Moreover, among the relatively numerously occurring mites (dominant species) were Tyrophagus putrescentiae (9.84%), Lepidoglyphus destructor (9.3%), cheyletids (Prostigmata: Cheyletidae) (6.55%), and Chortoglyphus arcuatus (Glycyphagoidea: Chortoglyphidae) (5.9%). Acarus immobilis (Acaridae) was a subdominant species and accounted for 2.91% of all mites. Other species of storage mites were generally less abundant. Gohieria fusca, Lepidoglyphus michaeli, and Ctenoglyphus plumiger belong to the recedent species group, while, L. fustifer, Tyrophagus longior, Gymnoglyphus longior, Hirstia chalidonis, as well as, other members of Oribatida sensu lato and other Prostigmata (Table 3; Figures 1-2) to the subrecedent group. The most frequent species found in samples from the examined farm buildings were A. siro, A. farris, T. putrescentiae, G. domesticus, and L. destructor with relative frequency 87.9%, 81.03%, 74.14%, 62.1% and 60.34%, respectively (Table 3). Moreover, Cheyletidae (65.5%) and some other potentially allergenic mites in the cohort Astigmatina, such as Ch. arcuatus (41.4%), Ct. plumiger (37.9%), L. michaeli (29.3%), A. immobilis (27.6%), and G. fusca (25.9%) also belong to the euconstant group (Table 3).









Mites from barns
A total of 1,007 mites were collected from barns, including 13 species from the cohort Astigmatina (Figure 2). Mite fauna was dominated by A. siro (48.2% of mites collected from barns) and A. farris (22.8%), both of which were eudominant species. Moreover, allergenic predatory mites of the family Cheyletidae (9.3%) and T. putrescentiae (7.8%) were dominants, while L. destructor (2.9%) and G. domesticus (2.8%) were subdominants. The remaining astigmatic mites belonged to recedent and subrecedent species. Mesostigmata constituted only 1.7% of the collected mites (Figures 1 and 2).


Acarus siro was also the most frequent species (86.7%), followed by A. farris (73.3%) and T. putrescentiae (46.7%). Among the euconstant species were also cheyletids (60.0%), other astigmatic mites – L. destructor and G. domesticus (33.33% in both cases), G. fusca and Ch. arcuatus (26.7% in both cases), as well as representatives of Mesostigmata (53.33%) (Table 4).
Mites from pigsties
Acarofauna from pigsties included 989 specimens of mites. Among them, there were eudominant species, like L. destructor (17.5% of the total count), followed by A. siro (16.6%), A. farris (14.1%), Ch. arcuatus (12.5%) and T. putrescentiae (11.5%) (Figure 2). In general, 14 species of astigmatic mites were found, including dominant species (G. domesticus), subdominant species (G. fusca, A. immobilis), recedents (L. fustifer) and subrecedents (T. longior, Ct. plumiger, Rhizoglyphus echinopus, L. michaeli, and G. ornatus). Among the remaining mite taxa were cheyletids, which constituted 8.6% of the total mite population of pigsties (dominants), and Mesostigmata (subdominants, 2.2%) (Figures 1 and 2).
Acarus siro was the most frequent species collected from pigsties, and was found in all examined samples (Table 4). Moreover, other allergenic mite species, like T. putrescentiae and L. destructor (81.8% in both cases), A. farris and G. domesticus (72.7% in both cases), G. fusca (63.6%), Ch. arcuatus (54.5%), and taxa, such as cheyletids (63.6%) and Mesostigmata (54.5%) also belong to the euconstant group (Table 4).
Mites from cowsheds
Acarofauna from cowsheds was also dominated by the astigmatic mites. Out of 14 identified Astigmatina species, A. siro was an eudominant (22.04%), followed by G. domesticus (18.8%), A. farris (16.2%), and L. destructor (11.02%). Moreover, among the numerously occurring astigmatic mites there were also the subdominant species – T. putrescentiae (4.5%), L. michaeli (2.2%), L. fustifer (2.2%), and one recedent – Ch. arcuatus (1.2%). Cheyletid mites were dominants, and constituted 6.5% of the total count from cowsheds (Figures 1 and 2).
It should be stressed, that the most frequently occurring mites (euconstants) once again were allergenic species A. siro, A. farris, T. putrescentiae, and G. domesticus (83.3% in all cases), followed by L. destructor (66.7%) (Table 4).
Mites from chaff-cutter buildings
A total of 756 mites were isolated from samples collected from chaff-cutter buildings. Acarofauna of chaff-cutters was also dominated by the mites of the families Acaridae and Glycyphagidae, especially A. farris (21.8%), A. siro (19.8%), G. domesticus (17.1%), T. putrescentiae (12.2%), A. immobilis (11.2%) and L. destructor (7.3%) (Figure 2).
Acarus farris was the most frequent species, and other euconstant taxa were T. putrescentiae (88.9% of all samples), A. siro, A. immobilis (77.8% in both cases), G. domesticus (66.7%), L. destructor, and L. michaeli (55.5% for both species), Cheyletidae and Mesostigmata (55.5% in both cases) (Table 4).
Mites from poultry houses
Of the 741 mites collected, 11 mite species of the cohort Astigmatina were found in poultry houses. A. siro was the eudominant (14.2% of the total count), followed by other species, such as Ch. arcuatus and G. domesticus (13.5%, in both cases), T. putrescentiae (11.5%), and A. farris (10.8%). Moreover, Ct. plumiger, G. fusca, Lepidoglyphus fustifer, L. michaeli, and Sancassania berlesei were reported, while Mesostigmata and Cheyletidae constituted 14.4% and 5.4% respectively of all mites collected from poultry houses (Figures 1 and 2).
The most frequent species in poultry houses were A. siro and G. domesticus (84.6% of all samples examined in both cases). Among the most frequent species were also some other species of allergenic or potentially allergenic mites – T. putrescentiae, A. farris, and L. destructor (76.9%), Ch. arcuatus (61.5%), Ct. plumiger (53.8%), L. fustifer and L. michaeli (30.8%), and G. fusca (23.1%). Among relatively frequent taxa were also cheyletids (69.2%) and Mesostigmata (53.8%) (Table 4).
Mites from pigeon houses
A total of 209 mites were collected from pigeon houses, including 10 species in the cohort Astigmatina. Mite fauna was dominated by two species (eudominant) of the genus Acarus – A. siro (34.45% of mites collected from pigeon houses) and A. farris (25.8%). A significant part of collected material constituted T. putrescentiae (7.8%), L. destructor (4.8%), as well as, nonastigmatic mites of the Cheyletidae family (7.18%). The remaining astigmatic mites were less numerous and belonged to the subdominant group – Ct. plumiger (2.4%), the recedent group – Ch. arcuatus, and G. fusca (1.4% in both cases), and the subrecedent group – G. domesticus and L. michaeli (approx. 1.0%, in both cases). Mesostigmata constituted only about 1.0% of all mites collected (Figures 1 and 2).
The allergenic mite species, like A. siro, A. farris, and T. putrescentiae were euconstants and they occurred in all examined samples from pigeon houses. Other frequently occurring taxa were L. destructor, Ct. plumiger (50.0% in both samples), as well as, Cheyletidae and Mesostigmata (75.0% in both cases), while G. domesticus, L. michaeli, G. fusca, Ch. arcuatus, and R. echinopus were constant species (25.0% in these cases) (Table 4).
Exposure of farmers and citizens to allergenic or potentially allergenic mites
The risk of exposure of farmers and other citizens to allergenic mites seems to be highest in barns and pigsties, and lowest in pigeon houses (Tables 3-5). However, it should be stressed that in all examined farm buildings a high number of allergenic mites were found, including the most medically important taxa such as Acarus siro complex, T. putrescentiae, G. domesticus, L. destructor, and Ch. arcuatus. In barns, the risk of exposure concerned was mainly connected with A. siro, A. farris, and then T. putrescentiae, L. destructor, and G. domesticus (Figure 2). In pigsties, farmers are exposed mainly to L. destructor, A. siro, A. farris, Ch. arcuatus, and T. putrescentiae, whereas in cowsheds the main exposure is to A. siro, G. domesticus, A. farris, and L. destructor (Figure 2). In chaff-cutter buildings apart from members of the Acarus siro complex, farmers are exposed to high numbers of G. domesticus, and in poultry houses to G. domesticus, Ch. arcuatus, T. putrescentiae, and mites of the genus Lepidoglyphus (Figure 2). Among species of the Acarus siro complex, A. siro was the most numerous in poultry houses, whereas A. farris was the most numerous in chaff-cutters. Pigeon houses were dominated by mites of the family Acaridae – A. siro, A. farris, and T. putrescentiae, respectively (Figure 2). It should be stressed that two species of the pyroglyphid mites were also found. H. chelidonis was found only in barns, whereas G. longior was found in barns, cowsheds, and chaff-cutter buildings (Table 4). Farmers and citizens living in the Żywiecki district (Silesian Province, Poland) are also exposed to high numbers of allergenic predatory mites of the family Cheyletidae, especially in barns and pigsties (Figure 1; Table 4).
Occurrence and frequency of particular astigmatic mite species in different farm buildings in relation to forensic analyses
Acarus siro was the most frequently occurring mite in almost all the types of examined farm buildings except for chaff-cutters, where it was preceded only by A. farris and T. putrescentiae. In cowsheds, these three species occurred with the same frequency (Table 4). The frequency of A. siro was the highest in pigsties and pigeon houses (100%), followed by barns (87%), poultry houses (85%), cowsheds (83%), and chaff-cutters (78%). G. domesticus occurred most frequently in poultry houses (85% positive samples) and cowsheds (83% samples) (Table 4).
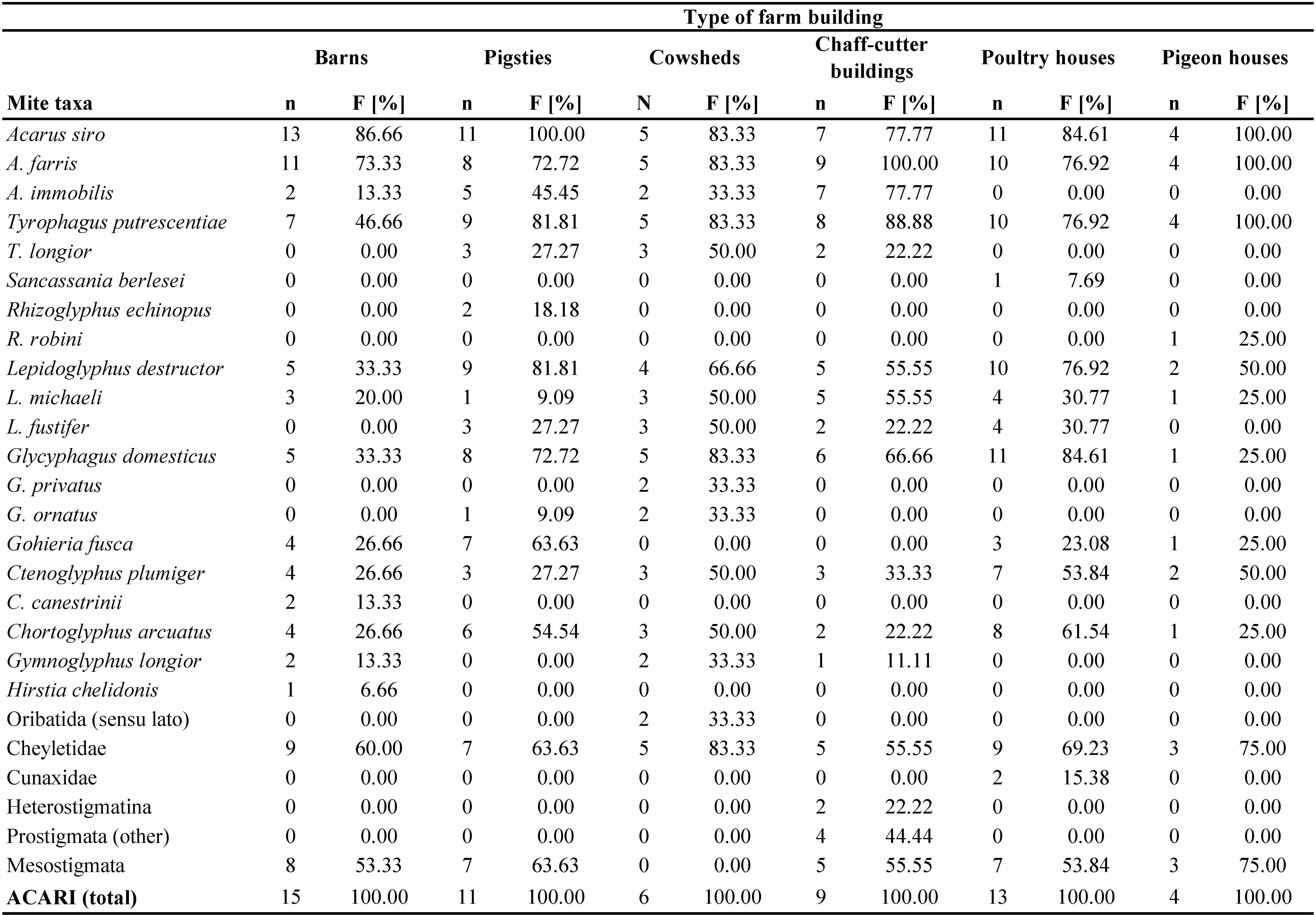


In summary, it should be stressed that A. siro was the most abundant species per sample in barns, cowsheds, and pigeon houses, whereas Ch. arcuatus was the most abundant in pigsties and poultry houses. In chaff-cutter buildings, the most abundant species were G. domesticus and A. siro (Table 5).



Discussion
Up until now many groups of mites have been reported as occupational biohazards for humans (Arlian 2002). This study describes the composition of storage mite communities collected from farm buildings in the southwestern Poland. The greatest exposure to storage mites usually occurs in agricultural settings, and therefore agricultural work is considered a major risk factor for occupational diseases (van Hage-Hamsten and Johansson 1998; Arlian 2002; Berger et al. 2005; Cichecka et al. 2006). Our previous studies suggest that allergenic mites belonging to Acaridae, Glycyphagidae, and Chortoglyphidae should be described as occupational risk factors contributing to respiratory and dermal diseases among farmers in Poland (Solarz and Pająk 2019; Cichecka et al. 2006; Solarz et al. 1997). In this study, we confirmed the presence of allergenic mite species in the examined material. The predominant species in all the examined farm buildings were Acarus siro and A. farris. Additionally, such species as G. domesticus, Ch. arcuatus, L. destructor, as well as T. putrescentiae were also frequently reported. These results confirm the presence of allergenic mites in cowsheds, barns, chaff-cutter buildings, pigsties, pigeon, and poultry houses (Solarz and Pająk 2019; Solarz et al. 1997; Terho et al. 1982; Mumcuoglu and Lutsky 1990; Stejskal and Hubert 2008). Thus, all the mentioned farm buildings should be regarded as potential sources of storage mites in rural environments. The stated prevalence of acarids, glycyphagids, and cheyletids in the investigated buildings are not in accordance with earlier data published by Hallas (1981), Terho et al. (1982), and Solarz et al. (1997).
Beyond their negative role as allergic agents, mites may also be used as professional markers in forensic analyses (Perotti et al. 2009; Solarz 2011b). Several genera of the family Acaridae can be encountered in forensic situations, particularly as allergenic taxa, in relation to workers, who are occupationally exposed to mites. However, many mites are also involved in the decomposition of animal carcasses and human corpses at every stage. Some of these species will be represented by just a few specimens, whereas others, such as Tyrophagus putrescentiae, Acarus farris, A. immobilis, and Sancassania berlesei, identified in this study, may increase in number to millions (Szelecz et al. 2018; Solarz 2011; OConnor 2009; Perotti 2009a; Braig and Perotti 2009; Perotti and Braig 2009; Russell et al. 2004). It is widely accepted that the first arthropod scavengers who colonised a dead body were flies with phoretic mite species. The flies complete their life cycle in and around the corpse, while the mites may feed on their immature stages. Since mites reproduce much faster than their carriers, they may offer themselves as valuable timeline markers. Among the mites, which were identified in this study, were species such as A. siro, A. farris, A. immobilis, T. putrescentiae, T. longior, S. berlesei, R. echinopus, L. destructor, G. domesticus, and other Glycyphagus spp, that are often found at every stage of decomposition on animal carcasses and human corpses. Many of them are known from forensic investigation cases (Russel et al. 2004). Members of the Astigmata are more frequently found during the dry decay stage of decomposition. Some of them belong to the phoretic mites of scavengers, while the majority travel on late-arriving carrion insects, such as moths, hide, and skin beetles (Perotti and Braig, 2009). Even though insects are larger, and hence more easily identifable, in particular situations, an informative diversity of phoretic mites may be found on a decaying carcass at any given time and their composition may provide valuable information in forensic investigations (OConnor 2009). The genus Tyrophagus was one of the first mite taxa found on human corpses. It has been used to estimate a post-mortem interval (PMI). The question of how this genus arrives on a carcass is still unresolved (OConnor 2009; Perotti 2009a). Mites of the genus Tyrophagus are not considered to be phoretic, but their long setae might allow them to be lifted and carried by mammals (Marzouk et al. 2006; Serpa et al. 2004; Perotti and Braig, 2009). It should be stressed that except for T. formicetorum, the remaining species of this genus do not go through the hypopial stage (heteromorphic deutonymph) in their life cycle. Tyrophagus species can be easily confused with other Acaridae species, such as T. longior. This species normally occurs together with another acarid mite – A. farris, which presents the deutonymphal phoretic stage (or hypopus). A. farris hypopi is even attached to the adults of T. longior (Hughes 1976; OConnor 2009). Sancassania berlesei, another reported mite species, may produce the hypopial stage, which could attach to chaffers (Scarabaeidae) and stored product beetles (Tenebrioniidae), e.g. the flour beetle (Tenebrio molitor) (Szelecz et al. 2018). Moreover, mites of the genus Rhizoglyphus also have the ability to develop heteromorphic deutonymphs, which are morphologically similar to those of Sancassania. They are phoretically associated with several species of Scarabaeidae (Coleoptera), such as Osmoderma eremicola, Bothynus gibbosus, and Phyllophaga spp. – insects, which belong to the group of opportunistic colonisers of human and animal remains (Rai et al. 2020). Many of mites described above belong to the most numerous species in the analysed farm buildings. Further studies are needed to clarify the relationship between indoor conditions in farm buildings and the species composition of the storage mite acarofauna. Our study may increase awareness of the occurrence of many allergenic mite species in the locations investigated in the Żywiecki district, as well as being of use in forensic medicine cases.
In conclusion, the acarofauna from the analysed farm buildings seems to be dominated by the allergenic mites of the families Acaridae (Acarus siro, A. farris) and Glycyphagoidae (G. domesticus, L. destructor). Moreover, all examined farms were sources of storage mites and therefore posed a potential risk of exposure to the mite allergens for farmers. The study suggests that the mites found in farm buildings, particularly the allergenic taxa should be considered occupational risk factors contributing to the occurrence of occupational respiratory and dermal diseases among farmers. This knowledge may be very useful in forensic and occupational medicine as well as in criminal investigations.
Conflict of interests
No conflict of interest
acarologia_4486_Supplementary-Table6.pdf
References
- Arlian L.G. 2002. Arthropod allergens and human health. Annu. Rev. Entomol., 47: 395-433. https://doi.org/10.1146/annurev.ento.47.091201.145224
- Berger I., Schierl R., Ochmann U., Egger U., Scharrer E., Nowak D. 2005. Concentrations of dust, allergens and endotoxin in stables, living rooms and mattresses from cattle farmers in Southern Bavaria. Ann. Agric. Environ. Med., 12: 101-107.
- Berlese A. 1905. Apparecchio per raccogliere presto ed in gran numero piccoli Artropodi [Apparatus for gathering early and in large numbers small arthropods] (in Italian). pp. 85-90. OCLC 79048180.
- Boström S., Johansson E., Härfast B., Lundqvist L., Bäckman I., von Rosen E., van Hage-Hamsten M. 1997. Characterization of the mite fauna (Acari) in Swedish barn dust. Internat. J. Acarol., 23: 127-132. https://doi.org/10.1080/01647959708683109
- Braig H.R., Perotti M.A. 2009. Carcasses and mites. Exp. Appl. Acarol., 49: 45-84. https://doi.org/10.1007/s10493-009-9287-6
- Cichecka E., Maniurka H., Szilman P., Szilman E., Solarz K., Sieroń A.L. 2006. Sensitization to storage mites in urban and subagricultural population of Upper Silesia. In: Buczek A., Błaszak Cz. (Eds) Arthropods. Epidemiological Importance. Koliber, Lublin. p. 287-293.
- Dutkiewicz J., Jabłoński L., Olenchock S.A. 1988. Occupational biohazards: a review. An. J. Ind. Med., 14: 605-623. https://doi.org/10.1002/ajim.4700140511
- Fain A., Guerin B., Hart B.J. 1990. Mites and Allergic Disease. Allerbio, Varennes en Argonne. p. 190.
- Fan Q.H., Zhang Z.Q. 2007a. Revision of some species of Tyrophagus (Acari: Acaridae) in the Oudemans Collection. System. Appl. Acarol., 12: 253-280. https://doi.org/10.11158/saa.12.3.11
- Fan Q.H., Zhang Z.Q. 2007b. Tyrophagus (Acari: Astigmata: Acaridae). In: TK Crosby (Ed) Manaaki Whenua Press, Lincoln, New Zealand. p. 291.
- Franz J.T., Masuch G., Musken H., Bergmann K.C. 1997. Mite fauna of German farms. Allergy, 52: 1233-1237. https://doi.org/10.1111/j.1398-9995.1997.tb02529.x
- Hallas T.E. 1981. Mites of stored hay in Iceland. J. Agr. Res. Icel., 13: 61-67.
- Henszel Ł., Kalisińska E., Kosik-Bogacka D.I., Kuźna-Grygiel W. 2011. Preliminary Studies on Storage Mites in Litter and Animal Fodder Collected from Farm Buildings in Northwestern Poland. Polish J. of Environ. Stud., 20: 795-800.
- Hughes A.M. 1976. The Mites of Stored Food and Houses. HMSO, London. p. 400.
- Krantz G.W., Walter D.E. 2009. A manual of acarology. Third edition. Texas Tech University Press, Lubbock, USA. p. 807.
- Marzouk A.S., Main A.J., Soliman S.S., Montasser A.A. 2006. Ectoparasites infesting commensal rodents in a rural area of Egypt, with special reference to newly recorded species. Int. J. Sci. Res., 15: 67-72.
- Mehl R. 1998. Occurrence of mites in Norway and the rest of Scandinavia. Allergy, 53: 28-35. https://doi.org/10.1111/j.1398-9995.1998.tb04993.x
- Mumcuoglu K.Y., Lutsky I. 1990. A prevalence survey of poultry house mites in Israel. Acarologia, 31: 51-56.
- OConnor B.M. 2009. Astigmatid mites (Acari: Sarcoptiformes) of forensic interest. Exp. Appl. Acarol., 49: 125-133. https://doi.org/10.1007/s10493-009-9270-2
- Perotti M.A. 2009a. Preface. Exp. Appl. Acarol. 49: 1-2. https://doi.org/10.1007/s10493-009-9289-4
- Perotti M.A., Braig H.R. 2009. Phoretic mites associated with animal and human decomposition. Exp. Appl. Acarol. 49: 85-124. https://doi.org/10.1007/s10493-009-9280-0
- Perotti M.A., Goff M.L., Baker S.A., Turner B.D., Braig H.R. 2009. Forensic acarology: an introduction. Exp. Appl. Acarol. 49: 3-13. https://doi.org/10.1007/s10493-009-9285-8
- Pike A.J., Wickens K. 2008. The House Dust Mite and Storage Mite fauna of New Zealand dwellings. N. Z. Entomol. 31: 17-22. https://doi.org/10.1080/00779962.2008.9722161
- Rai J.K., Armendt J., Bernhardt V., Pasquerault T., Lindstrom A., Perotti M.A. 2020. Mites (Acari) as a Relevant Tool in Trace Evidence and Postmortem Analyses of Buried Corpses. J. Forensic Sci., 65: 2174-2183. https://doi.org/10.1111/1556-4029.14506
- Rajski A. 1993. Faunistic-Ecological Investigations on Moss Mites (Acari, Oribatei) in Several Plant Associations. I. Ecology. Poznan Society of Friends of Sciences, Poznan, 163 pp.
- Revsbech P., Dueholm M. 1990. Storage mite allergy among bakers. Allergy, 45: 204-208. https://doi.org/10.1111/j.1398-9995.1990.tb00484.x
- Russel D.J., Schulz M.M., OConnor B.M. 2004. Mass occurrence of astigmatid mites on human remains. Abh Ber Naturk-mus Gorlitz 76: 51-56.
- Sánchez-Ramos I., Hernández C.A., Castanera P., Ortego F. 2004. Proteolytic activities in body and faecal extracts of the storage mite, Acarus farris. Med. Vet. Entomol., 18: 378-386. https://doi.org/10.1111/j.0269-283X.2004.00518.x
- Serpa L.L.N., Franzolin M.R., Barros-Battesti D.M., Kakitani I. 2004. Tyrophagus putrescentiae predating adult insects of Aedes aegypti and Aedes albopictus in laboratory. Rev. Saude. Publica. 38: 735-737. https://doi.org/10.1590/S0034-89102004000500019
- Solarz K., Senczuk L. 2003. Allergenic acarofauna of synanthropic outdoor environments in a densely populated urban area in Katowice, Upper Silesia, Poland. Internat. J. Acarol. Vol. 29, No. 4. https://doi.org/10.1080/01647950308684358
- Solarz K. 2009. Indoor mites and forensic acarology. Exp. Appl. Acarol. 49: 135-142. https://doi.org/10.1007/s10493-009-9292-9
- Solarz K. 2011a. Domestic and storage mites. An identification guide and diagnoses of taxa. Medical University of Silesia in Katowice, Advert Studio, ISBN 978-83-7509-161-8, Ruda Slaska, Poland.
- Solarz K. 2011b. House dust mites, other domestic mites and forensic medicine. In: Vieira D.N. (Ed.) by. Forensic Medicine - from Old Problems to New Challenges. First edition. Intech. p. 327-358.
- Solarz K. 2012. House dust mites and storage mites (Acari: Oribatida: Astigmatina). Identification keys. Institute of Systematics and Evolution of Animals Polish Academy of Sciences, Cracow. p. 120.
- Solarz K., Pająk C. 2019. Risk of exposure of a selected rural population in South Poland to allergenic mites. Part II: acarofauna of farm buildings. Exp. Appl. Acarol. 77: 387-399. https://doi.org/10.1007/s10493-019-00355-7
- Solarz K., Szilman P., Szilman E. 1997. Preliminary study on the occurrence and species composition of astigmatic mites (Acari: Astigmata) in samples of dust, debris and residues from farming environments in Poland. Ann. Agric. Environ. Med. 4: 249-252.
- Stejskal V., Hubert J. 2008. Risk of occupational allergy to stored grain arthropods and false pest-risk perception in Czech grain stores. Ann. Agric. Environ. Med. 15: 29-35.
- Szelecz I., Losch S., Seppey C.V.W., Lara E., Singer D., Sorge F., Tschui J., Perotti M.A., Mitchell E.A.D. 2018. Comparative analysis of bones, mites, soil, chemistry, nematodes and soil micro-eukaryotes from a suspected homicide to estimate the post-mortem interval. Sci. Rep. 8, 25. https://doi.org/10.1038/s41598-017-18179-z
- Terho E.O., Leskinen L., Husman K., Kärenlampi L. 1982. Occurrence of storage mites in Finnish farming environments. Allergy, 37: 15-19. https://doi.org/10.1111/j.1398-9995.1982.tb04112.x
- Tullgren A. 1918. Ein sehr einfacher Ausleseapparat für terricole Tierformen. Zeitschrift für Angewandte Entomologie, 4: 149-150. https://doi.org/10.1111/j.1439-0418.1918.tb00820.x
- van Bronswijk J.E.M.H. 1981. House dust biology for allergists, acarologists and mycologists. N.I.B. Publishers, Zoelmond. p. 316
- van Hage-Hamsten M., Johansson S.G.O. 1998. Clinical and immunologic aspects of storage mite allergy. Allergy, 53: 49-53. https://doi.org/10.1111/j.1398-9995.1998.tb04997.x
- Zhang Z.Q. and Fan Q.H. 2005. Revision of Tyrophagus Oudemans (Acari: Acaridae) of New Zealand and Australia. Landcare Research New Zealand Ltd, Auckland, New Zealand. p. 189.



2020-09-20
Date accepted:
2022-01-03
Date published:
2022-01-10
Edited by:
Roy, Lise

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Solarz, Krzysztof; Pająk, Celina; Pawełczyk, Olga; Bobiński, Rafał; Ciechacka, Maria; Dutka, Mieczysław and Ulman-Włodarz, Izabela
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



