A new genus and species of Digamasellidae (Acari: Mesostigmata) displaying some extraordinary gnathosomal structures
Faraji, Farid  1
; Zare, Mohsen
1
; Zare, Mohsen  2
and Rahmani, Hasan
2
and Rahmani, Hasan  3
3
1✉ Eurofins MITOX BV, Science Park 408, 1098 XH Amsterdam, The Netherlands & Institute for Biodiversity and Ecosystem Dynamics, Department of Evolutionary and Population Biology, University of Amsterdam, Amsterdam The Netherlands.
2Department of Plant protection, Faculty of Agriculture, University of Zanjan, P. O. Box: 313, Zanjan, Iran.
3Invertebrate and Weed Sciences, Agriculture Victoria Research Division, Department of Jobs, Precincts and Regions, Tatura centre, Victoria, Australia.
2021 - Volume: 61 Issue: 4 pages: 967-977
https://doi.org/10.24349/o0sa-j8xyZooBank LSID: 44AF1A1E-DE98-40C7-9C18-E497FA14F849
Original research
Keywords
Abstract
Introduction
The family Digamasellidae was first erected by Evans (1957) and then fully defined by Lindquist (1975) based on a limited number of genera. For this family, Shcherbak (1980) recognised eight genera – Dendrolaelaps Halbert, 1915, Dendrolaelaspis Lindquist, 1975, Dendroseius Karg, 1965, Insectolaelaps Shcherbak, 1980, Longoseius Chant, 1961, Multidendrolaelaps Hirschmann, 1974, Oligodentatus Shcherbak, 1980, and Orientolaelaps Bregetova & Shcherbak, 1977. The classification of Digamasellidae attributed to Shcherbak (1980) does not include the type genus Digamasellus. In his unpublished thesis, Castilho (2012) presented a key for the 11 genera of Digamasellidae and added Digamasellus Berlese, 1905, Panteniphis Willmann, 1949, Pontiolaelaps Luxton, 1984 to those of Shcherbak (1980). Castilho et al. (2012) listed 13 genera considering Dendrolobatus Shcherbak, 1983 and Lindquistoseius Genis, Loots & Ryke, 1968 as valid genera. They briefly defined the family Digamasellidae as:
With two-tined palp tarsal claw; most genera with podonotal and opisthonotal shields separated; generally with scleronoduli; without desclerotised punctae bands on dorsal and ventral shields; with seta St4 on sternal shield; tibia I with 5 dorsal setae; and genu and tibia IV with 6 to 8 setae each.
Members of the family Digamasellidae are mainly found in decaying organic material, on the soil surface, in galleries of bark beetles, under bark of trees, manure, and in bracket fungi where they feed on small arthropods, nematodes and even fungi. Deutonymphs of some species are phoretic on insects (Karg, 1993; Lindquist et al., 2009). The purpose of this study is to described a new genus and species of digamasellid mite found in association with gilled fungi from Iran.
Material and methods
Mites were collected by direct removal from gilled fungi and then preserved in 70% ethanol. Specimens were cleared in a mixture of Nesbitt and lactophenol solutions 1:1, and mounted in modified Hoyer's medium as described by Faraji & Bakker (2008). Specimens were examined using a Leica DM 2500 light microscope equipped with differential interference contrast (DIC) and drawings were made with the aid of a camera lucida (drawing tube) attached to an Olympus phase contrast microscope. All measurements are in micrometres (μm). Fixed cheliceral digit measured from the anterior part of dorsal lyrifissure to apical hook. The mean of the measurements is given followed by the range in parentheses. The setal notations for the idiosoma follow Lindquist & Evans (1965), and leg chaetotaxy follows Evans (1963). Notations of idiosomal solenostomes (pore-like structures) as well as lyrifissures (poroids) follow Athias-Henriot (1975).
The type specimens are deposited in ANIC = Australian National Insect Collection, CSIRO, Canberra, Australia; BMNH = Natural History Museum, London; JAZM = Jalal Afshar Zoological Museum, Iran; SMNG = Senckenberg Museum of Natural History, Görlitz and OSAL = Ohio State Acarology Collection.
Results
Digamasellidae Evans, 1957
Bulbolaelaps n. gen.
ZOOBANK: E66CAB95-8D7E-41C3-8AE1-2F083488D393 ![]()
Type species: Bulbolaelaps bossei
Diagnosis Podonotal and opisthonotal shields separated in both sexes; in males, opisthonotal shield fused with opisthogastric shield; scleronoduli present; almost all dorsal shield setae barbed (except S5, long, simple and mainly coiled), most of them apically bulbous; sternal seta St3 migrated inwards; palps with bulbous swollen protuberance on the venter of the palptrochanter; corniculi weakly formed and epistome weakly sclerotised and fimbriated dorsally; hypostomal setae h1 and h2 aligned more or less transversely; deutosternal groove narrow; ventral base of female cheliceral digit with a spine-like projection; setae ad1 and pd1 on legs II–IV narrowly lanceolate apically.
Etymology The generic name Bulbolaelaps refers to the extraordinary bulbous shape of the protuberance on the venter of the palptrochanter.
Bulbolaelaps bossei n. sp.
ZOOBANK: D30B10CD-1081-4556-B9E3-94A09756195F ![]()
(Figs 1–5)
Description
Adult Female — seven specimens measured.
(Figs 1, 3A–D, 4A–C, E–G and 5)



Dorsal idiosoma (Fig. 1A). Relatively large mite, idiosoma 737 (723–756) long and 512 (505–524) wide; podosomal and opisthosomal shields separated and entirely reticulated; podonotal shield 297 (288–303) long and 421 (418–225) wide (at widest point: level of setae s6) with 22 pairs of setae, all setae barbed and mostly knobbed apically (except for z1, s1, s2 and r2 with either pointed or knobbed tip); lengths of podonotal setae: j1 37 (35–39), j2 57 (54–60), j3 71 (65–76), j4 48 (46–52), j5 45 (43–47), j6 57 (55–58), z1 50–51, z2 90 (88–92), z3 57 (54–60), z4 71 (66–77), z5 53–54, z6 60 (59–62), s1 50 (47–52), s2 57 (55–60), s3 85 (81–87), s4 83 (81–85), s5 90 (85–95), s6 107 (104–112), r2 56 (54–58), r3 103 (101–106), r4 91 (88–94), r5 102 (100–104), prepodonotal area (anterior to j1 setae) weakly sclerotised 37–38 long; podonotal shield with one pair of solenostomes (gd2) and 5 pairs of poroids (id1, id2, id4, id5, id6) as well as two pairs of relatively large scleronoduli between setae z5 and j6; opisthonotal shield 383 (380–385) long and 478 (473–485) wide (at widest point) with 19 pairs of barbed setae (except for S5 long, simple and coiled, mostly with one loop, occasionally with two loops or none but with irregular shape), mostly apically knobbed (except for Z4 and J5); lengths of opisthonotal setae: J1 60–61, J2 61 (57–64), J3 59 (57–61), J4 72 (68–74), J5 22–23, Z1 88 (87–90), Z2 87 (85–88), Z3 77 (73–81), Z4 32, Z5 71 (67–79), S1 63 (62–65), S2 89 (85–93), S3 86 (81–88), S4 67 (63–73), S5 254 (239–284), R2 82 (76–90), R3 84 (79–93), R4 83 (79–90), R5 68 (66–69); R1 94 (88–103) on lateral integument; opisthonotal shield with 1 pair of solenostomes (gd9) and 12 pairs of poroids (idm1, idm2, idm3, idm4, idm5, idm6, is1, idx, idl1, idl2, idl3, idl4).
Peritreme and peritrematal shield (Fig. 1C). Peritrematal shield reticulated fused anteriorly with podonotal shield and extending posteriorly behind coxa IV carrying setae r2, r3, poroid ip and gland gp; peritreme relatively short 151 (144–158) long, extending forward to level of mid coxa II.
Ventral idiosoma (Figs 1B, 4G). Tritosternum (Fig. 4G) 145 (140–147) long with laciniae serrated subapically free for ca. one third of the length; presternal area without any plate, ornamentation or patches of striae; sternal shield weakly sclerotised with striae and reticulations on its anterior half, posterior margin slightly concave, 91 (88–98) long and 131 (128–136) wide at level of setae St3, with four pairs of setae and three pairs of lyrifissures (iv1, iv2 and iv3); St1 36–38, St2 35–39, St3 36–39, St4 41–46; St2 and St3 slightly thicker than St1 and St4; St3 migrated inwards; two small platelets positioned on soft cuticle posterior to sternal shield; a pair of endopodal plates situated between coxae III–IV and genital shield; genital shield relatively short, width at widest point (posterior margin) 157 (153–161), St5 44–46 and iv5 off the shield; one pair of metapodal shields 53–63 x 12–13 associated with three small platelets posterior to coxa IV; ventrianal shield pear-shaped with transverse striae and reticulations on anterior half, length 291 (289–295), width at level of setae Zv2 (widest point) 203 (199–207); with four pairs of pre-anal setae (Jv1 42–44, Jv2 39–43, Jv3 43–44, Zv2 44–46), para-anal setae 60 (57–65) and post-anal seta 42 (41–44), all ventrianal setae simple except post-anal seta knobbed apically and barbed; solenostomes gv3, rod-like flanking ventrianal shield level with anterior margin of anal opening; three pairs of setae surrounding ventrianal shield on integument Zv1 simple Jv4 knobbed apically and Jv5 knobbed apically and barbed (Jv4 65–72, Jv5 94 (92–98), Zv1 41–43), three pairs of poroids (ivo) surrounding ventrianal shield.
Spermathecal apparatus. Not distinct; location of solenostome not discernible.
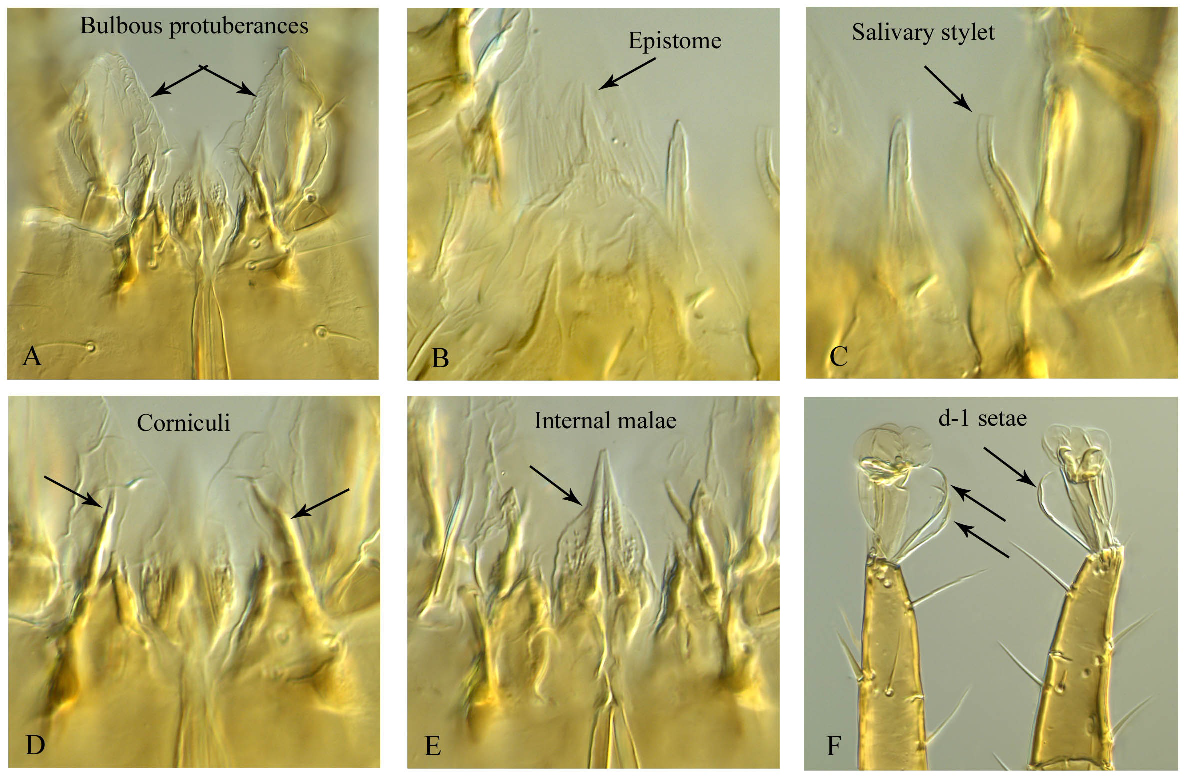
Gnathosoma. Corniculi (Plate 5D) weakly formed/sclerotised and difficult to detect; internal malae (Plate 5E) lanceolate and ventrally with spiculi; salivary stylets (Plate 5C) narrow, not sclerotised, and apically flatten; cheliceral digits relatively short, fixed digit 27–28 long, subapical ridge with two small teeth and apical hook and a pilus dentilis; movable digit 35–36 long with four teeth (two distal teeth are smaller), ventral base of female cheliceral digit with a paraxial spine-like projection (Figs 4A and B); deutosternum weakly developed and narrow with five transverse rows, most distal one smooth, three middle ones each unidentate, proximal row much wider and multidenticulate (Fig. 4F); hypostomatic setae h3 43–44 longer than h1 21–22, h2 24–25 and palpcoxal seta pc 35–36, h1 and h2 more or less transversely aligned; epistome triangular fimbriated dorsally, weakly sclerotised and difficult to detect (Fig. 4C, plate 5B); palpus 125 (124–127) long; number of setae on trochanter, femur, tibia and tarsus: 2, 5, 6, 7 and 14, respectively; venter of palptrochanter with a bulbous swollen protuberance (Fig. 4E, Plate 5A). Ventrolateral surface of papltrochanter split longitudinally, the split flanked with sclerotised lips, and a bulbous swollen protuberance emerging from the split.
Legs. Legs I–IV with paired claws and rounded pulvilli, lengths of legs: leg I 464 (460–466), leg II 401 (398–403), leg III 416 (403–425), leg IV 495 (487–509), all legs shorter than idiosoma; setation of legs I–II–III–IV: coxae 2–2–2–1, trochanters 6–5–5–5, femora 13–11–6–6, genua 12 (2 3/2 2/1 2) – 11(2 3/1 2/1 2) – 9 (2 2/1 2/1 1) – 7 (1 2/1 2/0 1), tibiae 12 (2 3/2 2/1 2) – 10 (2 2/1 2/1 2) – 8 (2 1/1 2/1 1) – 7 (1 1/1 2/1 1), tarsi II–IV with 18 setae; all setae aciculate except ad1 and pd1 on tarsi II–IV narrowly lanceolate apically (Plate 5F) and ventral setae of femora, genua and tarsi on legs II–IV slightly thickened (Figs 3A–D).
Adult male — Seven specimens measured.
(Figs 2, 3E, 4D and 4H)



Dorsal idiosoma (Fig. 2A). Idiosoma 629 (616–635) long and 457 (456–460) wide; podonotal and opisthosomal shields more or less similar to those of females, but slightly wider so to bear r2, r3 and R1; podonotal shield 306 (293–313) long and 452 (445–455) wide (at widest point: level of setae s6); lengths of podonotal setae: j1 36 (35–38), j2 52 (51–53), j3 58 (56–60), j4 45 (44–46), j5 42 (41–43), j6 48–49, z1 42 (41–43), z2 72 (70–74), z3 50 (48–51), z4 64 (61–67), z5 49–50, z6 51 (51–53), s1 38 (36–40), s2 47 (46–47), s3 69 (66–71), s4 66 (60–70), s5 72 (68–77), s6 75–76, r2 44 (40–49), r3 87–88, r4 69 (67–71), r5 67 (66–68); opisthonotal shield 335 (320–343) long and 452 (448–455) wide (at widest point: level of setae S1) with 20 pairs of setae (R1 on the shield); lengths of opisthonotal setae: J1 51–52, J2 51–52, J3 49–50, J4 58 (57–59), J5 22 (21–23), Z1 64 (63–66), Z2 69 (67–71), Z3 60–61, Z4 26 (25–28), Z5 62–63, S1 46–47, S2 67–68, S3 65–66, S4 52–53, S5 250 (228–269), R1 58–59, R2 53–54, R3 52–53, R4 55 (51–58), R5 45 (42–46).
Peritreme and peritrematal shield. Peritreme as in female 166 (150–175) long, peritrematal shield fused anteriorly and laterally with podonotal shield, and extending posteriorly behind coxa IV merging with triangular parapodal shields at level of setae St5.
Ventral idiosoma (Fig. 2B). Tritosternum 108 (106–111) long; sternogenital shield with some striae anteriorly and laterally, 243 (241–246) long and 136 (131–144) wide at level of setae St3, with four pairs of setae and three pairs of lyrifissures, St1 36–38, St2 34–36, St3 38, St4 42–43; St5 44–49 on a separate triangular parapodal shields fused with peritrematal shield; soft lateral cuticle between peritrematal shield and ventrianal shield with 4–6 pairs of small platelets; ventrianal shield reticulated, length 282 (261–296), width at widest point 445 (440–450); with seven pairs of pre-anal opisthogastric setae, all simple except Jv5 barbed and apically knobbed (Jv1 36–39, Jv2 36–43, Jv3 36–43, Jv4 46–51, Jv5 55–61, Zv1 38–39, Zv2 40–43); para-anal setae simple 55 (52–57) and post-anal seta barbed and apically knobbed 37 (32–39).
Gnathosoma. Corniculi, epistome (Fig. 4D), deutosternum and internal malae as in female; fixed cheliceral digit 31–32 long with one tooth and apical hook and pilus dentilis; movable digit 40–43 long with a single tooth (Fig. 4H); spermatodactyl stout, curved dorsally and apically pointed 46–49 long; palpus similar to that of females showing venter of palptrochanter with a bulbous swelling protuberance.
Legs. Lengths of legs: leg I 497 (486–510), leg II 418 (402–433), leg III 429 (420–442), leg IV 513 (505–518), setation of legs I–IV and form of setae on legs I, III and IV as in female; leg II showing sexual dimorphism, femur with seta av1 markedly enlarged spinelike and blunt with partial circular ribbing pattern, av1 genu, tibia and mv tarsus also thickened and blunt but shorter than on femur (Fig. 3E).








Etymology The specific name bossei is after Mr. Theo Bosse, the facility manager of Eurofins MITOX BV, who has kindly supported and provided the senior author with research tools, equipment and chemicals during many studies.
Type material and depository Holotype female, 22 July 2011, from fruiting bodies of wet gilled fungi (possibly a species of Pleurotus sp., fam. Pleurotaceae) on a tree trunk, Chiyar, Zanjan Province, Iran (36°43'47.1''N 48°18'28.6''E), deposited in JAZM, collector: Mohsen Zare; six females and eighteen males, same data as holotype: one paratype female and four paratype males deposited in JAZM; two paratype females and four paratype males deposited in ANIC; one paratype female and three paratype males deposited in SMNG; one paratype female and three paratype males deposited in BMNH; one paratype female and three paratype males deposited in OSAL.
Remarks The new genus mainly differs from the other genera of Digamasellidae by the following apmorphic attributes: (1) a bulbous (membranous) swollen protuberance at the venter of the palptrochanter. This feature is unique in the family Digamasellidae. A similar feature ''membranous flap'' was reported by Costa (1968), Moraza (2019) and Nemati et al. (2019) for the genus Reticulolaelaps Costa (Laelapidae). By dissecting the palps, Nemati et al. (2019) showed that membranous flap was connected to the inner part of the palptrochanter, more or less the same location as for the new genus here. The swollen protuberance in Bulbolaelaps is three-dimensional and kept its shape even after re-mounting and for a dissecting the specimen. For Reticulolaelaps the membranous flap might have been three-dimensional in living individuals but after mounting and dissecting it became flattened. (2) Corniculi weakly formed and epistome weakly sclerotised. Even with a DIC microscope and with dissected specimens, these two features are not easy to observe. Corniculi of the other genera of digamasellids are well sclerotised and mainly horn-like (bifid in the male of Longoseius Chant, 1961 and with a curved groove in the male of Panteniphis Willmann, 1949). The epistome of Bulbolaelaps n. gen. is triangular and fimbriated dorsally while the other genera have epistome mainly triramous or occasionally biramous (in Longoseius). Orientolaelaps Bregetova & Shcherbak, 1977 shows a triangular shape but in fact it can be considered triramous with reduced median and lateral tines; (3) Hypostomal setae h1 and h2 more or less transversely aligned. In all other genera of digamasellids, seta h1 is positioned far anterior to seta h2; (4) Deutosternal groove narrow and weakly developed with four transverse rows of denticles, the most distal one smooth, the three middle ones each unidentate, the proximal row much wider and multidenticulate. In all other genera of digamasellids the deutosternum has a relatively wider groove with five (rarely six) rows of denticles with the proximal row much widened; (5) Ventral base of female cheliceral digit with a spine-like projection. In the other genera of Digamasellidae this projection does not exist; (6) Setae ad1 and pd1 on tarsi II–IV narrowly lanceolate apically. This feature is not present in any other digamasellid genera or other closely related families; and (7) All dorsal shield setae barbed, and most knobbed apically. This feature does not exist in any other digamasellid genera except Dendrolaelaspis Lindquist, 1975, which shows some posterior opisthonotal setae stout, spatulate and a few setae serrate and Dendrolaelaps quadritorus (Robillard) shown to have two pairs of opisthonotal setae stout, spatulate (Lindquist 1975; Robillard 1971).
Discussion and conclusion
According to the key to families of order Mesostigmata (Lindquist et al., 2009) as well as definitions provided by Lindquist (1975), Shcherbak (1980) and Castilho et al. (2012), we have placed Bulbolaelaps bossei n. sp. in the family Digamasellidae based on the following key morphological characters: Dorsal shield divided; two pairs of scleronoduli present; S5 long and coiled; females with four pairs of setae and three pairs of poroids on the sternal shield; palptarsal apotele 2-tined; basal row of deutosternal denticles greatly widened and more multidenticulate; 13 setae on femur I; 12 setae on each of genu and tibia I, and seven setae on each genu and tibia IV; males with setae St5 on separate triangular parapodal shields; ventrianal shield of males merged laterally and posteriorly with opisthonotal shield; and femur, genu, tibia and tarsus of leg II in males each with ventral setae thickened and robust.
There are some key morphological characters in Digamasellidae to separate genera and to propose sister groups. These are: the basal row of deutosternal denticles being greatly widened and more multidenticulate, the male setae St5 on separate triangular parapodal shields, dorsal shields separated, presence of scleronoduli, location of spermathecal opening (coxa III or IV) and anterior migration of the opisthonotal shield without median notch. The first two characters seem to be strong apomorphic traits at the family level. Unfortunately, males of a few genera have not been described yet. Also, the location of spermathecal solenostome in Bulbolaelaps n. gen. is not known. Considering these characters, Bulbolaelaps n. gen. can be grouped with Insectolaelaps Shcherbak, 1980, Multidendrolaelaps Hirschmann, 1974 and Oligodentatus Shcherbak, 1980.
Because only the adults were collected from the fruiting bodies of gilled fungi, we are not confident about the true habitat of this species. The apparent absence of the juveniles from the fungi might suggest another habitat for development of juveniles. If immatures also inhabit fungi, their absence may be explained by two reasons: the collector picked up the large individuals (adults) which with naked eyes were easily detectable or almost all immatures had already reached to adulthood before the moment of sampling. The presence of a bulbous (membranous) swollen protuberance on the venter of the palptrochanter may be an adaptation for optimal feeding. This protuberance is located exactly in front of hypostomatic setae h1 and h2. Migration of seta h1 backwards might be an adjustment for the presence of this protuberance. It will be interesting to find whether juveniles show this feature as well. The reduced form of corniculi, which are to the contrary stronger among some gamasines associated with fungi, may indicate this species as a liquid feeder. As a matter of fact, some gilled fungi exude beads of moisture, called guttation (Parmasto and Voitk, 2010). Interestingly, this species was collected from gilled fungi completely covered by moisture and individuals were walking easily on the wet surface. It is possible that the gnathosoma has evolved in a way to consume guttated drops. On the other hand, the chelicerae of this species look very robust suitable for crushing fungal hyphae rather than sucking fluids. Catching live individuals and rearing them in the laboratory would solve feeding habits of this species.
Having most of dorsal shield setae relatively long, barbed and knobbed apically as well as modified form of apical setal pair d-1 of tarsi II-IV may give the mite an advantage to move freely among fungal gills as well as acting as tactile sensory giving information about the location of gills.
Dispersal of this species is another interesting topic that needs a further investigation, with the deutonymph being the phoretic instar in digamasellids. Thunes et al. (2000) found 36 species of beetles associated with a fungus (Fomitopsis pinicola) in a Norwegian spruce forest, and Yamashita et al. (2015) reported 82 coleopteran species associated with bracket fungi in a Bornean tropical rain forest. It is possible that deutonymphs of this new species uses adult coleopteran species for dispersal to new habitats.
A revision of the genera of Digamasellidae is still required especially for the status of the two enigmatic genera of Panteniphis and Pontiolaelaps. The definition for the family Digamasellidae should also be widened based on the new discoveries in this study. A comprehensive study including molecular research is needed to determine the phylogenetic relationship among digamasellid genera.
Acknowledgements
We would like to thank Drs. Bruce Halliday, Evert E. Lindquist, Maria L. Moraza and Edward A. Ueckermann for useful discussion and for help in preparing and reviewing the manuscript. Rik Delhem kindly took the photomicrographs displayed in this study.
References
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini II. Le relevé organotaxique de la face dorsale adulte (Gamasides Protoadenique, Phytoseiidae). Acarologia, 17: 20-29.
- Berlese A. 1905. Acari nuovi. Materiali pel Manipulus V. Redia, 2: 231-238.
- Bregetova N.G. Shcherbak G.I. 1977. New genus of Orientolaelaps (Gamasina, Rhodacaridae). Dopovidi Akad. Nauk Ukr. RSR (ser. B): 175-176. [in Russian]
- Castilho R.C. 2012. Taxonomy of Rhodacaroidea Mites (Acari: Mesostigmata). Doctoral Dissertation, Universidade de São Paulo, 579 pp.
- Castilho, R.C. Moraes G.J. de Halliday B. 2012. Catalogue of the mite family Rhodacaridae Oudemans, with notes on the classification of the Rhodacaroidea (Acari: Mesostigmata). Zootaxa, 3471, 1- 69. https://doi.org/10.11646/zootaxa.3471.1.1
- Chant D.A. 1961. A new genus and species of mite in the family Digamasellidae Evans (Acarina). Acarologia, 3(1): 11-13.
- Costa M. 1968. Little known and new litter-inhabiting Laelapine mites (Acari, Mesostigmata) from Israel. Israel J. Zool., 17: 1-30.
- Evans G.O. 1957. An introduction to the British Mesostigmata (Acarina) with key to families and genera. Journal of the Linnean Society of London, 43(291): 203-259. https://doi.org/10.1111/j.1096-3642.1957.tb01552.x
- Evans G.O. 1963. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata). Bull. Brit. Mus. (Nat. Hist.) Zool., 10(5): 277-303. https://doi.org/10.5962/bhl.part.20528
- Faraji F. Bakker F.M. 2008. A modified method for clearing, staining and mounting plant-inhabiting mites. Eur. J. Entomol., 105: 793-795. https://doi.org/10.14411/eje.2008.105
- Halbert J.N. 1915. Clare Island Survey - 39. Acarinida. Section II - Terrestrial and marine Acarina. Proc. Roy. Irish Acad., Dublin, v. 31, p. 45-136.
- Hirschmann W. 1974. Gangsystematik der Parasitiformes Teil 190. Die Gattung Dendrolaelaps Halbert 1915 Hirschmann nov. comb. Nova Subgenera Multidendrolaelaps, Tridendrolaelaps Hirschmann. Stadien von 4 neuen Dendrolaelaps-Arten. Acarologie, 20: 50-70.
- Karg W. 1965. Larvalsystematische und phylogenetische Untersuchung sowie Revision des Systems der Gamasina Leach, 1915 (Acarina, Parasitiformes). Mitt. Zool. Mus. Berlin, 41(2): 193-340. https://doi.org/10.1002/mmnz.19650410207
- Karg W. 1993. Acari (Acarina), Milben Parasitiformes (Anactinochaeta) Cohors Gamasina Leach. Raubmilbe. - Die Tierwelt Deutschlands: 59, Second Edition, Gustav Fischer Verlag, Jena, Germany: 523 pp.
- Lindquist E.E. 1975. Digamasellus Berlese, 1905, and Dendrolaelaps Halbert, 1915, with descriptions of new taxa of Digamasellidae (Acarina: Mesostigmata). Can. Entomol., 107(1): 1-43. https://doi.org/10.4039/Ent1071-1
- Lindquist E.E. Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entomol. Soc. Canada, 47: 1-64. https://doi.org/10.4039/entm9747fv
- Lindquist E.E. Krantz G.W. Walter D.E. 2009. Mesostigmata. In: Krantz G.W. Walter D.E. (Eds.). A manual of Acarology. 3rd ed. Lubbock: Texas Tech University Press, p. 124-232.
- Luxton M. 1984. More marine littoral mites from New Zealand. N. Z. J. Mar. Freshwat. Res. 18: 291-302. https://doi.org/10.1080/00288330.1984.9516051
- Moraza M.L. 2019. A new species of Reticulolaelaps Costa (Mesostigmata: Laelapidae) from the Iberian Peninsula, with a key to world species. Acarologia, 59(3): 374-382. https://doi.org/10.24349/acarologia/20194338
- Nemati A. Khalili-Moghadam A. Gwiazdowicz D. 2019. A review of the genus Reticulolaelaps Costa and redescription of R. elsae (Joharchi, Babaeian & Jalalizand) comb. nov. Persian J. Acarol., 8: 77-99.
- Parmasto, E. Voitk A. 2010. Why Do Mushrooms Weep? Fungi, Vol. 3(4): 15-17.
- Robillard J. 1971. A new species of Digamasellus (Acarina: Digamasellidae) from Louisiana. Can. Ent. 103: 1763-1774. https://doi.org/10.4039/Ent1031763-12
- Shcherbak G.I. 1980. The Palearctic Mites of the family Rhodacaridae. Naukova Durnka, Kiev, 215 pp.
- Shcherbak G.I. 1983. Taxonomic status and typical characters of Dendrolaelaspis (Parasitiformes, Rhodacaridae). Dopovidi Akad. Nauk Ukrayinskoyi RSR Seriya B Heolohichni Khimichni ta Biol. Nauky, 12: 72-74.
- Thunes K.H. Midtgaard F. Gjerde I. 2000. Diversity of coleoptera of the bracket fungus Fomitopsis pinicola in a Norwegian spruce forest. Biodiv. Conserv. 9: 833-852. https://doi.org/10.1023/A:1008927410513
- Willmann C. 1949. Über eine Milbenausbeute aus dem Naturschutzgebiet Verlorenes Wasser bei Panten (Kr. Liegnitz). Abh. naturw. Ver. Bremen 32: 339-358.
- Yamashita S. Ando K. Hoshina H. Ito N. Katayama Y. Kawanabe M. Maruyama M. Itioka T. 2015. Food web structure of the fungivorous insect community on bracket fungi in a Bornean tropical rain forest: Bornean fungivorous insect food webs. Ecol. Entomol. 40(4): 390-400. https://doi.org/10.1111/een.12200



2021-06-14
Date accepted:
2021-10-29
Date published:
2021-12-03
Edited by:
Roy, Lise

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Faraji, Farid; Zare, Mohsen and Rahmani, Hasan
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




