New Phytoseiidae (Acari: Mesostigmata) of Mascareignes and Comoros Archipelagos (Indian Ocean): one new record, three new species groups and description of six new species and of six unknown males
Kreiter, Serge  1
; Payet, Rose-My
1
; Payet, Rose-My  2
; Abo-Shnaf, Reham
2
; Abo-Shnaf, Reham  3
and Douin, Martial4
3
and Douin, Martial4
1✉ Institut Agro - Montpellier SupAgro, UMR CBGP INRA/ IRD/ CIRAD/ IA (SupAgro), Université de Montpellier, 755 Avenue du Campus Agropolis (Baillarguet), CS 30016, 34988 Montferrier-sur-Lez cedex, France.
2CIRAD, Université de Montpellier, Unité Hortsys, Station de Bassin-Plat, 97410 Saint-Pierre, La Réunion, France.
3Institut Agro - Montpellier SupAgro, UMR CBGP INRA/ IRD/ CIRAD/ IA (SupAgro), Université de Montpellier, 755 Avenue du Campus Agropolis (Baillarguet), CS 30016, 34988 Montferrier-sur-Lez cedex, France & Plant Protection Research Institute (PPRI), Agricultural Research Centre (ARC), 7 Nadi El-Seid Street, 12611 Dokii, Giza, Egypt.
4Institut Agro - Montpellier SupAgro, UMR CBGP INRA/ IRD/ CIRAD/ IA (SupAgro), Université de Montpellier, 755 Avenue du Campus Agropolis (Baillarguet), CS 30016, 34988 Montferrier-sur-Lez cedex, France.
2021 - Volume: 61 Issue: 4 pages: 845-889
https://doi.org/10.24349/Krky-e23sZooBank LSID: 3D9E2C62-029D-48FD-AD63-210635DFF2FD
Original research
Keywords
Abstract
Introduction
Mites of family Phytoseiidae are all predatory species on phytophagous mites and small insects like thrips and whiteflies, on commercial plants and the wild vegetation, many of these arthropods being important pests for agriculture. Several species are biological control agents for the control of these pest organisms in both open and protected crops all around the world (McMurtry and Croft 1997; McMurtry et al. 2013; Knapp et al. 2018).
This family is widespread around the world, present on all continents except Antarctica, and consists of about 2,521 valid species in 95 genera, 15 tribes and three subfamilies (Demite et al. 2021).
Despite several interests of this family and its large distribution, many areas of the world are very poorly investigated or not investigated, some areas remaining white spots concerning the fauna of Phytoseiidae.
Thus, biodiversity surveys in these poorly investigated areas are still an urgent need and might result in the discovery of additional species potentially useful for biological control as well as having more information on the biodiversity of these areas for biodiversity practical purposes.
In these perspectives, the more interesting areas are probably those with a high level of biodiversity. Most of the Indian Ocean constitutes one of the highest world biodiversity areas, those areas called hotspots, concept defined by Myers (1988) in order to identify the most immediately important areas for biodiversity conservation. The common characteristics of these hotspots are that they hold high endemism levels and have lost at least 70% of their original natural vegetation (Myers et al. 2000). Knowledge of the phytoseiid diversity in these high interest areas in the context of global climate changes may contribute to identify potential biological control agents (BCAs) and future establishment of conservation programs.
Several Islands are located in the Indian Ocean, especially in two archipelagos, Mascareignes and Comoros. The former is constituted of several small Islands and three main Islands: La Réunion, Mauritius and Rodrigues. The later is constituted of some small Islands and four main Islands: Mayotte, Anjouan, Mohéli and Grande Comore.
Although these Islands, especially Mascareignes Islands, are a top destination for tourism and attracted the interest of many European naturalists, the fauna of phytoseiid mites remains poorly known (Ferragut and Baumann 2019).
These main Islands of the two Archipelagos (except La Réunion which was investigated before, see Kreiter et al. 2020b) were investigated from October 25th to December 12th, 2018. Results of Phytoseiidae records were already published in six papers; Kreiter and Abo-Shnaf 2020a, b for Rodrigues and Mauritius (in addition to Mauritius, see Kreiter et al. 2018a; Kreiter et al. 2020a, 2021b, c, d for Mayotte, Anjouan, Mohéli, and Grande Comore (in addition to Grande Comore, see Kreiter et al. 2018b), respectively.
This paper aims to give the description of six species new to science and six unknown males along with one new record collected during this survey.
Material and Methods
The survey took place during 2018 in: Mauritius (October 27th – November 6th), Rodrigues (November 8th – November 16th), Mayotte (November 23rd – November 27th), Anjouan (November 28th – December 1st), Mohéli (December 1st – December 5th) and Grande Comore (December 5th – December 11th).
Mites were directly collected on leaves with a fine brush with or without a pocket lens or a stereo-microscope when available (large leaves and herbaceous plants) or by beating the plants (mainly shrubs and trees with very small or spiny leaves) and collecting the mites in a black plastic rectangular saucer 45 x 30 cm (Ref. STR 45, BHR, 71370 Saint-Germain-du-Plain, France). Collected mites were then transferred with a fine brush into small plastic vials containing 1.5 ml of 70% ethanol.
The mites were then all slide-mounted in Hoyer's medium (Walter and Krantz 2009), the slides were dried at 45-50oC for at least two weeks and then all examined and identified using a phase and interferential contrast microscope (DMLB, Leica Microsystèmes SAS, Nanterre, France). Characters of specimens were measured using a Leica graded eyepiece.
Chant and McMurtry's (1994, 2007) concepts of the taxonomy of the family Phytoseiidae for identification and the world catalogue database of Demite et al. (2014, 2021) for distribution and information on descriptions and re-descriptions were used. The setal nomenclature system adopted was that of Lindquist and Evans (1965) and Lindquist (1994) as adapted by Rowell et al. (1978) and Chant and Yoshida-Shaul (1989) for the dorsal surface and by Chant and Yoshida-Shaul (1991) for the ventral surface. Pore (= solenostome) and poroid (= lyrifissure) notations are that of Athias-Henriot (1975). Macrosetal notation (Sge = genual macroseta; Sti = tibial macroseta; St = tarsal macroseta) are that of Muma and Denmark (1970). Numbers of teeth on the fixed and movable cheliceral digits do not include the respective apical teeth. Setae not referred to in results section should be considered as absent. All measurements are given in micrometres (µm) and presented with the mean in bold followed by the range in parenthesis. Type of spermatheca or insemination apparatus is that of Denmark and Evans (2011).
Classification of plants follows the APG IV classification of 2016 (ex. Byng et al. 2018).
Specimens of each species are deposited in the mite collections of Institut Agro (Montpellier SupAgro) conserved in UMR CBGP INRAE/IRD/CIRAD/SupAgro/University of Montpellier.
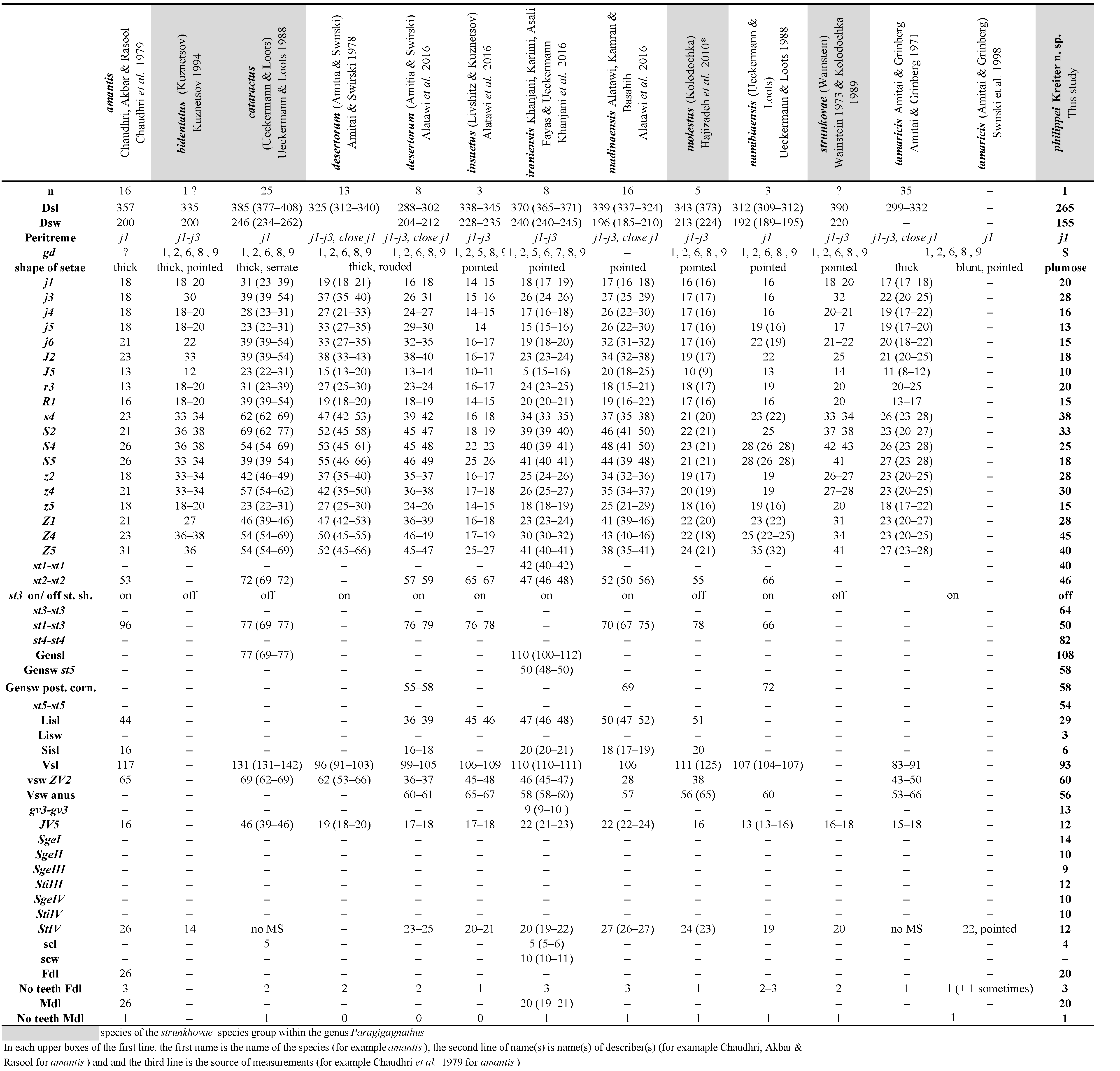
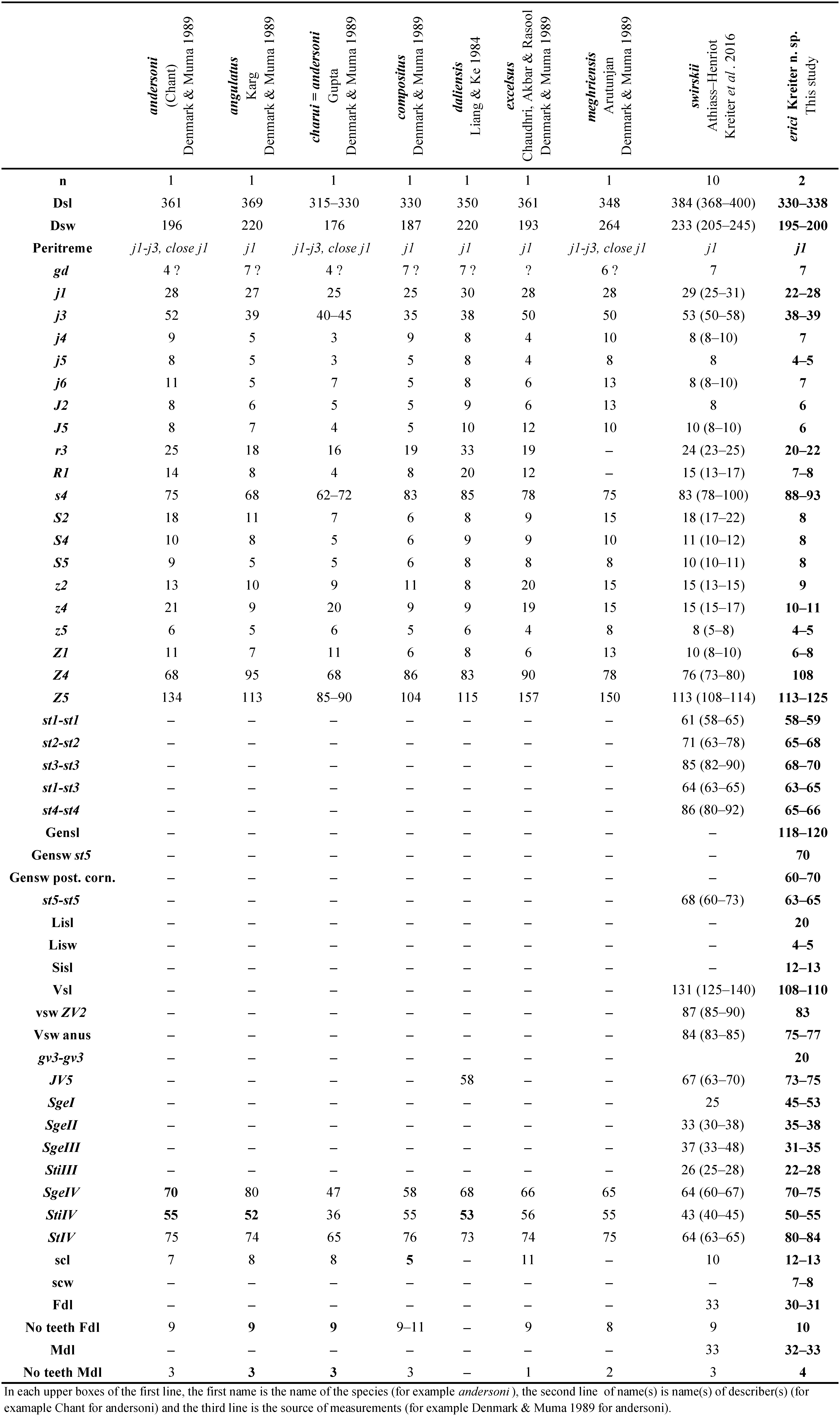
The following abbreviations are used in Tables (1–3) for morphological characters: n = number of individuals measured; dsl = dorsal shield length just above j1 to just below J5 in the mid line; dsw = dorsal shield width at the level of s4; Peritreme = level of the peritreme extension; gd = number of solenostomes; gensl = genital shield length; gensw st5 = genital shield width at level of setae st5; gensw post. cor. = genital shield width at level of posterior corners; lisl = primary or largest inguinal sigilla (= ''primary metapodal plate'') length; lisw = primary or largest inguinal sigilla (= ''primary metapodal plate'') width; sisl = secondary or smallest inguinal sigilla (= ''secondary metapodal plate'') length; vsl = ventrianal shield length; gv3-gv3 = distance between centres of each solenostome gv3 on the ventrianal shield; vsw ZV2 & vsw anus = ventrianal shield width at ZV2 level and at para-anal setae level; scl: largest calyx length; scw = calyx widest width; Fdl = fixed digit length; Mdl = movable digit length; Nb teeth Fd = number of teeth on the fixed digit; Nb teeth Md = number of teeth on the movable digit; Shaft = length of the shaft of spermatodactyl; branch = length of the branch; BCA = Biological Control Agent; aasl = altitude above sea level; imm. = immature.
The following abbreviations are used in this paper for institutions: CBGP = Centre de Biologie pour la Gestion des Populations; CIRAD = Centre International de Recherche Agronomique pour le Développement; IA = Institut Agro; INRAE = Institut National de Recherche pour l'Agriculture, l'Alimentation et l'Environnement; IRD = Institut de Recherche pour le Développement; MSA = Montpellier SupAgro, France; UMR = Unité Mixte de Recherche; UR = Unité de Recherche.
Results and Discussion
During the survey in Indian Ocean Islands, we found six new species to science and other six unknown males and one new record (in the chronological order of the survey: Mauritius, Rodrigues, Mayotte, Anjouan, Mohéli, and Grande Comore):
- One unknown male of Amblyseius haleakalus Prasad and one new record of Typhlodromips culmulus (van der Merwe) in Mauritius Island,
- One unknown male of Typhlodromus (Anthoseius) lobatus Zannou, Moraes and Oliveira in Mauritius and Rodrigues Islands,
- One new species of Ueckermannseius n. sp. 3 (different from the one in Mohéli and the one in Grande Comore Islands, see below) in Mayotte Island,
- One unknown male of Typhlodromus (Anthoseius) hartlandrowei Evans in Anjouan Island,
- One new species of Typhlodromalus , one unknown male of Amblyseius parasundi Blommers and one unknown male of Typhlodromus (Anthoseius) grewiae Zannou, Moraes & Oliveira in Mayotte and Mohéli Islands,
- One new species of Ueckermannseius n. sp. 1 (different from the one in Mayotte and the one in Grande Comore Islands, see below) and one new species of Paragigagnathus in Mohéli Island,
- One new species of each of Amblyseius and Ueckermannseius n. sp. 2 (different from the one in Mayotte and the one in Mohéli Islands, see below) in Grande Comore Island,
- One unknown male of Amblyseius duplicesetus Moraes & McMurtry in Mayotte, Mohéli and Grande Comore Islands.
These six new species and the six unknown males are all described and the new record mentioned thereafter.
Data follow the classification order of Chant and McMurtry (2007) and therefore the following taxonomical order: Paragigagnathus n. sp., Amblyseius n. sp. 1, Typhlodromips culmulus new record, Amblyseius n. sp. 2, unknown males of three species of Amblyseius, Typhlodromalus n. sp., new species groups of Ueckermannseius, Ueckermannseius n. sp. 1, Ueckermannseius n. sp. 2, Ueckermannseius n. sp. 3, unknown males of three species of Typhlodromus (Anthoseius).
Subfamily Amblyseiinae Muma
Amblyseiinae Muma 1961: 273.
Tribe Neoseiulini Chant & McMurtry
Neoseiulini Chant & McMurtry 2003: 6.
Genus Paragigagnathus Amitai & Grinberg
Paragigagnathus Amitai & Grinberg 1971: 327; Chant & McMurtry 2003: 39; Moraes et al. 2004b: 158.
Paragigagnathus philippei Kreiter n. sp.
ZOOBANK: 752EFED1-2498-4470-96FB-68C530C2C10E ![]()
Classification. Paragigagnathus philippei Kreiter n. sp. belongs to:
- the subfamily Amblyseiinae Muma (absence of dorsolateral setae z3 and s6 and the caudoventral seta JV3),
- to the tribe Neoseiulini Chant & McMurtry (seta S4 present, ratio s4/Z1 < 3.0, setae s4, Z4 and Z5 not greatly longer than other dorsal setae, usually slightly sclerotized, never with wide sternal shield, seta J2 always present),
- to the genus Paragigagnathus Amitai & Grinberg (female ventrianal shield reduced and/or markedly wider at the level of anus, with a prominent waist, chelicerae with teeth only on apical region, fixed digit with one to three teeth, movable digit with a single tooth, primary metapodal plate or unguinal sigillum elongate (Chant and McMurtry 2007). There are 12 species within this genus,
- Seta st3 is inserted off sternal shield of female on separate platelets (see below), which allows to classify this new species in the species group strunkhovae (Chant and McMurtry 2003). This species group contains four species (Chant and McMurtry 2003). The following list of characters of this new species is very different of all species of the genus and the species group. Despite the fact that we collected a single specimen, we consider this very original specimen as belonging to a new original species to science and we describe it thereafter.
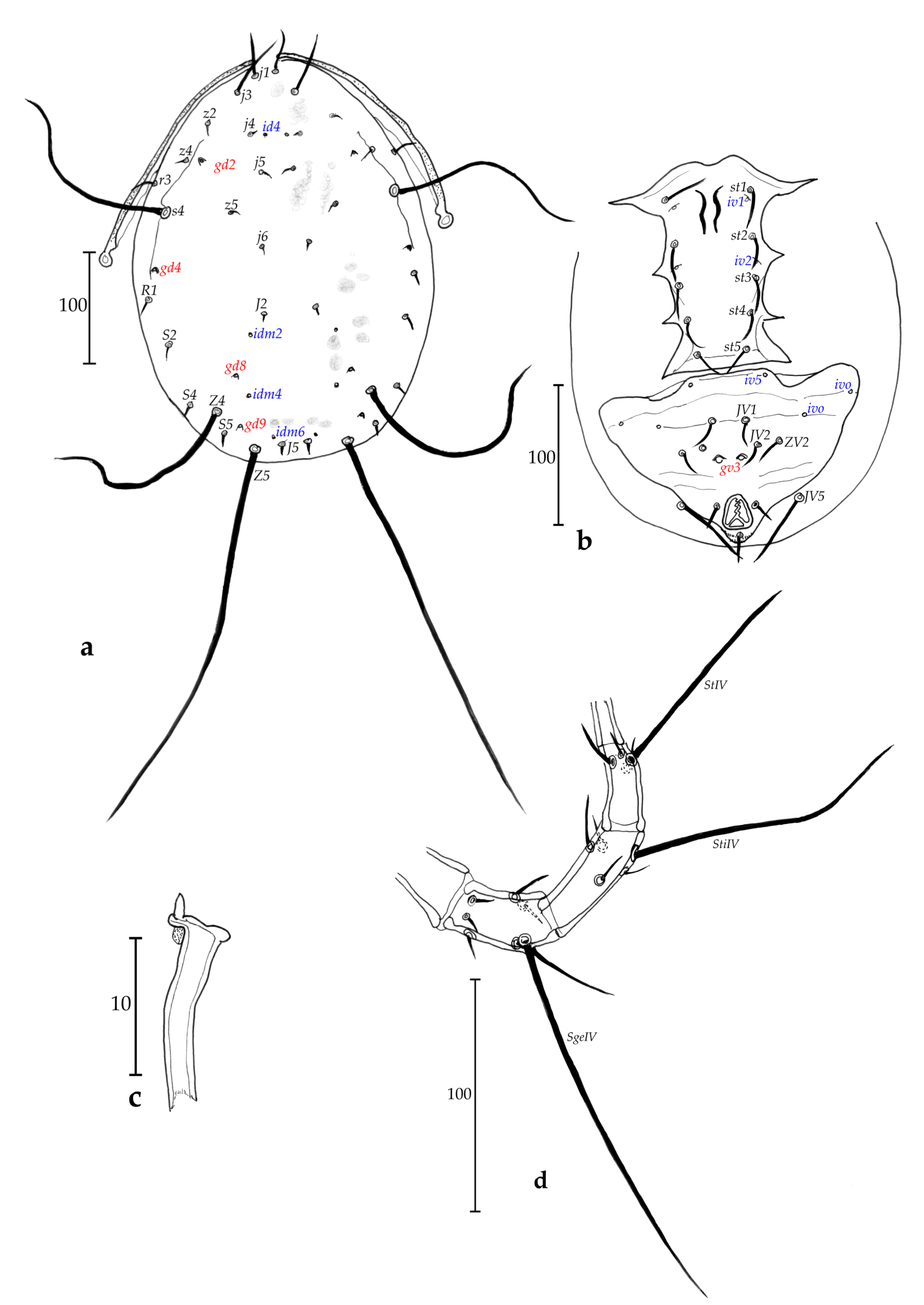
Description of adult female (n = 1, Figs. 1 a-e)
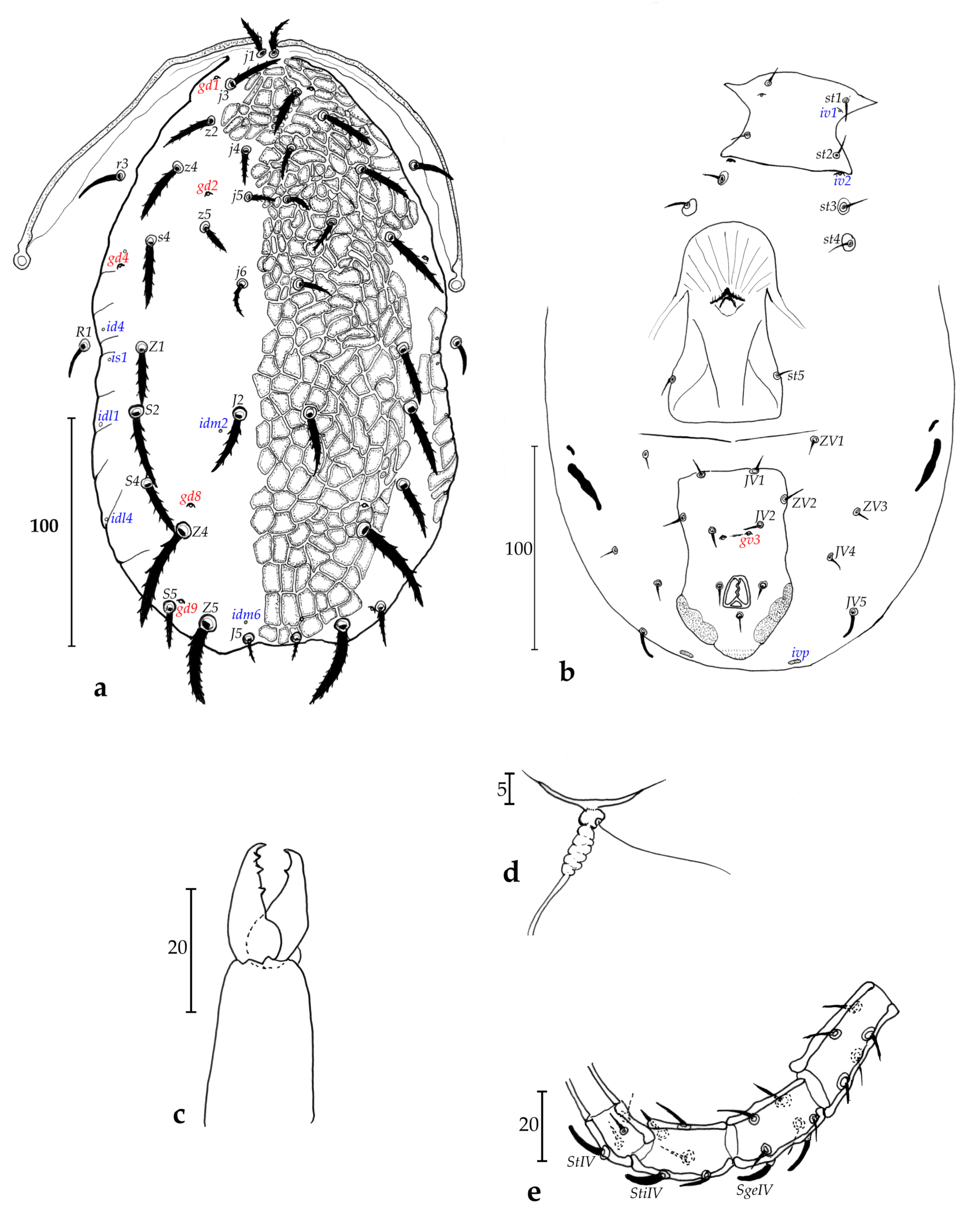


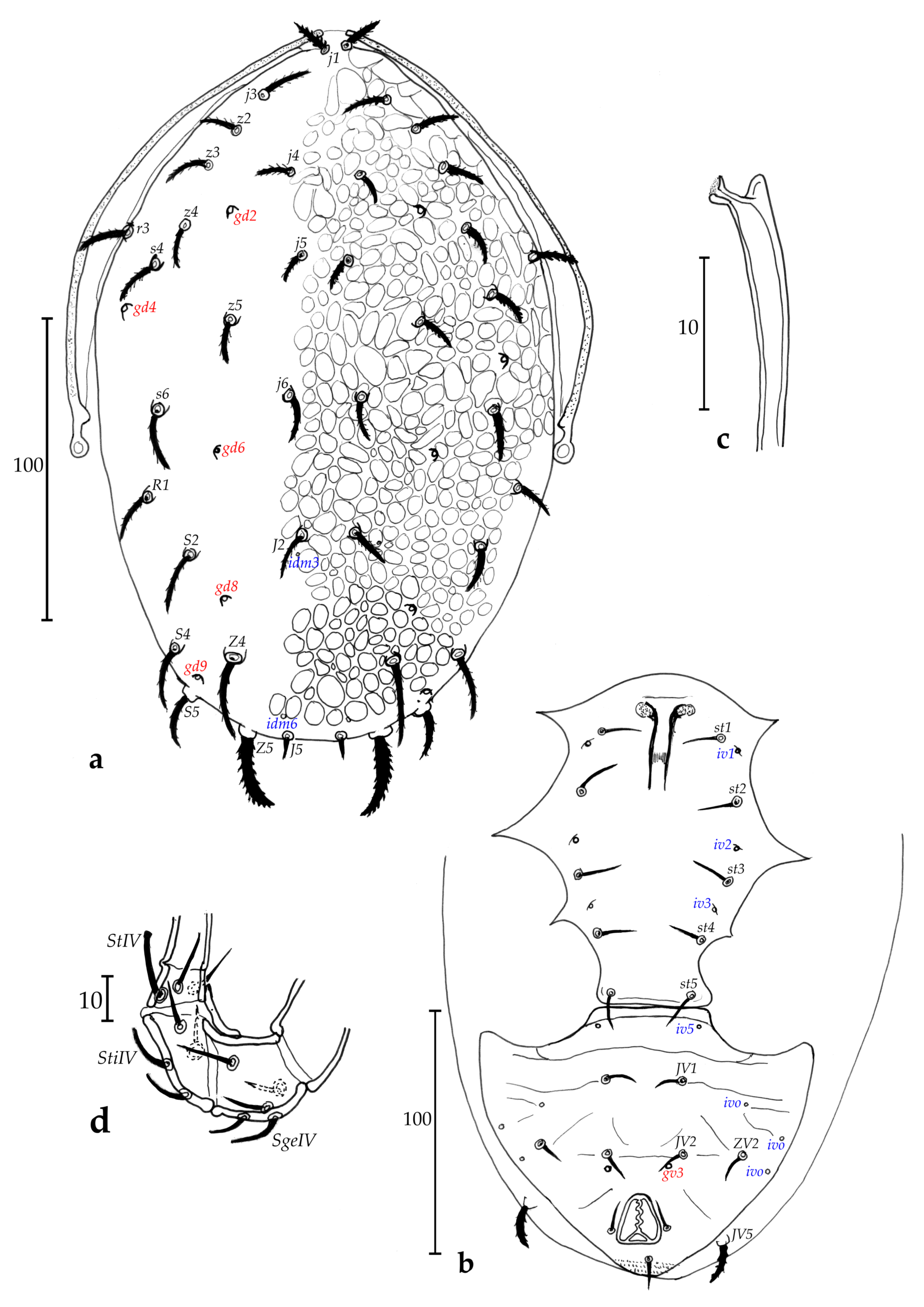
Dorsum (Fig. 1a) – Dorsal shield 285 long and 158 wide at level of s4, totally ornamented and reticulate, except on the posterior lateral margin from level of s4 to level of Z5 with less ornamentations and reticulations, with five solenostomes difficult to ascertain because of ornamentations and reticulations (gd1, gd2, gd4, gd8 and gd9), only six pairs of poroids difficult to see because of ornamentations and reticulations of the dorsal shield and possible to detect mainly on lateral sides, 17 pairs of dorsal setae and two pairs of sub-lateral setae on the membrane: j1 20, j3 28, j4 16, j5 14, j6 19, J2 30, J5 10, z2 28, z4 30, z5 15, Z1 28, Z4 45, Z5 40, s4 38, S2 33, S4 26, S5 18, r3 20, R1 15. All setae thick, plumose and serrate, except for r3 and R1 thick and smooth.
Peritreme and peritremal plate (Fig. 1a) – Extending to level of j1; peritremal plate fused with dorsal shield at level between j1 and j3.
Venter (Fig. 1b) – All ventral shields smooth. Sternal shield with two pairs of setae (st1 and st2) and a pair of poroids (iv1); two pairs of setae (st3 and st4) on two separate metasternal plates (no discernible pores on both of them); posterior margin of the sternal shield apparently straight; a pair of poroids (iv2) off sternal shield. Distances st1-st1 40, st2-st2 46, st3-st3 64, st1-st3 53, st4-st4 82. Genital shield length 108, width at level of st5 58, width at level of posterior corners 58, distance st5-st5 54. Two pairs of metapodal plates, primary metapodal plate moderately long compared to some other species (Table 1), 29 long and 3 wide and secondary short, 6 long and 2 wide. Ventrianal shield 93 long, 55 wide at level of anterior corners (ZV2), and 56 wide at level of para-anal setae. Ventrianal shield with three pairs of pre-anal setae (JV1, JV2 and ZV2), and a pair of small crateriform gv3, 13 apart. Unsclerotized cuticle arround ventrianal shield with four pairs of setae (JV4, JV5, ZV1 and ZV3), and apparently five pairs of round to oblong poroids difficult to see on our preparation, except for ivp on posterior part of the ventrianal shield. Seta JV5 short, thick and probably smooth (impossible to confirm on the single specimen), 12 long.
Chelicerae (Fig. 1c) – Fixed digit 20 long, with three strong teeth; and movable digit 20 long, with one strong tooth. Pilus dentilis not visible.
Spermatheca (Fig. 1d) – Pocular, 4 in length, with strong atrium at the basis of the calyx.
Legs (Fig. 1e) – Macrosetae are present on all legs and thick. Pointed thick macrosetae on genua I-III, tibia III, basitarsus, tibia and genu IV. Measurements: SgeI 14, SgeII 10, SgeIII 9, StiIII 12, SgeIV 10, StiIV 10, StIV 12. Genua II and III with seven and six setae, respectively. Chaetotactic formula of genu II: 2-2/0, 2/0-1; genu III: 1-2/0, 2/0-1.
Male. Unknown.
Material examined. A single ♀ in total collected during this study. One ♀ as type material. MOHELI ISLAND: Bangoma, top of the village (42 m aasl, 12°17′15″ S, 43°43′40″ E), 1 ♀ on Dendrocnide moroides (Weddel) Chew (Urticaceae), 4/XII/2018.
Type material. The holotype female is deposited in Institut Agro (Montpellier SupAgro) – INRAE Acarology collection, Montpellier, France.
Etymology. The name ''philippei'' refers to the first name of the senior author's second brother, Philippe Luc Kreiter, Engineer-Researcher in INRAE and specialist of biological control of mealybugs. The species is named in his honour.
Differential diagnosis and remarks. This species is unique in the genus Paragigagnathus by a set of unique characters (Table 1) and especially the small size of the body, the setae all plumose, thick and serrate, the reduce size of metapodal plates, the reduce size of ventrianal shield and the occurrence of macrosetae on all legs, the sternal shield with only two setae, along with an assemblage of specific setae lengths. No other species are closed to the new species, especially within the strunkhovae species group to which this new species belongs. For this reason, this species is described despite the single specimen collected. New surveys on Mohéli Island must occur in order to recover the species and to increase the description. Paragigagnathus philippei Kreiter n. sp. is the 13th species of the genus Paragigagnathus and the fifth species of the strunkhovae species group (the eight other species belonging to the desertorum species group).


Tribe Typhlodromipsini Chant & McMurtry
Typhlodromipsini Chant & McMurtry 2005c: 318.
Genus Typlodromips De Leon
Typhlodromips De Leon 1965: 23; Chant & McMurtry 2007: 61.
Typhlodromips culmulus (Van der Merwe)
Amblyseius (Amblyseius) culmulus van der Merwe 1968: 132; Ueckermann & Loots 1988: 157.
Typhlodromips culmulus, Moraes et al. 1986: 139, 2004b: 210; Chant & McMurtry 2005c: 327, 2007: 61.
This species belongs to the culmulus species group of the genus Typhlodromips with nine other species. It is also probably a type III species (McMurtry and Croft 1997; McMurtry et al. 2013), i.e., a polyphagous generalist predator. However, its biology remains totally unknown. It was already recorded on Mauritius Island, but only one record based on a single female and a single location (Kreiter et al. 2018a). It was also recorded in La Réunion Island, but with few specimens collected after intensive surveys (Kreiter et al. 2020b). This species seems rather rare.
Specimens examined. Two ♀♀ collected during this study. MAURITIUS ISLAND: Mare aux Vacoas (581 m aasl, 20°22′05″ S, 57°29′31″ E,), 2 ♀♀ on Ludwigia octovalvis (Jacquin) Raven (Onagraceae), 5/XI/2018.
Previous Records. Kenya, Lesotho, South Africa.
Remarks. Measurements of the two adult female specimens agree very well with measurements of the literature, with only very slight differences in the Mauritius specimen: smaller Z4, JV5, SgeII and StiIV setae.
Tribe Amblyseiini Muma
Amblyseiinae Muma 1961: 273 and Amblyseiini Muma, Wainstein 1962: 26.
Subtribe Amblyseiina Muma
Amblyseiina Muma, Chant & McMurtry 2004: 179.
Genus Amblyseius Berlese
Amblyseius Berlese 1914: 143.
Amblyseius erici Kreiter n. sp.
ZOOBANK: F011832D-AFE5-45B2-BA60-BB9DA7EA6A20 ![]()
Classification. Amblyseius erici Kreiter n. sp. belongs to:
- the subfamily Amblyseiinae (absence of dorsolateral setae z3 and s6 and the caudoventral setae JV3),
- to the tribe Amblyseiini (setae j3, s4, Z4 and Z5 longer than other setae, ratio s4/Z1 < 3.1, many teeth on the fixed cheliceral digit and macrosetae on legs I, II and/or III in addition to macrosetae on leg IV),
- to the subtribe Amblyseiina (sternal shield as long as wide, ventrianal shield longer than wide, seta J2 present, genital shield almost as wide as ventrianal shield, ventral shields generally smooth, macrosetae on all legs, setae j5, J2, S2, S4, S5 and Z1 present),
- to the genus Amblyseius (ratio s4/S2 < 3.0, chelicerae of normal size with fixed digit of the same size as movable digit, seta JV2 present, without incision in lateral margin of dorsal shield at level of s4, ventrianal shield not reduced to a simple anal shield, Ge III and Ti III each generally with a macroseta) (Chant and McMurtry 2007),
- to the species group obtusus as setae J2 and Z1 are present, dorso-central setae and setae z2, z4, Z1, S2, S4, and S5 are minute, setae s4, Z4 and Z5 are prominent, elongate and whip-like, female ventrianal shield usually pentagonal, as wide at level of anus than at level of ZV2 or wider at this later level (Chant and McMurtry 2004),
- to the large species subgroup andersoni with the calyx bell- to glass-shaped. This subgroup contains 120 species (in Chant and McMurtry 2004). Many of those species are very different from the new species and we compare it thereafter with closer related species.
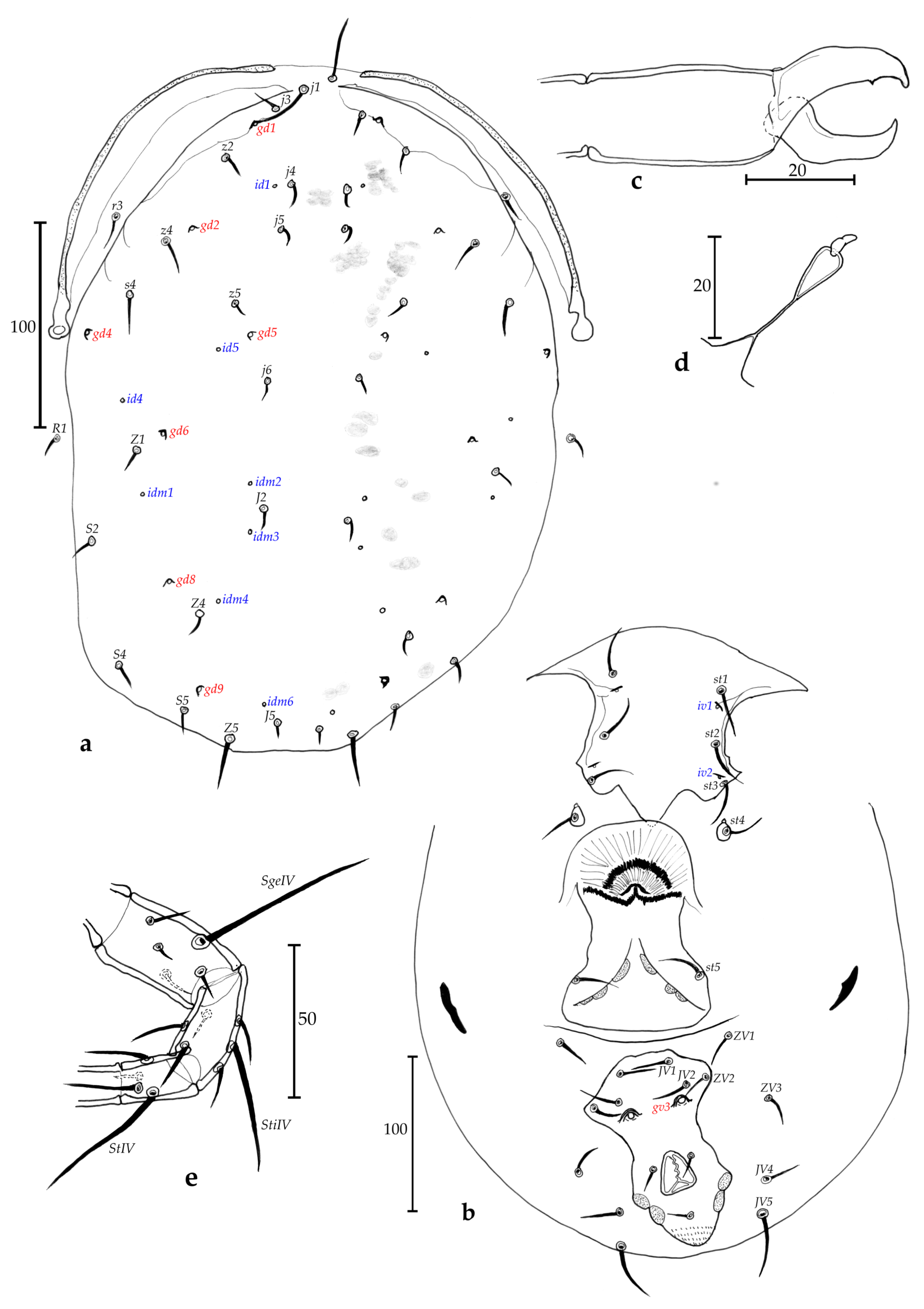
Description of adult female (n = 2, Figs. 2 a-e)
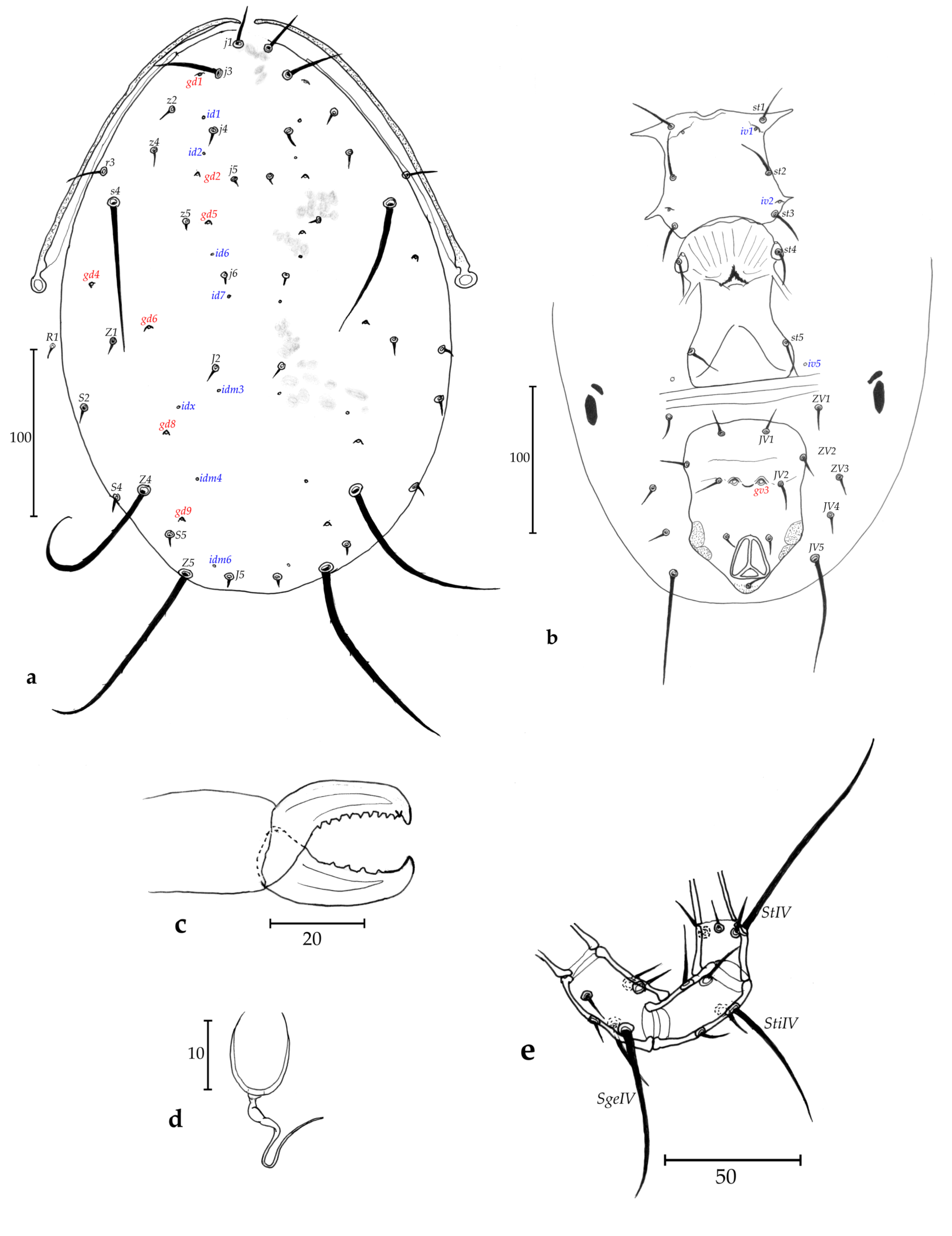


Dorsum (Fig. 2a) – Dorsal shield smooth, 330–338 long and 195–200 wide at level of s4, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9), eight pairs of poroids visible, lateral ones hidden, 17 pairs of dorsal setae and two pairs of sub-lateral setae on membranes: j1 22–28, j3 38–39, j4 7, j5 4–5, j6 7, J2 6, J5 6, z2 9, z4 10–11, z5 4–5, Z1 6–8, Z4 108, Z5 113–125, s4 88–93, S2 8, S4 8, S5 8, r3 20–22, R1 7–8; r3 and R1 apparently on the dorsal shield, but actually off on the unsclerotized cuticle. All setae smooth, except for Z4 and Z5 lightly serrate.
Peritreme and peritremal plate (Fig. 2a) – Extending to level of j1; peritremal plate fused with dorsal shield at level of j3.
Venter (Fig. 2b) – All ventral shields smooth. Sternal shield with three pairs of setae (st1-st3) and two pairs of poroids (iv1 and iv2); a pair of st4 and a pair of pores on a small pear-shaped metasternal plate; posterior margin of the sternal shield concave. Distances st1-st1 58–59, st2-st2 65–68, st3-st3 68–70, st1-st3 63–65, st4-st4 65–66. Genital shield length 118–120, width at level of st5 70, width at level of posterior corners 60–70, distance st5-st5 63–65. Two pairs of metapodal plates, the primary 20 long and 4–5 wide and the secondary 12–13 long and 2 wide. Ventrianal shield 110–120 long, 83 wide at level of anterior corners (ZV2), and 75–77 wide at level of para-anal setae. Ventrianal shield with three pairs of pre-anal setae (JV1, JV2 and ZV2), and a pair of large crateriform gv3, 20 apart. Unsclerotized cuticle around ventrianal shield with four pairs of setae (JV4, JV5, ZV1, and ZV3), and five pairs of round to oblong poroids not well discernible. Seta JV5 smooth, 73–75 long.
Chelicerae (Fig. 2c) – Fixed digit 30–31 long, with ten strong teeth; and movable digit 32–33 long, with four strong teeth. Pilus dentilis not visible.
Spermatheca (Fig. 2d) – Bell- to glass-shape, with a calyx swollen basally 12–13 long and 7–8 wide, an undifferentiated atrium and long major duct. Small minor duct not visible.
Legs (Fig. 2e) – Pointed strong and very visible whip-like macrosetae on genua I-III, on tibia III, and on basitarsus, tibia and genu IV. Measurements: SgeI 45–53, SgeII 35–38, SgeIII 31–35, StiIII 22–28, SgeIV 70–75, StiIV 50–55, StIV 80–84. Genua II and III both with seven setae. Chaetotactic formula of genua II: 2-2/0, 2/0-1; genu III: 1-2/1, 2/0-1.
Description of adult male (n = 1) (Figs. 3 a-d)



Dorsum (Fig. 3a) – Dorsal shield smooth, 250 long and 158 wide, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9), six pairs of poroids, 19 pairs of dorsal setae (r3 and R1 on dorsal shield): j1 23, j3 34, j4 5, j5 3, j6 5, J2 5, J5 5, z2 7, z4 9, z5 5, Z1 5, Z4 78, Z5 108, s4 65, S2 8, S4 7, S5 6, r3 15, R1 8. All setae similar to adult female.
Peritreme and peritremal plate (Fig. 3a) – Extending to level of j1; peritremal plate fused with dorsal shield at level of j1.
Venter (Fig. 3b) – Sternogenital shield smooth. Distances st1-st1 48, st2-st2 53, st3-st3 50, st1-st5 105, st4-st4 40, st5-st5 30, with three pairs of poroids (iv1-iv3). Ventrianal shield 105 long, 128 wide at anterior corners and 50 wide at level of para-anal setae. Ventrianal shield reticulate anteriad JV1 with three pairs of pre-anal setae (JV1, JV2 and ZV2) and a pair of small crateriform gv3, between JV2 bases, 14 apart. Two pairs of poroids ivo discernible. Unsclerotized cuticle arround ventrianal shield with a pair of seta (JV5). Seta JV5 smooth, 38 long.
Chelicerae (Fig. 3c) – Fixed digit 20 long, with nine teeth discernible; and movable digit 20 long, with two teeth discernible. Spermatodactyl shaft 19 and branch 5.
Legs (Fig. 3d) – One macroseta on legs I and II, two macrosetae on leg III and three macrosetae on legs IV similar to adult female. All macrosetae sharp-tipped. Measurements: SgeI 33, SgeII 30, SgeIII 23, StiIII 18, SgeIV 50, StiIV 43, StIV 78. Chaetotactic formula of genua II and III similar to adult female.
Specimens examined and measured. Two ♀♀ and one ♂ collected during this study measured and type material. GRANDE COMORE ISLAND: Mvouni, University of Comoros (434 m aasl, 11°43′11″ S, 43°16′31″ E), 1 ♀ and 1 ♂ on Clidemia hirta L. (Melastomataceae), 6/XII/2018; Ivembeni, Banda Samlini (791 m aasl, 11°29′22″ S, 43°19′36″ E), 1 ♀ on Rubus rosifolius Smith (Rosaceae), 7/XII/2018.
Type material. One holotype ♀ on one slide, one paratype ♀ and one paratype ♂ on another slide are deposited in Institut Agro (Montpellier SupAgro) – INRAE Acarology collection, Montpellier, France.
Etymology. The name ''erici'' refers to the first name of the senior author's youngest and third brother, Eric Kreiter. The species is named in his honour.
Differential diagnosis and remarks. None of the females of species of Amblyseius (of the obtusus species group and of the andersoni species subgroup) included in Table 2 share similar characters with females of Amblyseius erici Kreiter n. sp. The two closest species concerning setae length are A. angulatus Karg and A. compositus Denmark & Muma, but several other details are different: macrosetae lengths and number of teeth of these two species compared to the new species. But descriptions of these two new species are old and very poor and lacking information for a complete description. The shape of the spermatheca of the new species is unique and allows distinguishing this new species from all others in Table 2 and all species of the andersoni species subgroup. The following combination of characters, of the male indicated in the description of the male of this new species, is quite similar to that of the few described males of species of Amblyseius belonging to the obtusus species group and to the andersoni species subgroup.


Not many characters allow to distinguish it from all males of other species if no females are collected at the same time: the peritreme reaching the level of j1, an absence of reticulation of the dorsal shield, some dorsal setae lengths, especially z2, z4, r3 and S2 approximately of the same length (12–15), additional macrosetae on all other legs than leg IV, macrosetae of leg IV not subequal, a sternogenital shield smooth, ventrianal shield reticulate, only three pairs of pre-anal setae, a pair of crateriform gv3 between JV2. All described males of the large species subgroup andersoni have similar ventrianal shield reticulate with three pairs of pre-anal setae. Only the shape of the spermatodactyl allows distinction of the male of the species (Figure 3c).
Amblyseius duplicesetus Moraes & McMurtry
Amblyseius duplicesetus Moraes & McMurtry 1988: 13; Moraes et al. 2004a: 143, 2004b: 22; Zannou et al. 2007: 10; El-Banhawy & Knapp 2011: 25.
Amblyseius duplicisetus [sic], Chant & McMurtry 2004: 208, 2007: 78.
Description of adult male of Amblyseius duplicesetus Moraes & McMurtry (n = 10, five from Anjouan, three from Mohéli and two from Grande Comore Islands, Figs 4 a-d)



Dorsum (Fig. 4a) – Dorsal shield smooth, 271 (262–295) long and 174 (150–193) wide, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9) similar to adult female, seven pairs of poroids visible, but probably more present, 19 pairs of dorsal setae (r3 and R1 on dorsal shield): j1 31 (29–33), j3 44 (43–45), j4 8 (6–8), j5 5 (5–6), j6 8 (7–8), J2 9 (8–10), J5 8 (8–9), z2 9 (8–10), z4 9 (8–10), z5 5 (5–6), Z1 9 (8–11), Z4 61 (56–70), Z5 218 (200–238), s4 68 (63–73), S2 11 (10–12), S4 10 (9–11), S5 8 (7–9), r3 10 (8–13), R1 11 (9–14). All setae sharp-tipped and smooth, except for Z4 and Z5 lightly serrate.
Peritreme and peritremal plate (Fig. 4a) – Extending to level of j1 insertion; peritremal plate fused with dorsal shield at level between j1 and j3.
Venter (Fig. 4b) – Sternogenital shield smooth with only few striae in the anterior part and lateral margings, with five pairs of setae (st1-st5) and two pairs of poroids (iv1 and iv2). Distances st1-st1 52 (50–55), st2-st2 55 (50–58), st3-st3 55 (51–58), st1-st5 114 (112–118), st4-st4 36 (31–40), st5-st5 31 (30–34). Ventrianal shield 112 (108–118) long, 148 (138–158) wide at anterior corners and 59 (50–75) wide at level of para-anal setae. Ventrianal shield anteriorly reticulate (before the line constituted by JV2), with three pairs of pre-anal setae (JV1, JV2 and ZV2) and a pair of small crateriform gv3, between JV2 just below the line between their bases, 22 (20–25) apart. Shield also with a pair of iv5 and three pairs of poroids ivo. Unsclerotized cuticle arround ventrianal shield with a pair of seta (JV5). Seta JV5 smooth, 38 (34–45) long.
Chelicerae (Fig. 4c) – Fixed digit 23 (21–24) long, with eight teeth; and movable digit 23 (21–25) long, with one tooth. Spermatodactyl shaft 18 (14–20) and branch 8 (8–9). Pilus dentilis not visible.
Legs (Fig. 4d) – All legs with macrosetae sharp-tipped. Measurements: SgeI 36 (33–40), SgeII 31 (28–33), SgeIII 38 (37–40), StiIII 33 (30–38), SgeIV 96 (90–100), StiIV 75 (68–85), StIV 55 (50–58). Chaetotactic formula of genua II and III similar to adult female.
Specimens examined. Twenty-three ♂♂ collected during this study, 10 ♂♂ measured, 13 ♂♂ as complementary voucher material. ANJOUAN ISLAND (5 ♂♂): Chandra, inside the village (436 m aasl, 12°12′36″ S, 44°27′09″ E), 1 ♂ on Acalypha wilkesiana Müller Argoviensis (Euphorbiaceae), 29/XI/2018; Pomoni, exit of the village (29 m aasl, 12°17′01″ S, 44°34′37″ E), 1 ♂ on Artocarpus heterophyllus Lamarck (Moraceae), 1 ♂ on Artocarpus altilis (Parkinson) Fosberg (Moraceae) and 2 ♂♂ on an uncknown tree with alternate leaves, 30/XI/2018. MOHELI ISLAND (11 ♂♂): Fomboni, inside the town (15 m aasl, 12°17′29″ S, 43°44′35″ E), 1 ♂ on Annona muricata L. (Annonaceae), 2/XII/2018; Fomboni, Les-Hauts (60 m aasl, 12°17′29″ S, 43°44′35″ E), 2 ♂♂ on an unknown host plant, 2/XII/2018; Hoani, inside village (38 m aasl, 12°17′3″ S, 43°44′34″ E), 1 ♂ on the same unknown host plant than above, 1 ♂ on A. muricata, 2 ♂♂ on Artocarpus altilis J.R. Forster and G. Forster (Moraceae) and 1 ♂ on Theobroma cacao L. (Malvaceae), 3/XII/2018; Bangoma, Les Hauts (137 m aasl, 12°17′18″ S, 43°43′41″ E), 1 ♂ on Cinnamomum odoratum Schäffer (Lauraceae), 1 ♂ on A. altilis and 1 ♂ on Persea americana Miller (Lauraceae), 4/XII/2018. GRANDE COMORE ISLAND (7 ♂♂): Mdé, INRAPE (51 m aasl, 11°44′12″ S, 43°14′59″ E), 1 ♂ on Mangifera indica L. (Anacardiaceae), 6/XII/2018; Mvouni, University of Comoros (434 m aasl, 11°43′11″ S, 43°16′31″ E), 1 ♂ on Myristica fragans Houttuyn (Myristicaceae) and 1 ♂ on Citrus sinensis (L.) Osbeck (Rutaceae), 6/XII/2018; Dzahani, village (209 m aasl, 11°46′32″ S, 43°16′40″ E), 1 ♂ on Carica papaya L. (Caricaceae), 1 ♂ on Artocarpus altilis Parkinson Fosberg (Moraceae), 7/XII/2018; Mdjoiyezi (230 m aasl, 11°50′19″ S, 43°18′29″ E), 1 ♂ on M. indica, 10/XII/2018; Mdé, INRAPE (51 m aasl, 11°44′12″ S, 43°14′59″ E), 1 ♂ on Spondias dulcis Solander ex. Parkinson (Anacardiaceae), 11/XII/2018.
Voucher material. Twenty-three ♂♂ on 20 slides are deposited in Institut Agro (Montpellier SupAgro) – INRAE Acarology collection, Montpellier, France.
Differential diagnosis and remarks. The male of this species was mentioned in El-Banhawy and Knapp (2011), but it is not indicated that this is the first mention of the male of this species, the male was illustrated, but the description lacks detail (El-Banhawy and Knapp 2011). We thus decide on a more detailed description of the male of this species.
This species belongs to the largoensis species group as setae J2 and Z1 are present, seta s4 is minute and the ventrianal shield of the female is vase-shaped. It belongs to the largoensis species subgroup as seta Z4 is long, spermatheca has the calyx elongate mostly tubular and the female ventrianal shield is entire (Chant and McMurtry 2004).
The following combination of characters, indicated in the description of the male of this species, is quite similar to the few described males of species of Amblyseius belonging to the largoensis species group and to the largoensis species subgroup.
Not many characters allow to distinguish it from all males of other species if no females are collected in the same time (all the males used for description were collected with females of this species): the peritreme reaching level of j1, absence of reticulation of the dorsal shield, all dorsal setae including J5 length approximately of the same length (8–11), except for j1, j3, s4, Z4, Z5 longer and z5 shorter, additional macrosetae on all other legs than leg IV, macrosetae of leg IV not sub-equal, a sternogenital shield mostly smooth, a ventrianal shield reticulate, only three pairs of pre-anal setae, a pair of crateriform gv3 between JV2.
All described males of the large species subgroup largoensis have very similar ventrianal shield reticulate with three pairs of pre-anal setae.
Amblyseius haleakalus Prasad
Amblyseius haleakalus Prasad 1968: 1516; Moraes et al. 1986: 14, 2004b: 27; Denmark & Muma 1989: 97; Chant & McMurtry 2004: 199, 2007: 78.
Amblyseius (Multiseius) haleakalus, Denmark & Evans 2011: 75.
Description of adult male of Amblyseius haleakalus Prasad (n = 1, Figs 5 a-d)



Dorsum (Fig. 5a) – Dorsal shield smooth, 300 long and 193 wide, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9), seven pairs of poroids visible, 19 pairs of dorsal setae (r3 and R1 on dorsal shield): j1 33, j3 33, j4 4, j5 4, j6 8, J2 8, J5 5, z2 8, z4 9, z5 6, Z1 8, Z4 85, Z5 (half-broken), s4 80, S2 10, S4 9, S5 8, r3 8, R1 8. All setae smooth, except for Z4 lightly serrate and Z5 probably slightly serrate, but not all visible because both members of Z5 are damaged.
Peritreme and peritremal plate (Fig. 5a) – Extending to level of j1; peritremal plate fused with dorsal shield at level of z2.
Venter (Fig. 5b) – Sternogenital shield smooth with only few striae, five pairs of setae (st1-st5) and two pairs of poroids (iv1 and iv2). Distances st1-st1 54, st2-st2 61, st3-st3 60, st1-st5 117, st4-st4 48, st5-st5 38. Ventrianal shield 140 long, 148 wide at anterior corners and 75 wide at level of para-anal setae. Ventrianal shield reticulate with three pairs of pre-anal setae (JV1, JV2 and ZV2), and a pair of small crateriform gv3, between JV2 bases, 16 apart. A pair of poroids iv5 and three pairs of poroids ivo also discernible. Unsclerotized cuticle arround ventrianal shield with a pair of setae (JV5). Seta JV5 smooth, 48 long.
Chelicerae (Fig. 5c) – Fixed digit 23 long, with at least ten teeth discernible; and movable digit 23 long, with no teeth discernible. Spermatodactyl shaft 21 and branch 7. Pilus dentilis not visible.
Legs (Fig. 5d) – All legs with pointed macrosetae similar to adult female. Measurements: SgeI not measured, SgeII 24, SgeIII 30, StiIII 30, SgeIV 60, StiIV 55, StIV 57. Chaetotactic formula of genua II and III similar to adult female.
Specimens examined. One single ♂ collected during this study, measured and deposited as a complementary voucher specimen.
MAURITIUS ISLAND. Curepipe, Anderson Street (560 m aasl, 20°19′11′′ S, 57°31′52′′ E), one ♂ (along with eight ♀♀ on the same leaves of the same plant collected in the same time) on Araucaria columnaris (Forster) Hook (Araucariaceae), 4/XI/2018.
Voucher material. One male on one slide is deposited in Institut Agro (Montpellier SupAgro) – INRAE Acarology collection, Montpellier, France.
Differential diagnosis and remarks. This species belongs to the obtusus species group as seta z4 is minute and female ventral shield is not vase-shaped or divided. It belongs to the andersoni species subgroup as the spermatheca has a differentiated atrium, a calyx not dotted or annulated, not swollen basally and calyx dish-, cup-, bell- or V-shaped. The following combination of characters, indicated below in the description of the male of this species, is quite similar to the few described males of species of Amblyseius belonging to the obtusus species group and to the andersoni species subgroup.
Not many characters allow to distinguish it from all males of other species if no females are collected in the same time: the peritreme reaching level of j1, absence of reticulation of the dorsal shield, some dorsal setae lengths, especially z2, z4, r3 and S2 approximately of the same length (12–15), additional macrosetae on all other legs than leg IV, macrosetae of leg IV not subequal, a sternogenital shield smooth, ventrianal shield reticulate, only three pairs of pre-anal setae, a pair of crateriform gv3 between JV2. All described males of the large species subgroup andersoni have similar ventrianal shield reticulate with three pairs of pre-anal setae.
Characters of males are very similar to that of adult females, except of course for length of setae and few other characters. The only difference is that ventrianal shield of the male is moderately reticulate, while the ventrianal shield of the female is not.
Amblyseius parasundi Blommers
Amblyseius (Proprioseiopsis) parasundi Blommers 1974: 144.
Amblyseius (Amblyseius) parasundi, Denmark & Muma 1989: 19.
Amblyseius parasundi, Moraes et al. 1986: 27, 2004b: 46.
Description of adult male of Amblyseius parasundi Blommers (n = 8, Figs 6 a-d)


Dorsum (Fig. 6a) – Dorsal shield smooth, 265 (253–275) long and 179 (170–213) wide, with only four solenostomes difficult to distinguish (gd2, gd4, gd8 and gd9), four pairs of poroids, 18 pairs of dorsal setae (r3 and R1 on dorsal shield): j1 29 (26–33), j3 39 (38–41), j4 4 (4–5), j5 4, j6 5 (4–5), J2 5 (5–6), J5 7 (5–8), z2 7 (6–8), z4 7 (7–8), z5 5 (4–5), Z4 142 (130–150), Z5 364 (350–383), s4 140 (133–146), S2 7 (6–8), S4 8 (6–8), S5 6 (5–7), r3 13 (11–15), R1 7 (6–8). All setae sharp-tipped and smooth, except for Z4 and Z5 lightly serrate.
Peritreme and peritremal plate (Fig. 6a) – Extending to level of j1; peritremal plate fused with dorsal shield at level between z2 and z4.
Venter (Fig. 6b) – Sternogenital shield smooth with very few striae, five pairs of setae (st1-st5) and two pairs of poroids (iv1 and iv2). Distances st1-st1 54 (49–58), st2-st2 62 (60–63), st3-st3 58 (50–59), st1-st5 117 (113–120), st4-st4 43 (40–45), st5-st5 38 (34–41). Ventrianal shield 119 (115–125) long, 153 (145–160) wide at anterior corners and 64 (58–70) wide at level of para-anal setae. Ventrianal shield striate, with three pairs of pre-anal setae (JV1, JV2 and ZV2) and a pair of small rounded gv3, between JV2 just below the line between their bases, 16 (13–20) apart. A pair of iv5 and two pairs of poroids ivo also discernible. Unsclerotized cuticle around ventrianal shield with a pair of setae (JV5). Seta JV5 smooth, 65 (60–69) long.
Chelicerae (Fig. 6c) – Fixed digit 24 (22–25) long, with 11 teeth discernible; and movable digit 27 (25–28) long, with three teeth discernible. Spermatodactyl shaft 15 (13–18) long and branch 5 (4–5). Pilus dentilis not visible.
Legs (Fig. 6d) – All legs with sharp-tipped macrosetae similar to adult female. Measurements: SgeI 61 (55–65), SgeII 39 (37–40), SgeIII 56 (48–58), StiIII 46 (43–49), SgeIV 171 (160–180), StiIV 128 (122–138), StIV 94 (90–98). Chaetotactic formula of genua II and III similar to adult female. One erected seta on femur IV.
Specimens examined. Eight ♂♂ collected during this study, measured and deposited as complementary voucher material. MAYOTTE ISLAND: Coconi, Maison de l'Office National des Forêts (156 m aasl, 12°50′1″ S, 45°8′5″ E), 1 ♂ on Terminalia catappa L. (Combretaceae), 24/XI/2018; Combani, gîte du Mont-Combani (437 m aasl, 12°48′23′′ S, 45°9′17′′ E), 1 ♂ on Cocos nucifera L. (Arecaceae), and 1 ♂ on Cananga odorata L. (Annonaceae), 25/XI/2018; L'Abattoir, Dziani lake (23 m aasl, 12°46′14′′ S, 45°17′18′′ E), 1 ♂ on Artocarpus altilis (Parkinson) Fosberg (Moraceae), 27/XI/2018. MOHELI ISLAND: Hoani, inside village (38 m aasl, 12°17′3′′ S, 43°44′34′′ E), 1 ♂ on Theobroma cacao L. (Malvaceae), 3/XII/2018; Bangoma, Les Hauts (137 m aasl, 12°17′18′′ S, 43°43′41′′ E), 1 ♂ and 1 im. on Cinnamomum odoratum Schäffer (Lauraceae), 1 ♂ on Annona muricata L. (Annonaceae) and 1 ♂ on Litchi chinensis Sonnerat (Sapindaceae), 4/XII/2018.
Voucher material. Eight males on eight slides are deposited in Institut Agro (Montpellier SupAgro) – INRAE Acarology collection, Montpellier, France.
Differential diagnosis and remarks. This species has no seta Z1 and consequently belongs to the sundi species group and having the spermatheca elongate, tube-like, it belongs to the sundi species subgroup. The following combination of characters indicated below in the description of the male of this species is quite similar to the unique described males of species of Amblyseius belonging to the sundi species group and to the sundi species subgroup. Not many characters allow distinguishing it from the single described male of this sundi subgroup, the male of A. sundi Pritchard & Baker. If no females are collected in the same time, the identification will be impossible. These characters are: the peritreme reaching level of j1, absence of reticulation of the dorsal shield, some dorsal setae length, especially j-J serie starting to j4, z2 to z5, R1 and S series (after s4) approximately of the same length (4–8), additional macrosetae on all other legs than leg IV, macrosetae of leg IV not subequal and long, sternogenital shield smooth, ventrianal shield reticulate, only three pairs of pre-anal setae, a pair of round gv3 between JV2, a macroseta present also on genu lI, only three teeth on the movable digit and 11 on the fixed digit of chelicera instead of one and six in the male chelicera of A. sundi, respectively.
Characters of males are very similar to adult females, except of course for lengths of setae and other few characters. The only difference is that the ventrianal shield of the male is lightly reticulate in the anterior part and the ventrianal shield of the female is not.
Blommers and Gutierrez (1975) found this species very abundant on fruit trees preying on several species of tetranychid mites. Amblyseius sundi is reported by Blommers (1974) as being a thelytokous species in mass-rearing and field collected specimens and similar information is also mentioned by Denmark and Muma (1989). In nature, reproduction of A. parasundi seems more complicated. Males were not so rare in fields of the two Islands where they were found (Mayotte and Mohéli). This suggests further fundamental studies on the biology of this species.
Tribe Euseiini Chant & McMurtry
Euseiini Chant & McMurtry 2005a: 191.
Subtribe Typhlodromalina Chant & McMurtry
Typhlodromalina Chant & McMurtry 2005a: 195.
Genus Typhlodromalus Muma
Amblyseius (Typhlodromalus) Muma 1961: 288;
Typhlodromalus De Leon 1966: 87.
Typhlodromalus baillodi Kreiter n. sp.
ZOOBANK: 7BDF4768-99F0-4CDE-A946-574CFB26D1A0 ![]()
Classification. Typhlodromalus baillodi Kreiter n. sp. belongs to:
- the subfamily Amblyseiinae (absence of dorsolateral setae z3 and s6 and the caudoventral seta JV3),
- to the tribe Euseiini (sternal shield with median posterior projection, deutosternal groove < 5 µm in width, forward migration of pre-anal setae JV2 and ZV2),
- to the subtribe Typhlodromalina (chelicera of normal size and shape, with prominent teeth evenly distributed along fixed digit, peritreme usually extending to level of j1, deutosternal groove narrow, 4–7 µm width),
- to the genus Typhlodromalus (female ventrianal shield with more than one pair of pre-anal setae, GeI usually with a macroseta, GeII and III with macrosetae, leg IV with three macrosetae usually stout, often knobbed or blunt, male ventrianal shield with three pairs of pre-anal setae, most dorsal setae either setiform or thickened, thorn like, tapering distally, without terminal knobs, fixed digit with 6–12 teeth evenly distributed along the digit, BtI without erected seta, female ventrianal shield with three pairs of pre-anal setae, ratio s4/Z1 < 3.0 : 1.0, dorsal setae of medium length subequal, dorsal shield ornamented in addition to anterolateral striations, seta Z4 longer than distance between its base and that of S4,
- to the peregrinus species group as seta S5 is present (Chant and McMurtry 2007) which includes 16 species (Chant and McMurtry 2005a but incomplete): T. araucariae Gonçalves & Ferla, T. aripo De Leon, T. clavicus Denmark & Muma, T. erigeronus Denmark & Evans, T. etiennei (Kreiter & Ueckermann), T. feresi Lofego, Moraes & McMurtry, T. feresisimilis Moraes, Barbosa & Castro, T. ingae Moraes, Barbosa & Castro, T. jucundus (Chant), T. marmoreus (El-Banhawy), T. olombo (Pritchard & Baker), T. peregrinus (Muma), T. planetarius (De Leon), T. pumilus Denmark & Evans, T. rosayroi Denmark & Muma and T. simus Denmark & Muma.
Description of adult female (n = 15 of 44 collected during this study, Figs. 7 a-e)



Dorsum (Fig. 7a) – Dorsal shield strongly ornamented and reticulate, with margins of posterior part slightly indented at level of S5 creating a slight ''trilobite appearance'', with an expansion on each lateral side at level of s4-Z1 and with a constriction at level of R1, 310 (283–333) long and 187 (165–210) wide at level of s4, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9), 14 pairs of poroids, 17 pairs of dorsal setae and two pairs of sub-lateral setae: j1 24 (23–25), j3 24 (20–30), j4 14 (13–15), j5 14 (12–15), j6 18 (15–20), J2 20 (18–23), J5 8 (7–10), z2 22 (20–25), z4 26 (23–28), z5 18 (15–20), Z1 21 (18–24), Z4 32 (29–38), Z5 60 (54–68), s4 33 (29–35), S2 29 (25–33), S4 24 (18–28), S5 15 (13–18), r3 21 (18–24), R1 19 (16–23). All setae thickened and smooth, except for Z5 strongly serrate.
Peritreme and peritremal plate (Fig. 7a) – Extending to level of j1; peritremal plate fused with dorsal shield at level between j1 and j3, much closer to the former.
Venter (Fig. 7b) – All shields smooth. Sternal shield with three pairs of setae (st1-st3) and two pairs of rounded poroids (iv1 and iv2); a pair of st4 and a pair of rounded pores (iv3) on a metasternal plate; posterior margin of the sternal shield convex, with a posterior projection. Distances st1-st1 52 (44–58), st2-st2 60 (55–65), st3-st3 69 (63–75), st1-st3 61 (53–65), st4-st4 69 (61–83). Genital shield length 107 (103–119), width at level of st5 70 (63–75), width at level of posterior corners 75 (68–80), distance st5-st5 66 (63–70). Two pairs of metapodal plates 16 (10–19) long and 4 (2–5) wide for the larger and 8 (5–10) long and < 1 wide for the slender. Ventrianal shield 101 (90–113) long, 64 (58–70) wide at level of anterior corners (ZV2), and 65 (61–70) wide at level of para-anal setae. Ventrianal shield smooth, with three pairs of pre-anal setae (JV1, JV2 and ZV2), and a pair of evolved and oblong crateriform gv3, 23 (19–25) apart. Unsclerotized cuticle around ventrianal shield with four pairs of setae (JV4, JV5, ZV1 and ZV3,), and five pairs of round to oblong poroids ivo and ivp. Seta JV5 thickened and smooth, 39 (30–43) long.
Chelicerae (Fig. 7c) – Fixed digit 26 (25–28) long, with five teeth in row and one subapical tooth; and movable digit 27 (25–28) long, with two teeth. Pilus dentilis not visible.
Spermatheca (Fig. 7d) – Resembles that of Ueckermannseius payetae Kreiter n. sp. in the new species group havu of the genus Ueckermannseius, with the atrium bulbous and elongate, the calyx basally swollen, bladder-like and then elongate and slender, 36 (30–45) long and 9 (8–11) wide at the widest of the calyx, small minor duct visible.
Legs (Fig. 7e) – Thickened blunt macrosetae on tibia III, tarsus III and tibia IV, thickened knobbed macrosetae on genua I-III, genu and basitarsus IV. Measurements: SgeI 10 (9–11), SgeII 11 (9–13), SgeIII 20 (16–22), StiIII 15 (13–15), StIII 14 (13–16), SgeIV 29 (23–33), StiIV 18 (15–20), StIV 50 (45–58). Genua II and III both with seven setae. Chaetotactic formula of genu II: 2-2/0, 2/0-1; genu III: 1-2/1, 2/0-1.
Description of adult male (n = 9, Figs. 8 a-d)



Dorsum (Fig. 8a) – Dorsal shield similar to adult female, 238 (218–275) long and 154 (140–173) wide, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9) similar to adult female, 14 pairs of poroids visible, 19 pairs of dorsal setae (r3 and R1 on dorsal shield): j1 20 (18–23), j3 21 (18–23), j4 12 (10–14), j5 12 (11–13), j6 13 (12–15), J2 15 (13–16), J5 7 (7–8), z2 17 (15–19), z4 20 (18–22), z5 13 (13–15), Z1 16 (15–20), Z4 23 (21–25), Z5 40 (36–43), s4 25 (23–28), S2 21 (20–24), S4 16 (15–18), S5 12 (11–13), r3 16 (14–18), R1 14 (11–16). All setae thickened and smooth, except for Z5 slightly serrate.
Peritreme and peritremal plate (Fig. 8a) – Extending to level of j1; peritremal plate fused with dorsal shield at level between j3 and z2.
Venter (Fig. 8b) – Sternogenital shield smooth, except for edges that are very slightly striate, with five pairs of setae (st1-st5) and two pairs of poroids (iv1 and iv2). Distances st1-st1 45 (43–46), st2-st2 53 (50–56), st3-st3 56 (53–59), st1-st5 100 (94–103), st4-st4 46 (43–49), st5-st5 36 (34–38). Ventrianal shield 96 (88–108) long, 132 (123–140) wide at anterior corners and 63 (55–75) wide at level of para-anal setae. Ventrianal shield with three pairs of pre-anal setae (JV1, JV2 and ZV2), and a pair of large crateriform solenostome gv3, between JV2, 18 (15–20) apart. A pair of iv5 and four pairs of poroids ivo discernible. Unsclerotized cuticle arround ventrianal shield with a pair of setae (JV5). Seta JV5 pointed and smooth, but not thickened as in adult female, 20 (19–22) long.
Chelicerae (Fig. 8c) – Fixed digit 20 (16–21) long, with six or seven teeth discernible; and movable digit 19 (18–21) long, apparently edentate. Spermatodactyl shaft 18 (14–20) and branch 5 (4–6).
Legs (Fig. 8d) – All legs with at least one macroseta similar to adult female, except that in male only genu III has a macroseta, not tibia III. Measurements: SgeI 9 (8–10), SgeII 10 (9–12), SgeIII 14 (13–16), SgeIV 20 (19–23), StiIV 14 (13–15), StIV 36 (34–38). Chaetotactic formula of genua II and III similar to adult female.
Material examined. Fourty-four ♀♀, nine ♂♂ and two imm. collected during this study, fifteen ♀♀ and nine ♂♂ measured, 43 ♀♀, nine ♂♂ and two imm. as type material. MAYOTTE ISLAND (29 ♀♀ and 1 ♂): Coconi, Maison de l'Office National des Forêts (156 m aasl, 12°50′1″ S, 45°8′5″ E), 1 ♀ on Terminalia catappa L. (Combretaceae) and 1 ♀ on Cananga odorata L. (Annonaceae), 24/XI/2018; Combani, gîte du Mont-Combani (437 m aasl, 12°48′23″ S, 45°9′17″ E), 1 ♀ on Psidium guajava L. (Myrtaceae), 2 ♀♀ on Hydrangea aspera Buchanan-Hamilton ex D. Don (Hydrangeaceae) and 8 ♀♀ on Bidens pilosa L. (Asteraceae), 25/XI/2018; Coconi, Lycée Agricole (189 m aasl, 12°50′7″ S, 45°8′11″ E), 3 ♀♀ on Solanum melongena L. (Solanaceae), 2 ♀♀ on Ageratum conizoides L. (Asteraceae), 26/XI/2018; L'Abattoir, Dziani lake (23 m aasl, 12°46′14″ S, 45°17′18″ E), 11 ♀♀ and 1 ♂ on Ricinus communis L. (Euphorbiaceae), 27/XI/2018. MOHELI ISLAND (15 ♀♀, 8 ♂♂ and 2 imm.): Fomboni, University (25 m aasl, 12°17′3′′ S, 43°44′34′′ E), 2 ♀♀ on Zyzyphus mauritiana Lamarck (Malvaceae) and 12 ♀♀, 8 ♂♂ and 2 imm. on Ricinus communis L. (Euphorbiaceae), 3/XII/2018; Hoani, inside village (38 m aasl, 12°17′3″ S, 43°44′34″ E), 1 ♀ on Amaranthus viridis L. (Amaranthaceae), 3/XII/2018.
Type material. The holotype female, 43 paratype females, nine paratype males and two immatures are deposited in Institut Agro (MSA) – INRAE Acarology collection, Montpellier, France.
Etymology. The name ''baillodi'' refers to the family name of the researcher Dr Marc Baillod, who has worked during his career at the Station Fédérale de Recherche Agronomique de Changins in Switzerland (now called Agroscope) and has published many useful papers on plant inhabiting mites in agrosystems. He contributed towards the senior author's knowledge of the Phytoseiidae (taxonomy, biology, ecology, side effects of pesticides, etc.) more than 35 years ago. Marc Baillod was a real Master and deserves billions of billions of thanks! This new species is named in his honour.
Differential diagnosis and remarks. This species is very original by the set of characters described above. Lengths of most of the major setae are very similar to those obtained by Yoshida-Shaul and Chant (1991) for T. fragosoi Yoshida-Shaul & Chant and by Kreiter et al. (2002) for T. etiennei Kreiter & Ueckermann. However, the unique shape of the spermatheca not only distinguishes if from the latter two species, but also from all known species of the genus Typhlodromalus. The spermatodactyl also distinguishes it from that of T. spinosus Meyer and Rodrigues, allowing an easy distinction between the two species mentioned from this region.
Genus Ueckermannseius Chant & McMurtry
Ueckermannia Chant & McMurtry 2005a: 201. Preoccupied by Ueckermannia Kaźmierski, 1996 (Tydeidae).
Ueckermannseius Chant & McMurtry 2005b: 337, 2007: 115.
We describe here three new species groups within the genus Ueckermannseius and three new species belonging to the same species group.
Ueckermannseius gutierrezi Kreiter n. sp.
ZOOBANK: A56A4712-1C00-4F5B-84BE-2B9592180D24 ![]()
Classification. Ueckermannseius gutierezzi Kreiter n. sp. belongs to:
- the subfamily Amblyseiinae (absence of dorsolateral setae z3 and s6 and the caudoventral seta JV3),
- to the tribe Euseiini (sternal shield with median posterior projection, deutosternal groove < 5 µm in width, forward migration of pre-anal setae JV2 and ZV2),
- to the subtribe Typhlodromalina (chelicera of normal size and shape, with prominent teeth evenly distributed along fixed digit, peritreme usually extending to level of j1, deutosternal groove narrow, 4–7 µm width),
- to the genus Ueckermannseius (dorsal setae short/minute, shorter than distances between their bases, seta Z4 not as long as distance between its base and that of S4, dorsal shield smooth, except for anterolateral striation) (Chant and McMurtry 2007),
- to the new species-group havu Kreiter, with spermatheca with the atrium bulbous, the calyx basally swollen, bladder-like and then elongate and slender. This kind of spermatheca is shared by 13 African species of Ueckermannseius we proposed to include in the new species group havu: U. bundibugyoensis Moraes, Zannou & Oliveira, U. eastafricae Moraes, Zannou & Oliveira, U. havu (Pritchard & Baker), U. lugula El-Banhawy & Irungu, U. macrosetosus (van der Merwe), U. mangrovei El-Banhawy & Knapp, U. nesiotus (Ueckermann & Kreiter), U. neohavu Moraes, Zannou & Oliveira, U. parahavu Moraes, Zannou & Oliveira, U. quilicii (Ueckermann & Kreiter), U. sabatiae El-Banhawy & Knapp, U. saltus (Denmark & Matthysse) and U. ueckermanni Moraes, Zannou & Oliveira.
The two other new species groups proposed are:
- the species group ultimus Kreiter, with spermatheca elongate, tubular, flared distally with an atrium prominent, but small. This kind of spermatheca is shared by six African species of Ueckermannseius we proposed to include in the ultimus species-group: U. aequidens Blommers, U. bunyalae El-Banhawy and Knapp, U. kiminini El-Banhawy and Knapp, U. munsteriensis (van der Merwe), U. tenuiscutus McMurtry and Moraes and U. ultimus (Chant and Baker),
- the species group danhomensis Kreiter, with spermatheca with calyx short, funnel-shaped, with an atrium distinctly bulbous. This kind of spermatheca is shared by only two species of Ueckermannseius we proposed to include in the danhomensis species-group: U. danhomensis Moraes, Zannou and Oliveira and U. musoli El-Banhawy and Knapp.
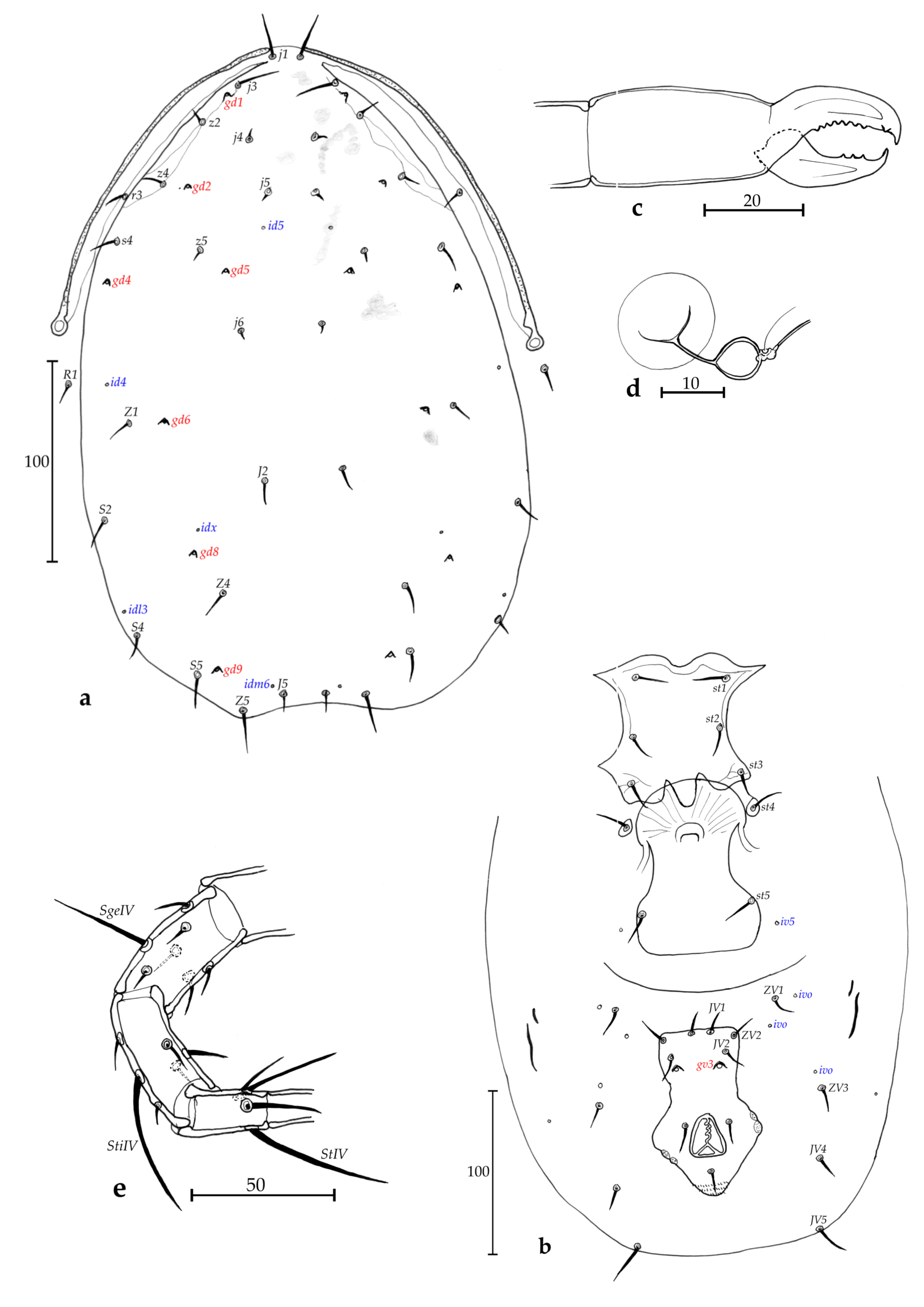
Description of adult female (n = 13, Figs. 9 a-e)



Dorsum (Fig. 9a) – Dorsal shield smooth with only few striae anterolaterally, 330 (318–353) long and 214 (170–240) wide at level of s4, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9), 12 pairs of poroids, 17 pairs of dorsal setae and two pairs of sub-lateral setae: j1 36 (34–40), j3 24 (22–25), j4 10 (8–10), j5 10 (8–10), j6 11 (9–13), J2 13 (10–14), J5 8 (6–9), z2 14 (13–15), z4 15 (13–16), z5 10 (9–11), Z1 12 (11–13), Z4 14 (13–16), Z5 42 (35–47), s4 23 (20–25), S2 14 (13–15), S4 13 (12–15), S5 14 (12–16), r3 18 (15–20), R1 13 (10–16). All setae smooth.
Peritreme and peritremal plate (Fig. 9a) – Extending to level between j3 and z2; peritremal plate fused with dorsal shield at a level between j3 and z2.
Venter (Fig. 9b) – Sternal shield smooth with few anterolateral striae, with three pairs of setae (st1-st3) and two pairs of poroids (iv1 and iv2); a pair of setae (st4) and a pair of pores (iv3) on a small metasternal shield; posterior margin of the sternal shield convex, with a central projection. Distances st1-st1 58 (55–60), st2-st2 66 (62–70), st3-st3 78 (75–84), st1-st3 60 (54–64), st4-st4 85 (75–90). Genital shield smooth, 133 (125–143) long, width at level of st5 82 (78–93), width at level of posterior corners 95 (85–105), distance st5-st5 76 (70–81). One pair of metapodal plate 25 (19–28) long and 2 (1–4) wide. Ventrianal shield 100 (88–118) long, 61 (55–70) wide at level of anterior corners (ZV2), and 74 (65–80) wide at level of para-anal setae. Ventrianal shield smooth, with three pairs of pre-anal setae (JV1, JV2 and ZV2), and a pair of evolved and crateriform gv3, 34 (30–38) apart. Unsclerotized cuticle around ventrianal shield with four pairs of setae (JV4, JV5, ZV1 and ZV3), and four pairs of round to oblong poroids (ivo). Seta JV5 smooth, 34 (28–40) long.
Chelicerae (Fig. 9c) – Fixed digit 24 (23–26) long, with five teeth visible; and movable digit 26 (25–28) long, with one tooth. Pilus dentilis not visible.
Spermatheca (Fig. 9d) – With the atrium c-shaped, calyx basally swollen, bladder-like and then elongate and slender 34 (30–37) long and 8 (6–8) wide in the wider part, a small atrium adjacent to the calyx, small minor duct visible.
Legs (Fig. 9e) – Pointed whip-like macrosetae on genua I-III, tibia III, and basitarsus, tibia and genu IV. Measurements: SgeI 21 (18–25), SgeII 24 (22–25), SgeIII 34 (30–43), StiIII 28 (28–30), SgeIV 51 (43–55), StiIV 43 (38–47), StIV 76 (70–80). Genua II and III both with seven setae. Chaetotactic formula of genua II: 2-2/0, 2/0-1; genu III: 1-2/1, 2/0-1.
Description of adult male (n = 1, Figs. 10 a-c)



Dorsum (Fig. 10a) – Dorsal shield similar to adult female, 248 long and 175 wide, with seven solenostome well visible (gd1, gd2, gd4, gd5, gd6, gd8 and gd9), with only five poroids visible, 19 pairs of dorsal setae (r3 and R1 on dorsal shield): j1 25, j3 25, j4 8, j5 8, j6 8, J2 10, J5 5, z2 12, z4 13, z5 8, Z1 10, Z4 10, Z5 38, s4 20, S2 13, S4 12, S5 12, r3 17, R1 10. All setae smooth, except for Z5 lightly serrate.
Peritreme and peritremal plate (Fig. 10a) – Extending to level between j1 and j3; peritremal plate fused with dorsal shield at level between j3 and z2.
Venter (Fig. 10b) – Sternogenital shield smooth, except for few striae posteriolaterally, with five pairs of setae (st1-st5) and two pairs of poroids (iv1 and iv2). Distances st1-st1 47, st2-st2 55, st3-st3 53, st1-st5 103, st4-st4 48, st5-st5 39. Ventrianal shield 100 long, 145 wide at level of anterior corners, and 83 wide at level of para-anal setae. Ventrianal shield reticulate in the anterior part, above pores gv3, with three pairs of pre-anal setae (JV1, JV2, and ZV2), and a pair of evolved and crateriform gv3 between JV2, 23 apart. A pair of poroids iv5 and four pairs of poroids ivo on the ventrianal shield. Unsclerotized cuticle around ventrianal shield with a pair of setae (JV5). Seta JV5 smooth, 28 long.
Chelicerae – Fixed digit 20 long, no discernible teeth; and movable digit 20 long, with no discernible teeth. Spermatodactyl shaft renders measurement and illustration impossible.
Legs (Fig. 10c) – Pointed whip-like macrosetae on genua II and III and basitarsus, tibia and genu IV. Measurements: SgeII 13, SgeIII 20, SgeIV 35, StiIV 30, StIV 50. Chaetotactic formula of genua II and III similar to adult female.
Material examined. Thirteen ♀♀ and one ♂ collected during this study, measured and type material. ANJOUAN ISLAND: Pomoni, exit of the village (29 m aasl, 12°17′01′′ S, 44°34′37′′ E), 1 ♀ on Gliricidia sepium (Jacquin), Kunth ex Walpers (Fabaceae) and 1 ♀ Hibiscus tiliaceus L. (Malvaceae), 30/XI/2018.MOHELI ISLAND: Bandar-Es-Salam, Les Abous Inn (23 m aasl, 12°17′37″ S, 43°45′27″ E), 11 ♀♀ and 1 ♂ on Carica papaya L. (Caricaceae), 2/XII/2018.
Type material. The holotype ♀, twelve paratype ♀♀ and one paratype ♂ are deposited in Institut Agro (MSA) – INRAE Acarology collection, Montpellier, France.
Etymology. The name ''gutierrezi'' refers to the family name of the researcher Dr Jean Gutierrez, who has worked during his career at ORSTOM (= IRD for now) and have published many papers on plant inhabiting mites, mainly tetranychid mites, from Indian Ocean among many other sites. He has helped the senior author in many aspects at the beginning of his career, especially with exciting and stimulating scientific discussions on mites and many other subjects. This species is named in his honour.
Differential diagnosis and remarks. This species closely resembles U. neohavu concerning length of setae on dorsal shield. However, it differs from the latter in having: setae j4-j6, R1, s4, z2, z4 and Z5 shorter with Z5 serrate, ventrianal shield and calyx of spermatheca shorter, cheliceral digits also shorter with less teeth (5/1 in the new species compared to 11/4 in U. neohavu) (Table 3). It is also close to U. macrosetosus, but differs in shorter dorsal setae especially s4, z2, z4, Z5 and all macrosetae, except for StIV longer, by fewer teeth on both digits of chelicera and the shape of macrosetae that are all pointed and not knobbed as in U. macrosetosus.
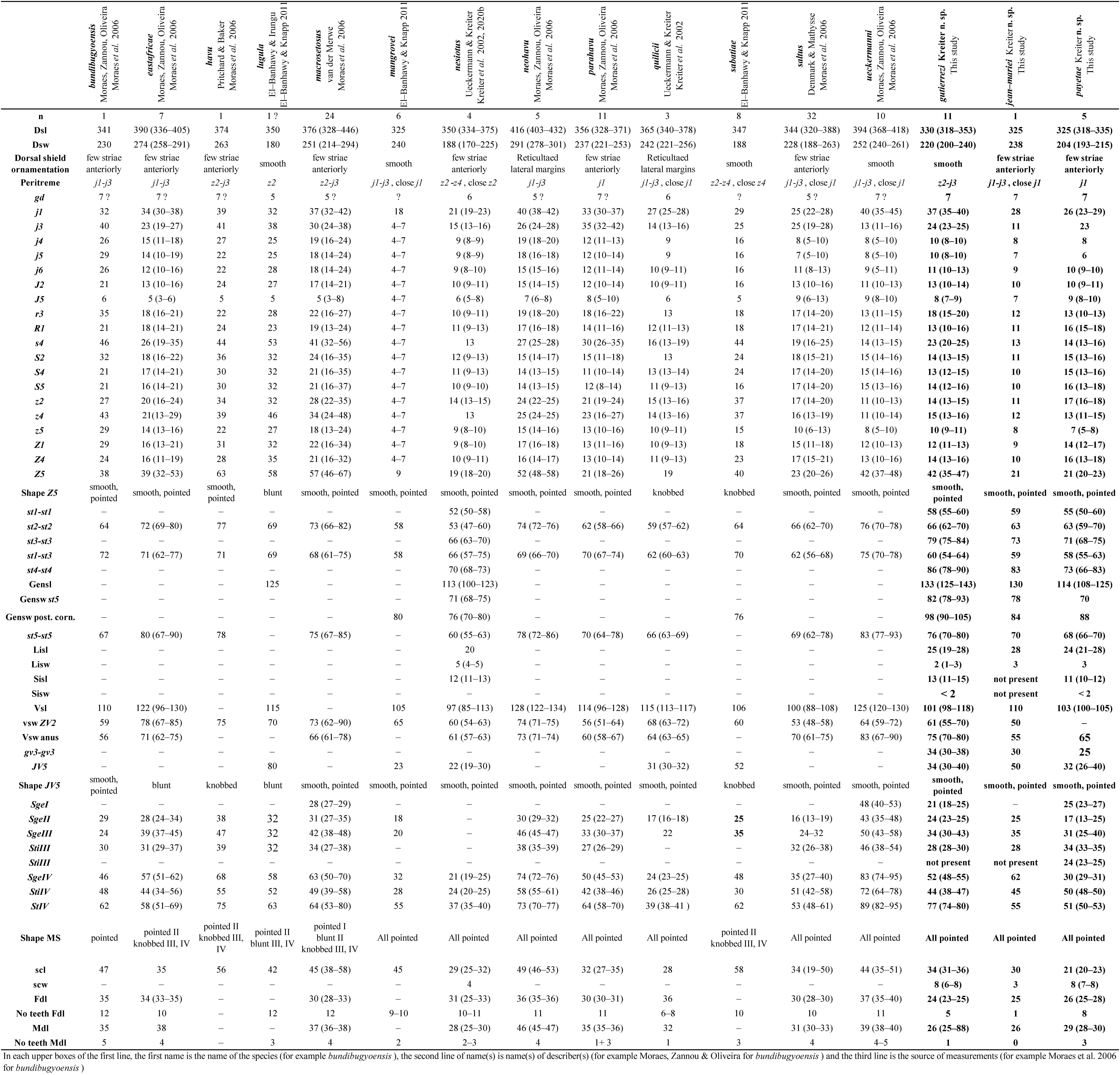


It also closely resembles U. eastafricae Moraes, Zannou & Oliveira, but differs by shorter setae z2 and z4 and a longer Z5, a longer StIV, by the fewer teeth on both digits of chelicera and the shape of macrosetae that are all pointed in the new species and not knobbed as in U. eastafricae, the shape of macrosetae being considered as a diagnostic character in all previous descriptions.
This species was identified as U. eastafricae in two previous papers (Kreiter et al. 2021a, c) for fauna of Anjouan and Mohéli Islands, but here it is considered a new species in the new havu species group Kreiter (Table 3) and named U. gutierezzi Kreiter n. sp.
Ueckermannseius jean-mariei Kreiter n. sp.
ZOOBANK: 4506C84E-D900-46A5-AAC3-1D9A9288EA06 ![]()
Classification. Ueckermannseius jean-mariei Kreiter n. sp. belongs to:
- the subfamily Amblyseiinae (absence of dorsolateral setae z3 and s6 and the caudoventral seta JV3),
- to the tribe Euseiini (sternal shield with median posterior projection, deutosternal groove < 5 µm in width, forward migration of pre-anal setae JV2 and ZV2),
- to the subtribe Typhlodromalina (chelicera of normal size and shape, with prominent teeth evenly distributed along fixed digit, peritreme usually extending to level of j1, deutosternal groove narrow, 4–7 µm width),
- to the genus Ueckermannseius (dorsal setae short/minute, shorter than distances between their bases, seta Z4 not as long as distance between its base and that of S4, dorsal shield smooth, except for anterolateral striation) (Chant and McMurtry 2007),
- Like the two previous species and for the same reasons, to the species-group havu Kreiter new species group (see text for U. gutierrezi Kreiter n. sp.).
The following list of characters of this new species is very different from all other species of the genus and the species group. So, despite the fact that we collected a single specimen, we still consider to describe this very original specimen as belonging to a very original new species.
Description of adult female (n = 1, Figs. 11 a-e)


Dorsum (Fig. 11a) – Dorsal shield smooth with only very few anterior striae, 325 long and 238 wide at level of waist, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9), eight pairs of poroids visible, 17 pairs of dorsal setae and two pairs of sub-lateral setae; all setae subequal in length (7–13), except for j1 which is the longest: j1 28, j3 11, j4 8, j5 7, j6 9, J2 10, J5 7, z2 11, z4 12, z5 8, Z1 9, Z4 10, Z5 21, s4 13, S2 11, S4 10, S5 10, r3 12, R1 11. All setae smooth.
Peritreme and peritremal plate (Fig. 11a) – Extending to level between j1 and j3, but much closer to j1; peritremal plate fused with dorsal shield at level of j1.
Venter (Fig. 11b) – Sternal shield smooth with few lateral striae, with three pairs of setae (st1-st3) and two pairs of poroids (iv1 and iv2); a pair of seta (st4) and a pair of pores (iv3) on a small metasternal plate; posterior margin of the sternal shield with a central posterior projection. Distances st1-st1 59, st2-st2 63, st3-st3 73, st1-st3 59, st4-st4 83. Genital shield smooth, 130 long, width at level of st5 78, width at level of posterior corners 84, distance st5-st5 70. A pair of metapodal plates, 28 long and 3 wide. Ventrianal shield 110 long, 50 wide at level of anterior corners (JV2), and 55 wide at level of para-anal setae. Ventrianal shield smooth, with three pairs of pre-anal setae (JV1, JV2 and ZV2), and a pair of evolved and crateriform gv3 posteromesad JV2, 30 apart. Unsclerotized cuticle around ventrianal shield with four pairs of setae (JV4, JV5, ZV1 and ZV3) and four pairs of round to oblong poroids not well discernible. Seta JV5 smooth, 50 long.
Chelicerae (Fig. 11c) – Fixed digit 25 long, with one tooth not well visible because digit not well positioned; and movable digit 26 long, edentate, but digit also not well positioned. Pilus dentilis not visible.
Spermatheca (Fig. 11d) – Like the two other new species of Ueckermannseius, spermatheca is with the atrium bulbous, the calyx basally swollen, bladder-like and then elongate and slender, this shape of the spermatheca is shared by ten African species of Ueckermannseius of the species group havu Kreiter new species group. Spermatheca 30 long and 3 wide at the widest base of calyx.
Legs (Fig. 11e) – Pointed whip-like macrosetae on genua II and III, on tibia III, basitarsus, tibia and genu IV. Measurements: SgeII 25, SgeIII 35, StiIII 28, SgeIV 62, StiIV 45, StIV 55. Genua II and III both with seven setae. Chaetotactic formula of genu II: 1-2/1, 2/0-1; genu III: 1-2/1, 2/0-1.
Male. Unknown.
Material examined. One single female collected during this study, measured and type material. GRANDE COMORE ISLAND: Ivembeni, Banda Samlini (791 m aasl, 11°29′22″ S, 43°19′36″ E), 1 ♀ on Rubus rosifolius Smith (Rosaceae), 7/XII/2018.
Type material. The holotype female on one slide together with the holotype female of Amblyseius erici Kreiter n. sp., two females of Typhlodromalus spinosus (Meyer & Rodrigues) and two females of Amblyseius herbicolus (Chant) (see above) are deposited in Institut Agro (Montpellier SupAgro) – INRA Acarology collection, Montpellier, France.
Etymology. The name ''jean-mariei'' refers to the first name of the eldest brother of the senior author after him, Jean-Marie Kreiter. The species is named in his honour.
Differential diagnosis and remarks. This species is very similar to U. quilicii concerning length of setae (Table 3). However, comparison with the available characters listed in Table (3) shows that the new species has: macrosetae all pointed (against knobbed in U. quilicii), a longer seta JV5, longer macrosetae on leg IV, both digits of chelicera longer and with less teeth (1/0 in the new species compared to 6–8/1 in U. quilicii). Other species of the species group havu Kreiter new species group are very different in many aspects concerning measurements and shape of characters (Table 3).
Ueckermannseius payetae Kreiter n. sp.
ZOOBANK: 467E989B-C03A-4997-8E25-823EECB3A126 ![]()
Classification. Ueckermannseius payetae Kreiter n. sp. belongs to:
- the subfamily Amblyseiinae (absence of dorsolateral setae z3 and s6 and the caudoventral seta JV3),
- to the tribe Euseiini (sternal shield with median posterior projection, deutosternal groove < 5 µm in width, forward migration of pre-anal JV2 and ZV2),
- to the subtribe Typhlodromalina (chelicera of normal size and shape, with prominent teeth evenly distributed along fixed digit, peritreme usually extending to level of j1, deutosternal groove narrow, 4–7 µm width),
- to the genus Ueckermannseius (dorsal setae short/minute, shorter than distances between their bases, seta Z4 not as long as distance between its base and that of S4, dorsal shield smooth, except for anterolateral striation) (Chant and McMurtry 2007),
- it also belongs to the species-group havu Kreiter new species group for the same reasons as the previous species (see text for previous species).
Description of adult female (n = 5, Figs. 12 a-e)


Dorsum (Fig. 12a) – Dorsal shield smooth with only few striae in anterior part, 325 (318–335) long and 204 (193–215) wide at level of s4, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9), five visible poroids, 17 pairs of dorsal setae and two pairs of sub-lateral setae on the membrane: j1 26 (23–29), j3 23, j4 8, j5 6, j6 10 (9–10), J2 10 (9–11), J5 9 (8–10), z2 17 (16–18), z4 13 (11–15), z5 7 (5–8), Z1 14 (12–17), Z4 16 (13–18), Z5 21 (20–23), s4 14 (13–16), S2 15 (13–16), S4 15 (13–16), S5 16 (13–18), r3 13 (10–13), R1 16 (15–18). All setae smooth.
Peritreme and peritremal plate (Fig. 12a) – Extending to level of j1; peritremal plate fused with dorsal shield at level between j1 and j3.
Venter (Fig. 12b) – Sternal shield smooth with few anterolateral striae, with a posterior projection well visible, with three pairs of setae (st1-st3) and no visible pairs of poroids; a pair of setae (st4) on a pair of metasternal plate with no visible pores (iv3). Distances st1-st1 55 (50–60), st2-st2 63 (59–70), st3-st3 71 (68–75), st1-st3 58 (55–63), st4-st4 73 (66–83). Genital shield smooth, 114 (108–125) long, width at level of st5 70, width at level of the posterior corners 88, distance st5-st5 68 (66–70). Two pairs of metapodal plates 24 (21–28) long and 3 wide for the primary and 11 (10–12) long and 1 wide for the secondary plate. Ventrianal shield 103 (100–105) long, level at of anterior corners (ZV2) not visible because of eggs present in five female specimens collected and so, not measurable, and 65 wide at level of para-anal setae. Ventrianal shield smooth with three pairs of pre-anal setae (JV1, JV2 and ZV2), and a pair of crateriform gv3, 25 apart. Unsclerotized cuticle around ventrianal shield with four pairs of setae (JV4, JV5, ZV1 and ZV3) and five pairs of round to oblong poroids (ivo). Seta JV5 smooth and sharp-tipped, 32 (26–40) long.
Chelicerae (Fig. 12c) – Fixed digit 26 (25–28) long, with eight teeth; and movable digit 29 (28–30) long, with apparently three teeth. Pilus dentilis not visible.
Spermatheca (Fig. 12d) – With the atrium bulbous, the oblong calyx basally swollen, bladder-like and then elongate and slender, 21 (20–23) long and 8 (7–8) wide, atrium adjacent to calyx, small minor and slender major ducts visible.
Legs (Fig. 12e) – Pointed whip-like macrosetae on genua I-IV, tibia and basitarsus III and IV. Measurements: SgeI 25 (23–27), SgeII 17 (13–25), SgeIII 31 (25–40), StiIII 34 (33–35), StIII 24 (23–25), SgeIV 30 (29–31), StiIV 50 (48–50), StIV 51 (50–53). Genua II and III with seven and six setae, respectively. Chaetotactic formula of genu II: 2-2/0, 2/0-1; genu III: 1-2/0, 2/0-1.
Description of adult male (n = 2, Figs. 13 a-d)



Dorsum (Fig. 13a) – Dorsal shield smooth, 228–248 long and 155–160 wide at level of s4, with no solenostome visible, but probably with the same number as in adult female, no visible poroids either, 19 pairs of dorsal setae (r3 and R1 on dorsal shield): j1 23–25, j3 18, j4 4–5, j5 5, j6 6–8, J2 5–8, J5 5–7, z2 6–9, z4 7–8, z5 5–6, Z1 8–10, Z4 8–10, Z5 25–30, s4 10, S2 8–10, S4 6–9, S5 5–8, r3 10, R1 8. All setae smooth.
Peritreme and peritremal plate (Fig. 13a) – Extending almost to level of j1; peritremal plate fused with dorsal shield at level between j1 and j3.
Venter (Fig. 13b) – Sternogenital shield smooth with five pairs of setae (st1-st5) and two pairs of poroids (iv1 and iv2); distances st1-st1 45–49, st2-st2 53, st3-st3 53, st1-st5 100–125, st4-st4 41–45, st5-st5 34–38. Ventrianal shield 100 long, 120–125 wide at level of anterior corners, and 50–58 wide at level of para-anal setae. Ventrianal shield reticulate anteriorly, anteriad pores gv3, suddenly narrows at level above anus, with three pairs of pre-anal setae (JV1, JV2 and ZV2) and a pair of crateriform gv3 just under the line JV2-ZV2, 23 apart. Two pairs of poroids (ivo). Unsclerotized cuticle around ventrianal shield with a pair of setae (JV5). Seta JV5 smooth and short, 17–20 long.
Chelicerae (Fig. 13c) – Fixed digit 23 long, with eight teeth; and movable digit 23–25 long, with two teeth. Spermatodactyl shaft 21–22.
Legs (Fig. 13d) – Pointed whip-like macrosetae on genua I-III, tibia and basitarsus III, basitarsus, tibia and genu IV. Measurements: SgI 20, SgeII 20, SgeIII 24, StiIII 28, StIII 18–20, SgeIV 38–40, StiIV 40, StIV 40. Chaetotactic formula of genua II and III similar to adult female.
Material examined. Five ♀♀ and two ♂♂ collected during this study, measured and type material. MAYOTTE ISLAND: Coconi, Maison de l'Office National des Forêts (156 m aasl, 12°50′1″ S, 45°8′5″ E), 1 ♀ and 1 ♂ on Carica papaya L. (Caricaceae), 24/XI/2018; Coconi, Lycée Agricole (189 m aasl, 12°50′7″ S, 45°8′11″ E), 2 ♀♀ on Carica papaya L. (Caricaceae) and 2 ♀♀ and 1 ♂ on Trema orientalis (L.) Blume (Cannabaceae), 26/XI/2018.
Type material. The holotype ♀, four paratype ♀♀ and two paratype ♂♂ are deposited in Institut Agro (MSA) – INRAE Acarology collection, Montpellier, France.
Etymology. The name ''payetae'' refers to the family name of the researcher Rose-My Payet, co-author of this paper, who has worked during her career at CIRAD. She has helped the senior author in many aspects concerning fauna of Phytoseiidae of Indian Ocean Island. This species is named in her honour.
Differential diagnosis and remarks. This species is very similar to U. saltus concerning length of setae (Table 3). The description of U. saltus is however quite incomplete. But comparison with available characters listed in Table (3) allows that the new species can be distinguished by having: peritreme ending at level of j1 (and not between j1 and j3 as illustrated by Mathysse and Denmark in 1981, or very close, but anteriorly to j1 as illustrated by Moraes et al. in 2006 for U. saltus), setae r3 and s4 slightly shorter, occurrence of clear macrosetae on genu I and tibia III, a shorter calyx of spermatheca and both digits of chelicera with less teeth (8/3 in the new species compared to 10/4 in U. saltus). However, the other species of the species group havu Kreiter new species group can be clearly distinguished (Table 3).
Subfamily Typhlodrominae Wainstein
Typhlodromini Wainstein 1962: 26 and Typhlodrominae Chant & McMurtry 1994: 235.
Tribe Typhlodromini Wainstein
Typhlodromini Wainstein 1962: 26.
Genus Typhlodromus Scheuten
Typhlodromus Scheuten 1857: 111.
Subgenus Anthoseius De Leon
Typhlodromus (Anthoseius) De Leon 1959: 258; van der Merwe 1968: 20; Karg 1982: 194; Chant & McMurtry 1994: 250, 2007: 149.
Typhlodromus (Anthoseius) grewiae Zannou, Moraes & Oliveira
Typhlodromus (Anthoseius) grewiae Zannou, Moraes & Oliveira in Ueckermann et al. 2008: 48.
Diagnosis. The male of this species has five solenostomes (gd2, gd4, gd6, gd8 and gd9) similar to adult female, all dorsal setae lanceolate, strongly serrate and inserted on tubercules, except for J5 smooth, setiform and sharp-tipped, peritreme extending to level of j1, three setae on the ventrianal shield with small punctiform pre-anal solenostomes, three thick macrosetae strongly knobbed. This is a unique combination of characters which make specimens of this species very different from all other species within the genus Typhlodromus, subgenus Anthoseius.
Description of adult male (n = 4, Figs. 14 a-d)


Dorsum (Fig. 14a) – Dorsal shield strongly ornamented, 231 (220–240) long and 136 (130–150) wide, with five solenostome well visible (gd2, gd4, gd6, gd8 and gd9), only two pairs of poroids visible (probably because of the strong ornamentation), 20 pairs of dorsal setae (r3 and R1 on dorsal shield): j1 13 (12–14), j3 13 (11–15), j4 12 (11–13), j5 13 (11–14), j6 15 (14–15), J2 17 (15–20), J5 8 (8–9), z2 12 (10–12), z3 12 (9–13), z4 15 (13–18), z5 14 (13–15), Z4 21 (19–23), Z5 26 (24–29), s4 14 (14–15), s6 17 (15–18), S2 18 (18–19), S4 19 (18–20), S5 16 (14–18), r3 14 (13–15), R1 12 (12–13). All setae lanceolate, plumose, strongly serrate and inserted on tubercules with presence of these tubercules starting back to a line constituted of setae r3, s4 and z5 until the posterior part of the dorsum. Seta J5 is the only seta smooth, setiform and sharp-tipped.
Peritreme and peritremal plate (Fig. 14a) – Extending to level of j1; peritremal plate fused with dorsal shield at level of j3.
Venter (Fig. 14b) – Sternogenital shield smooth with few anterior and posterior striae, with five pairs of setae (st1-st5) and three pairs of poroids (iv1-iv3); distances st1-st1 37 (35–38), st2-st2 52 (50–54), st3-st3 50 (49–50), st1-st5 95 (93–98), st4-st4 36 (34–38), st5-st5 28 (25–30). Ventrianal shield 88 (83–93) long, 111 (105–115) wide at level of anterior corners, and 56 (50–63) wide at level of para-anal setae. Ventrianal shield with few striae, with three pairs of pre-anal setae (JV1, JV2 and ZV2), and a pair of small punctiform gv3 mesad JV2, 19 (18–20) apart. Unsclerotized cuticle around ventrianal shield with a pair of setae (JV5). Seta JV5 lanceolate and strongly serrate, 15 long.
Chelicera (Fig. 14c) – Fixed digit 16 (15–17) long, with no teeth visible; and movable digit 18 (17–19) long, with one tooth visible. Spermatodactyl shaft straight, shaft 18 (17–18) long, branch 3.
Legs (Fig. 14d) – One macroseta only on leg IV: St IV 15 (13–15), blunt and knobbed. Genua II and III both with seven setae. Chaetotactic formula of genu II: 2-2/0, 2/0-1; genu III: 1-2/1, 2/0-1.
Specimens examined. Four ♂♂ collected during this study, measured and deposited as complementary voucher material. MAYOTTE ISLAND: Combani, gîte du Mont-Combani (437 m aasl, 12°48′23″ S, 45°9′17″ E), 1 ♂ on Cananga odorata (Lamark) Hooker & Thomson (Annonaceae) or Ylang-Ylang, 25/XI/2018. MOHELI ISLAND: Bangoma, top of the village (42 m aasl, 12°17′15″ S, 43°43′40″ E), 3 ♂♂ (and 1 ♀ on the same slide) on Dendrocnide moroides (Weddel) Chew (Urticaceae), 4/XII/2018.
Voucher material. Four males on two slides (one with one female) are deposited in Institut Agro (Montpellier SupAgro) – INRAE Acarology collection, Montpellier, France.
Remarks. Characters of males are very similar to that of females, except of course for length of setae and few other characters. Ventrianal shield of the male is slightly striate and the ventrianal shield of the female is entirely smooth. Morphological characteristics of this species are so unique within the genus Typhlodromus, subgenus Anthoseius that is possible to identify the species based on a single male alone.
Typhlodromus (Anthoseius) hartlandrowei Evans
Typhlodromus (Typhlodromus) hartlandrowei Evans 1958: 580; Chant 1959: 60.
Clavidromus hartlandrowei, Muma 1961: 296.
Typhlodromus (Neoseiulus) hartlandrowei, Pritchard & Baker 1962: 222.
Typhlodromus (Anthoseius) hartlandrowei, Moraes et al. 2004b: 328; Chant & McMurtry 2007: 155; Ueckermann et al. 2008: 50.
Diagnosis. The male of this species has four solenostomes (gd2, gd6, gd8 and gd9), all dorsal setae serrate and knobbed, except for j1, z2, S5 and r3 sharp-tipped, J5 smooth and sharp-tipped similar to adult female, peritreme extending to level of z2, three setae on the ventrianal shield with small punctiform pre-anal solenostomes, three macrosetae strongly knobbed. This is a unique combination of characters, which make specimens of this species very different from all other species within the genus Typhlodromus, subgenus Anthoseius.
Description of adult male (n = 1, Figs. 15 a-d)



Dorsum (Fig. 15a) – Dorsal shield slightly striate anteriorly and behind setae S2-J2 line, 225 long and 150 wide, with four solenostomes well visible (gd2, gd6, gd8 and gd9), with eight visible poroids, 20 pairs of dorsal setae (r3 and R1 on dorsal shield): j1 21, j3 34, j4 23, j5 22, j6 28, J2 30, J5 9, z2 35, z3 38, z4 42, z5 23, Z4 40, Z5 50, s4 45, s6 45, S2 45, S4 45, S5 18, r3 30, R1 33. All setae serrate and knobbed, except for j1, z2, S5 and r3 sharp-tipped, seta J5 smooth and sharp-tipped similar to adult female.
Peritreme and peritremal plate (Fig. 15a) – Extending to level of z2; peritremal plate fused with dorsal shield at level of z2.
Venter (Fig. 15b) – Sternogenital shield reticulate, except for smooth part between setae st1-st2, with five pairs of setae (st1-st5) and three pairs of poroids (iv1-iv3); distances between st1-st1 40, st2-st2 46, st3-st3 48, st1-st5 95, st4-st4 41, st5-st5 30. Ventrianal shield 95 long, 145 wide at level of anterior corners, and 43 wide at level of para-anal setae. Ventrianal shield reticultae, with three pairs of pre-anal setae (JV1, JV2 and ZV2) and a pair of small punctiform gv3 mesad JV2, 20 apart, two pairs of poroids (iv5 and ivo). Unsclerotized cuticle around ventrianal shield with a pair of setae (JV5). Seta JV5 smooth and knobbed, 48 long.
Chelicerae (Fig. 15c) – Fixed digit 20 long, with no teeth visible; and movable digit 20 long, with no teeth visible. Spermatodactyl shaft straight, shaft 15 long, branch with hook-shape 4.
Legs (Fig. 15d) – Three macrosetae only on leg IV all strongly knobbed. Measurements: Sge IV 20, Sti IV 15, St IV 36. Genua II and III both with seven setae. Chaetotactic formula of genu II: 2-2/0, 2/0-1; genu III: 1-2/1, 2/0-1.
Specimens examined. One single ♂ collected during this study, measured and deposited as complementary voucher material. ANJOUAN ISLAND: Pomoni, exit of the village (29 m aasl, 12°17′01″ S, 44°34′37″ E), 1 ♀ and 1 ♂ on Piper nigrum L. (Piperaceae), 30/XI/2018.
Voucher material. One male on one slide is deposited in Institut Agro (Montpellier SupAgro) – INRAE Acarology collection, Montpellier, France.
Remarks. Characters of males are very similar to that of females, except of course for length of setae and few other characters. Ventrianal shield of the male is reticulate as that of female, the sternogenital shield is almost reticulate, of normal size, macroseta StIV of leg IV in the male is the longer followed by SgeIV and StiIV as in adult female, seta JV5 smooth and sharp-tipped as in adult female. The only difference concerns dorsal setae: setae j1, z2, S5 and r3 are sharp-tipped in adult female, while also j3, j5, j6, z3 and z4 in the male.
Typhlodromus (Anthoseius) lobatus Zannou, Moraes & Oliveira
Typhlodromus (Anthoseius) lobatus Zannou, Moraes & Oliveira in Ueckermann et al. 2008: 59.
Diagnosis. The male of this species has four solenostomes (gd2, gd4, gd6 and gd9), all dorsal setae sub-equal in length, smooth, except for Z4 and Z5 serrate and knobbed, peritreme extending to level between j3 and z2, four setae on ventrianal shield, one macroseta on basitarsus of leg IV, knobbed. This is a unique combination of characters that makes specimens of this species very different from all other species within the genus Typhlodromus, subgenus Anthoseius.
Description of adult male (n = 2, Figs. 16 a-d)



Dorsum (Fig. 16a) – Dorsal shield with some reticulations close to edges of dorsum from seta j3 to anteriad of R1 and less marked at level of setae S2-S4, 200–203 long and 105–125 wide, with only four pairs of visible solenostomes (gd2, gd4, gd6 and gd9), five pairs of poroids visible, 20 pairs of dorsal setae (r3 and R1 on dorsal shield): j1 13–14, j3 23, j4 17, j5 17, j6 20, J2 23, J5 9–10, z2 14–15, z3 20, z4 23–25, z5 19, Z4 23–24, Z5 25, s4 23–24, s6 23–24, S2 25, S4 20–22, S5 18–20, r3 20, R1 18. All setae smooth and sharp-tipped, except for Z4 and Z5 serrate and knobbed.
Peritreme and peritremal plate (Fig. 16a) – Extending to level between j3 and z2, much closer to z2; peritremal plate fused with dorsal shield at level between z4 and s4.
Venter (Fig. 16b) – Sternogenital shield smooth with few lateral striae, with five pairs of setae (st1-st5) and three pairs of poroids (iv1-iv3). Distances st1-st1 32–38, st2-st2 38–39, st3-st3 38–40, st1-st5 90–91, st4-st4 31–33, st5-st5 28–30. Ventrianal shield 84–88 long, 80–100 wide at anterior corners and 40–50 wide at level of para-anal setae. Ventrianal shield reticulate with four pairs of pre-anal setae (JV1-JV3 and ZV2) and no solenostome; a pair of iv5 and two pairs of ivo discernible. Unsclerotized cuticle arround ventrianal shield with a pair of setae (JV5). Seta JV5 smooth, 16–18 long.
Chelicerae (Fig. 16c). Fixed digit 15 long, with three teeth discernible; and movable digit 16 long, with one tooth discernible. Spermatodactyl shaft 13–14 and branch 7.
Legs (Fig. 16d). Legs IV with one knobbed macroseta similar to adult female on basitarsus IV: StIV 19–20. Chaetotactic formula of genua II and III similar to adult female: genu II 2-2/0, 2/0-1; genu III 1-2/1, 2/0-1.
Specimens examined. Two ♂♂ collected during this study, measured and deposited as complementary voucher material. MAURITIUS ISLAND: Morne-Brabant (249 m aasl, 20°22′05′′ S, 57°29′31′′ E), 1 ♀ and 1 ♂ on Chromolaena odorata (L.) R.M. King and H. Robinson (Asteraceae), 5/XI/2018. RODRIGUES ISLAND: Mont Lubin (346 m aasl, 19°42′21′′ S, 63°26′40′′ E), 1 ♂ on Urena lobata L. (Malvaceae), 15/XI/2018.
Voucher material. Two ♂♂ on two slides are deposited in Institut Agro (Montpellier SupAgro) – INRAE Acarology collection, Montpellier, France.
Remarks. Characters of males are very similar to that of females, except of course for length of setae and other characters. Ventrianal shield of the male is reticulate, while that of female not. All other characters are similar to adult female, and the male of this species is very similar to males of other species, making difficult the identification of the species without collection of males and females together.
Conclusion
Six new species to science and six unknown males have been collected and described in this paper along with a new record, namely: Paragigagnathus philippei Kreiter n. sp., Amblyseius erici Kreiter n. sp., Typhlodromips culmulus new record, unknown males of three species of Amblyseius: A. duplicisetus, A. haleakalus and A. parasundi, Typhlodromalus baillodi Kreiter n. sp., Ueckermannseius gutierrezi Kreiter n. sp., Ueckermannseius jean-mariei Kreiter n. sp., Ueckermannseius payetae Kreiter n. sp., unknown males of three species of Typhlodromus (Anthoseius): T. (A.) grewiae, T. (A.) hartlandrowei and T. (A.) lobatus. These species have to be added to the species list of Archipelagos, Mascareignes and Comoros. A catalogue and a key for all species of both Archipelagos will be published in a following paper.
Acknowledgements
Firstly, we wish to thank the Institut Agro - Montpellier SupAgro for funding the senior author's research, travels and accommodations in Mauritius: UMR CBGP (Internal call for proposals 2018). Many thanks are also to Mrs Marie Anne Edouard for accomodation and her son for helping the senior author with transport. Grateful thanks are to Mrs Claudia Baider, responsible for Mauritius's Herbarium, who has identified some plants and supplied advices on Island locations and Mauritius biodiversity. Thanks are to National Authorities of Mauritius for the signature of a Memorandum of agreement for the supply of biological material by Government of Mauritius and a Phytosanitary certificate. Thanks also are to Le Vélo Vert Association, and especially Mrs Géraldine d'Unienville for e-mail exchanges and advices. Thanks also are to the I-SITE Montpellier Université d'Excellence (MUSE) for the international mobility support to the third author for the current work (Explore #2, the MUSE International Mobility program, 2019). Grateful thanks are to UR Hortsys and to the head, Dr Fabrice Le Bellec, who have allowed the second author Rose-My Payet to join the senior author on collection trips and partly funding field collecting trips. Field collections had been made with authorizations of the Government of Union des Comores by letters of the head of INRAPE, Union des Comores (ref. n°18/193/INRAPE/DG and n°18/210/INRAPE/DG, respectively). Finally thanks to Professor Marie-Stéphane Tixier for useful, valuable and interesting discussions on taxonomic issues.
References
- Alatawi F.J., Kamran M., Basahih J. 2016. First record of the genus Paragigagnathus Amitai and Grinberg, 1971 (Mesostigmata: Phytoseiidae) from Saudi Arabia with description of a new species. J. Natur. Hist., 50(11-12): 701-709. https://doi.org/10.1080/00222933.2015.1082656
- Amitai S., Grinberg T. 1971. Description of a new phytoseiid genus and species (Acarina: Mesostigmata) from Israel. Isr. J. Entomol., 6: 327-335.
- Amitai S., Swirski E. 1978. A new genus and new records of phytoseiid mites (Mesostigmata: Phytoseiidae) from Israel. Israel. Isr. J. Entomol., 12: 123-143.
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. Le relevé organotaxique de la face dorsale adulte (Gamasides protoadéniques, Phytoseiidae). Acarologia, 17(1): 20-29.
- Berlese A. 1914. Acari nuovi. Manipulus IX. Redia, 10: 113-150.
- Blommers L. 1974. Species of the genus Amblyseius Berlese, 1914, from Tamatave, east Madagascar (Acarina: Phytoseiidae). Bul. Zool. Mus. Univ. Amster., 3: 143-155.
- Blommers L., Gutierrez J. 1975. Les tétranyques vivant sur agrumes et avocatiers dans la région de Tamatave (Madagascar - est) et quelques-uns de leurs prédateurs. Fruits, 30: 191-200.
- Byng J.W., Smets E.F., van Vugt R., Bidault E., Davidson C., Kenicer G., Chase M.W., Christenhusz M.J.M. 2018. The phylogeny of angiosperms poster: a visual summary of APG IV family relationships and floral diversity. The Global Flora: 4-7.
- Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. Can. Entomol., 61(12): 1-166.
- Chant D.A., McMurtry J.A. 1994. A review of the subfamilies Phytoseiinae and Typhlodrominae (Acari: Phytoseiidae). Intern. J. Acarol., 20(4): 223-310. https://doi.org/10.1080/01647959408684022
- Chant D.A., McMurtry J.A. 2003. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part I. Neoseiulini new tribe. Intern. J. Acarol., 29(1): 3-46. https://doi.org/10.1080/01647950308684319
- Chant D.A., McMurtry J.A. 2004. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part III. The tribe Amblyseiini Wainstein, subtribe Amblyseiina n. subtribe. Intern. J. Acarol., 30(3): 171-228. https://doi.org/10.1080/01647950408684388
- Chant D.A., McMurtry J.A. 2005a. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae) Part VI. The tribe Euseiini n. tribe, subtribes Typhlodromalina n. subtribe, Euseiina n. subtribe, and Ricoseiina n. subtribe. Intern. J. Acarol., 31(3): 187-224. https://doi.org/10.1080/01647950508684424
- Chant D.A., McMurtry J.A. 2005b. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae) Part VII. Typhlodromipsini n. tribe. Intern. J. Acarol., 31(4): 315-340. https://doi.org/10.1080/01647950508683673
- Chant D.A., McMurtry J.A. 2005c. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae) Part VII. Typhlodromipsini n. tribe. Intern. J. Acarol., 31(4): 315-340. https://doi.org/10.1080/01647950508683673
- Chant D.A., McMurtry J.A. 2007. Illustrated keys and diognoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, 219 pp.
- Chant D.A., Yoshida-Shaul E. 1989. A world review of the tiliarum species group in the genus Typhlodromus Scheuten (Acari: Phytoseiidae). Can. J. Zool., 67(4): 1006-1046. https://doi.org/10.1139/z89-144
- Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Intern. J. Acarol., 17(3): 187-199. https://doi.org/10.1080/01647959108683906
- Chaudhri W.M., Akbar S., Rasool A. 1979. Studies on the predatory leaf inhabiting mites of Pakistan. University of Agriculture, Faisalabad, Pakistan, 243 pp.
- De Leon D. 1959. Two new genera of phytoseiid mites with a note on Proprioseius meridionalis Chant (Acarina: Phytoseiidae). Entomol. News, 70(10): 257-262.
- De Leon D. 1965. A note on Neoseiulus Hughes 1948 and new synonymy (Acarina: Phytoseiidae). Proceedings of the Entomological Society of Washington, 67(1): 23.
- De Leon D. 1966. Phytoseiidae of British Guyana with keys to species (Acarina: Mesostigmata). Stud. Fauna Suriname and other Guyanas, 8: 81-102.
- Demite P.R., McMurtry J.A., Moraes G.J. de. 2014. Phytoseiidae Database: a website for taxonomic and distributional information on phytoseiid mites (Acari). Zootaxa, 3795 (5): 571-577. https://doi.org/10.11646/zootaxa.3795.5.6
- Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2021. Phytoseiidae Database. Available from: www.lea.esalq.usp.br/phytoseiidae
 (Last access 28/VI/2021).
(Last access 28/VI/2021). - Denmark H.A., Evans G.A. 2011. Phytoseiidae of North America and Hawaii (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, USA, 451 pp.
- Denmark H.A., Muma M.H. 1989. A revision of the genus Amblyseius Berlese, 1914 (Acari: Phytoseiidae). Occas. Pap. Fla State Coll. Arthropods, USA, 4, 149 pp.
- El-Banhawy E.M., Knapp M. 2011. Mites of the family Phytoseiidae Berlese from Kenya (Acari: Mesostigmata). Zootaxa, 2945: 1-176. https://doi.org/10.11646/zootaxa.2945.1.1
- Evans G.O. 1958. Some mesostigamatid mites from a nest of social spiders in Uganda. Ann. Mag. Nat. Hist., Ser. 13(1-9): 580-590. https://doi.org/10.1080/00222935808650985
- Ferragut F., Baumann J. 2019. New phytoeiid mites (Mesostigmata: Phytoseiidae) of Mauritius, with the description of two new species. Syst. Appl. Acarol., 24(5): 825-856. https://doi.org/10.11158/saa.24.5.8
- Hajizadeh J., Faraji F., Rafatifardf M., Kamranfard F. 2010. The genus Paragigagnathus Amitai & Grinberg in Iran, with key to the known species. Syst. Appl. Acarol., 15: 222-227. https://doi.org/10.11158/saa.15.3.6
- Karg W. 1982. Diagnostic and systematics of predatory mites of the family Phytoseiidae Berlese in orchards. Zool. Jahrb. Syst., 109: 188-210.
- Kaźmierski A. 1996. A revision of the subfamilies Pretydeinae and Tydeinae (Tydeidae). Part III. Seven new genera and some new species of the Tydeinae, with a generic key. Mitt. Hamburg. Zool. Mus. Inst., 93: 199-227.
- Khanjani J., Karimi M., Asali Fayaz B., Ueckermann E.A. 2016. Paragigagnathus iraniensis n. sp. (Acari: Phytoseiidae) from Western Iran. Intern. J. Acarol., 56(2): 195-201. https://doi.org/10.1051/acarologia/20162246
- Knapp M., Van Houten Y., Van Baal E., Groot T. 2018. Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia, 58 (Suppl): 72-82. https://doi.org/10.24349/acarologia/20184275
- Kolodochka L.A. 1989. A revision of the phytoseiid mites of the genus Pamiroseius Wain. (Parasitiformes, Phytoseiidae). Entomol. Obozr., 68(1): 221-229 [in Russian].
- Kreiter S., Abo-Shnaf R.I.A. 2020a. Phytoseiid mites of Rodrigues Island. Acarologia, 60(2): 449-468. https://doi.org/10.24349/acarologia/20204376
- Kreiter S., Abo-Shnaf R.I.A. 2020b. New records of phytoseiid mites from Mauritius Island (Acari: Mesostigmata). Acarologia 60(3): 520-545. https://doi.org/10.24349/acarologia/20204382
- Kreiter S., Abo-Shnaf R.I.A., Payet R.-M. 2020a. Phytoseiid mites of Mayotte Island (Acari: Mesostigmata). Acarologia, 60(3): 622-642. https://doi.org/10.24349/acarologia/20204391
- Kreiter S., Douin M., Tixier M.-S. 2021a. New records of phytoseiid mites (Acari: Mesostigmata) from Madeira Island. Acarologia, 61(2): 217-240. https://doi.org/10.24349/acarologia/20214428
- Kreiter S., Fontaine O., Payet R.-M. 2018a. New records of Phytoseiidae (Acari: Mesostigmata) from Mauritius. Acarologia, 58(4): 773-785. https://doi.org/10.24349/acarologia/20184273
- Kreiter S., Payet R.-M., Douin M., Fontaine O., Fillâtre J., Le Bellec F. 2020b. Phytoseiidae of La Réunion Island (Acari: Mesostigmata): three new species and two males described, new synonymies, and new records. Acarologia, 60(1): 111-195. https://doi.org/10.24349/acarologia/20204361
- Kreiter S., Payet R.-M., Abdou Azali H. 2021b. Phytoseiid mites of Anjouan Island (Acari: Mesostigmata). Acarologia, 61(1): 62-83. https://doi.org/10.24349/acarologia/20214418
- Kreiter S., Payet R.-M., Abdou Azali H. 2021c. Phytoseiid mites of Mohéli Island (Acari: Mesostigmata). Acarologia, 61(1): 94-114. https://doi.org/10.24349/acarologia/20214419
- Kreiter S., Payet R.-M., Fillâtre J., Abdou Azali H. 2018b. First records of Phytoseiidae from one island of the Comoros Archipelago. Acarologia, 58(3): 529-545. https://doi.org/10.24349/acarologia/20184256
- Kreiter S., Payet R.-M., Mouigni H., Douin M., Tixier M.-S., Abdou Azali H. 2021d. New records of phytoseiid mites (Acari: Mesostigmata) from Grande Comore Island (Comoros Archipelago). Acarologia, 61 (2): 241-273. https://doi.org/10.24349/acarologia/20214429
- Kreiter S., Ueckermann E.A., Quilici S. 2002. Seven new phytoseiid species, with a new generic assignement and a key to the species of La Reunion Island (Acari: Mesostigmata). Acarologia, 42(4): 335-350.
- Kreiter S., Vicente V., Tixier M.-S., Fontaine O. 2016. An unexpected occurrence of Amblyseius swirskii Athias-Henriot in La Reunion Island (Acari: Phytoseiidae). Acarologia, 56(2): 175-181. https://doi.org/10.1051/acarologia/20162254
- Kuznetsov N.N. 1994. Two new phytoseiid mite species (Parasitiformes, Phytoseiidae) from Armenia and Tadjikistan. Vest. Zool., (2): 78-81 [in Russian].
- Liang L.-R., Ke L.-S. 1984. Notes on three new species of Amblyseius Berlese (Acari: Phytoseiidae). Acta Zootaxon. Sin., 9(2): 151-155 [in Chinese with English abstract].
- Lindquist E.E. 1994. Some observations on the chaetotaxy of the caudal body region of gamasine mites (Acari: Mesostigmata), with a modified notation for some ventrolateral body setae. Acarologia, 35: 323-326.
- Lindquist E.E., Evans G.W. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina. Mem. Entomol. Soc. Can., 47: 1-64. https://doi.org/10.4039/entm9747fv
- McMurtry J.A., Croft B.A. 1997. Life-styles of phytoseiid mites and their roles in biological control. Ann. Rev. Entomol., 42: 291-321. https://doi.org/10.1146/annurev.ento.42.1.291
- McMurtry J.A., Moraes G.J. de, Sourassou N.F. 2013. Revision of the life styles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Moraes G.J. de, Lopes P.C., Fernando C.P. 2004a. Phytoseiid mite (Acari: Phytoseiidae) of coconut growing areas in Sri Lanka, with descriptions of three new species. J. Acarol. Soc. Japan, 13(2): 141-160. https://doi.org/10.2300/acari.13.141
- Moraes G.J. de, McMurtry J.A. 1988. Some phytoseiid mites from Kenya, with description of three new species. Acarologia, 29(1): 13-18.
- Moraes G.J. de, McMurtry J.A., Denmark H.A. 1986. A catalog of the mite family Phytoseiidae. References to taxonomy, synonymy, distribution and habitat. EMBRAPA - DDT, Brasilia, Brazil, 353 pp.
- Moraes G.J. de, McMurtry J.A., Denmark H.A., Campos C.B. 2004b. A revised catalog of the mite family Phytoseiidae. Zootaxa, 434: 1-494. https://doi.org/10.11646/zootaxa.434.1.1
- Moraes G.J. de, Zannou I.D., Oliveira A.R., Yaninek J.S., Hanna R. 2006. Phytoseiid mites of the subtribes Typhlodromalina and Euseiina (Acari: Phytoseiidae: Euseiini) from sub-Saharan Africa. Zootaxa, 1114: 1-52. https://doi.org/10.11646/zootaxa.1114.1.1
- Muma M.H. 1961. Subfamiles, genera, and species of Phytoseiidae. Fla St. Mus. Bull., 5(7): 267-302.
- Muma M.H., Denmark H.A. 1970. Phytoseiidae of Florida. Arthropods of Florida and neighbouring land areas, 6. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville, USA, 150 pp.
- Myers N. 1988. Threatened biotas: hostspots in tropical forests. Environmentalist, 8: 187-208. https://doi.org/10.1007/BF02240252
- Myers N., Mittermeier R.A., Mittermeier C.G., Da Fonseca G.A., Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. https://doi.org/10.1038/35002501
- Prasad V. 1968. Amblyseius mites from Hawaii. Ann. Entomol. Soc. Amer., 61(6): 1514-1521. https://doi.org/10.1093/aesa/61.6.1514
- Pritchard A.E., Baker E.W. 1962. Mites of the family Phytoseiidae from Central Africa, with remarks on the genera of the world. Hilgardia, 33(7): 205-309.v33n07p205 https://doi.org/10.3733/hilg.v33n07p205
- Rowell H.J., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae. Can. Entomol., 110: 859-876. https://doi.org/10.4039/Ent110859-8
- Scheuten A. 1857. Einiges uber Milben. Archiv für Naturg., 23: 104-112.
- Ueckermann E.A., Loots G.C. 1988. The African species of the subgenera Anthoseius De Leon and Amblyseius Berlese (Acari: Phytoseiidae). Entomol. Mem., Dep. Agric. Water Supply, Rep. South Africa 73, 168 pp.
- Ueckermann E.A., Zannou I.D., Moraes G.J. de, Oliveira A.R. de, Hanna R., Yaninek J.S. 2008. Phytoseiid mites of the tribe Typhlodromini (Acari: Phytoseiidae) from sub-Saharan Africa. Zootaxa, 1901: 1-122. https://doi.org/10.11646/zootaxa.1901.1.1
- van der Merwe G.G. 1968. A taxonomic study of the family Phytoseiidae (Acari) in South Africa with contributions to the biology of two species. Entomol. Mem. South Africa Dep. Agric. Techn. Serv., 18: 1-198.
- Wainstein B.A. 1962. Révision du genre Typhlodromus Scheuten, 1857 et systématique de la famille des Phytoseiidae (Berlese 1916) (Acarina: Parasitiformes). Acarologia, 4: 5-30.
- Wainstein B.A. 1973. New genus and species of Phytoseiidae (Parasitiformes). Zoologicheskii Zhurnal, 52: 953-955 [in Russian].
- Walter D.E., Krantz G.W. 2009. Collecting, rearing and preparing specimens. In: Krantz G.W., Walter D.E. (Eds.). A manual of acarology, 3rd ed. Texas Tech Univ. Press, Lubbock, Texas, USA: 83–96.
- Yoshida-Shaul E., Chant D. A. 1991. Five new species of Phytoseiidae from Central and South America (Acari: Gamasina). Intern. J. Acarol., 17(2): 93-102. https://doi.org/10.1080/01647959108683888
- Zannou I.D., Moraes G.J. de, Ueckermann E.A., Oliveira A.R., Yaninek J.S., Hanna R. 2007. Phytoseiid mites of the subtribe Amblyseiina from sub-Saharan Africa. Zootaxa, 1550: 1-47. https://doi.org/10.11646/zootaxa.1550.1.1



2021-06-30
Date accepted:
2021-08-13
Date published:
2021-10-21
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Kreiter, Serge; Payet, Rose-My; Abo-Shnaf, Reham and Douin, Martial
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



