Contribution to the knowledge of the oribatid mite genus Setoppia (Acari, Oribatida, Oppiidae), with description of a new species from South Africa
Ermilov, Sergey G.1 ; Hugo-Coetzee, Elizabeth A.2 and Khaustov, Alexander A.3
1✉ Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Tyumen, Russia.
2National Museum, Bloemfontein, South Africa & University of the Free State, Bloemfontein, South Africa.
3Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Tyumen, Russia.
2020 - Volume: 60 Issue: 4 pages: 892-902
https://doi.org/10.24349/acarologia/20204409ZooBank LSID: 4D6F6355-D016-450F-9453-B56C661945AF
Original research
Keywords
Abstract
Introduction
During taxonomic identification of oribatid mites (Acari, Oribatida) from South African Golden Gate Highlands National Park, we found a new species, belonging to the genus Setoppia (family Oppiidae). The main goals of our paper are: to describe and illustrate this new species based on adults; to summarize generic morphological traits; to provide identification key to known species of Setoppia; to present data on distribution and habitats of representatives of the genus.
The genus Setoppia was proposed by Balogh (1983) with Oppia toroki Balogh, 1982 as type species. At present, the genus comprises 19 species, which are distributed in the Afrotropical, Neotropical and Australasian regions (Subías, online version 2020). Of these, eight species were registered in South Africa earlier: S. antennata (Balogh & Mahunka, 1966), S. clavimera (Mahunka, 1985), S. fortis (Balogh & Mahunka, 1966), S. izinyosa Hugo-Coetzee, 2017, S. karinae (Mahunka, 1973), S. quattuor (Kok, 1967), S. tuberosa (Mahunka, 1984), and S. verrucosa (Mahunka, 1985).
Materials and methods
Specimens — Substrate samples containing oribatid mites were collected from Golden Gate Highlands National Park (28°30′S, 28°37′E) in South Africa (eastern highveld region in Free State near the Lesotho border). Mites were extracted from samples into 75% ethanol using Berlese's funnels with electric lamps in laboratory conditions during five days.
As detailed below, specimens are distributed among two institutions: the National Museum Bloemfontein, South Africa (NMB); and the Tyumen State University Museum of Zoology, Tyumen, Russia (TSUMZ).
Observation and documentation — Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration. Body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the gastronotum. Notogastral width refers to the maximum width of the notogaster in dorsal view. Lengths of body setae were measured in lateral aspect. All body measurements are presented in micrometers. Formulas for leg setation are given in parentheses according to the sequence trochanter-femur-genu-tibia-tarsus (famulus included). Formulas for leg solenidia are given in square brackets according to the sequence genu-tibia-tarsus.
Drawings were made with a camera lucida using a Leica transmission light microscope ''Leica DM 2500''.
Terminology — Morphological terminology used in this paper follows that of F. Grandjean: see Travé & Vachon (1975) for references; Norton (1977) for leg setal nomenclature; and Norton & Behan-Pelletier (2009) for overview.
Abbreviations — Prodorsum: ro, le, in, bs, ex = rostral, lamellar, interlamellar, bothridial, and exobothridial setae, respectively. Notogaster: c, la, lm, lp, h, p = notogastral setae; ia, im, ip, ih, ips = notogastral lyrifissures; gla = opisthonotal gland opening. Gnathosoma: a, m, h = subcapitular setae; or = adoral seta; d, l, v, cm, ul, sul, vt, lt = palp setae; ω = palp solenidion; cha, chb = cheliceral setae; Tg = Trägårdh's organ; Epimeral and lateral podosomal regions: 1a–c, 2a, 3a–c, 4a–c = epimeral setae; I = pedotectum I. Anogenital region: g, ag, an, ad = genital, aggenital, anal and adanal setae, respectively; iad = adanal lyrifissure; po = preanal organ. Legs: Tr, Fe, Ge, Ti, Ta = leg trochanter, femur, genu, tibia, tarsus, respectively; ω, φ, σ = leg solenidia; ɛ = leg famulus; d, l, v, bv, ev, ft, tc, it, p, u, a, s, pv, pl = leg setae; pa = porose area.
Systematics
Main generic traits of Setoppia
Adult — Size. Small, length about 300–800. Prodorsum. Rostrum rounded or medially incised (usually tripartite). Costula absent, rarely very short. Transcostula absent, but slight transverse line sometimes developed. Two or more pairs of interbothridial tubercles often present. Postbothridial tubercle and several pairs of interbothridial muscle sigillae present or absent. Rostral and bothridial setae long, setiform. Lamellar and interlamellar setae short to long, setiform. Notogaster. Without humeral tooth and crista. Ten pairs of setiform setae, c developed or represented by alveolus, often dorsal setae la, lm, lp, and h2 located in two longitudinal rows and clearly longer than other setae. Gnathosoma. Subcapitulum diarthric. Adoral seta present. Chelicera chelate-dentate. Epimeral and lateral podosomal regions. Epimeral border IV present. Epimeral setal formula: 3-1-3-3, setae setiform. Ventrosejugal tubercle absent. Pedotectum I represented by small lamina. Discidium slightly developed or absent. Anogenital region. With six pairs of genital, one pair of aggenital, two pairs of anal and three pairs of adanal setae, all setiform. Adanal setae ad1 posterior, ad2 lateral, ad3 lateral or anterolateral to anal plate, distance between ad3–ad3 longer than ag–ag and ad2–ad2. Adanal lyrifissure diagonal, located close to anal aperture. Legs. Setae l'' and v' present on tarsus I, l'' present on tarsus II. Tarsus II with two solenidia.
Juveniles — Unknown.
Description
Setoppia paraquattuor n. sp.
ZOOBANK: 3CCF98C5-C43B-4D7D-9BC7-57FA7CAB91BF ![]()
(Figures 1, 2)






Diagnosis — Body size: 381–465 × 215–249. Rostrum tripartite. Rostral, lamellar and interlamellar setae long, setiform, barbed; in shorter than ro and le. Bothridial seta very long, setiform, shortly ciliate. Interbothridial region with two pairs of muscle sigillae and two pairs of interbothridial tubercles. Notogastral seta c minute; h1 and h3 very short, setiform, smooth; p1, p2 and p3 of medium length, setiform, slightly barbed; la, lm, lp, and h2 long, subequal in length, setiform, barbed, inserted in two longitudinal rows. Epimeral and anogenital setae setiform, slightly barbed. Discidium not observed.
Description — Measurements – Body length 415 (holotype), 381–465 (nine paratypes); body width 215 (holotype), 215–249 (nine paratypes). No distinct difference between male and female in body size.
Integument (Figs 1A, 1C) – Body color light brown to brown. Body surface microporose (visible only under high magnification in dissected specimens, × 1000). Lateral part of rostrum foveolate (diameter of foveola up to 1). Lateral parts of body between bothridium and acetabula I-III with numerous cuticular granules (their diameter up to 4).
Prodorsum (Figs 1A, 1C) – Rostrum tripartite, teeth small, tightly pressed to each other, incision between teeth vary narrow, median tooth smallest or reduced. Rostral (53–61), lamellar (53–61) and interlamellar (41–49) setae setiform, barbed; le equally removed from ro and in. Exobothridial seta (20) setiform, thin, slightly barbed. Bothridial seta (184–196) setiform, shortly ciliate. Interbothridial region with two pairs of muscle sigillae and two pairs (anterior and posterior) of interbothridial tubercles. Postbothridial tubercle slightly developed. Longitudinal row, comprising several muscle sigillae, present in front of the bothridium.
Notogaster (Figs 1A–1C) – Anterior border convex medially. Ten pairs of notogastral setae present: c (4), h1 (8–12) and h3 (8–12) setiform, thin, smooth; p1 (24–28), p2 (16–20) and p3 (16–20) setiform, thin, slightly barbed; la, lm, lp, and h2 (114–135) setiform, barbed, inserted in two longitudinal rows. All notogastral lyrifissures and opisthonotal gland opening, circumgastric scissure, and circumgastric sigillar band distinct.
Gnathosoma (Figs 2A–2C) – Subcapitulum longer than wide (86–94 × 69–77). Subcapitular setae setiform, barbed, a (22–24) shorter than m (28–32) and h (28–32). Adoral seta (6) setiform, thin, smooth. Palp (61–65) with setation 0-2-1-3-8(+1 solenidion). Solenidion swollen distally and connected with seta ul'. Postpalpal seta (4) spiniform, smooth. Chelicera (86–94) with two setiform, barbed setae, cha (26–28) longer than chb (18–20). Trägårdh's organ of chelicerae elongate triangular.
Epimeral and lateral podosomal regions (Figs 1B, 1C) – All epimeral setae setiform, slightly barbed, 1b, 1c, 3b, and 3c (45–49) longer than 1a, 2a and 3a (16–20) and others (24–28). Discidium not observed.
Anogenital region (Figs 1B, 1C) – Six pairs of genital (g1, 14–16; others, 10–12), one pair of aggenital (30–32), three pairs of adanal (30–32) and two pairs of anal (22–24) setae setiform, slightly barbed. Adanal lyrifissure diagonal, close and lateral to anal aperture.
Legs (Figs 2D–2G) – Leg claw smooth. Porose area on femora I-IV slightly visible. Formulas of leg setation and solenidia: I (1-5-2-4-20) [1-2-2], II (1-5-2-4-16) [1-1-2], III (2-3-1-3-15) [1-1-0], IV (1-2-2-3-12) [0-1-0]; homology of setae and solenidia indicated in Table 1. Setae p setiform on tarsi I, and very short, conical on tarsi II-IV. Famulus of tarsus I erect, slightly swollen and blunted distally, inserted between solenidia ω1 and ω2. Solenidia ω1 on tarsi I, ω1 and ω2 on tarsi II and σ on genua III bacilliform, other solenidia setiform.



Material examined — Holotype (male) and nine paratypes (two males and seven females): South Africa, Golden Gate Highlands National Park, 28°31′S, 28°39′E, \textasciitilde1900 m a.s.l., soil near termite nests of Trinervitermes trinervoides, 11.IX.2019 (collected by V.A. Khaustov, S.G. Ermilov, E.A. Hugo-Coetzee and A.A. Khaustov).
Type deposition — The holotype is deposited in the collection of the NMB; nine paratypes are deposited in the collection of the TSUMZ. All specimens are preserved in 70% solution of ethanol with a drop of glycerol.
Etymology — The name paraquattuor refers to the similarity between the new species and Setoppia quattuor Kok, 1967.
Remarks — The holotype and paratypes of S. quattuor, housed at NMB, were re-examined. It was observed that all types have a tripartite rostrum (incorrectly described and figured by Kok, 1967 as rounded), with wide lateral teeth, wide incision, and a medium sized middle tooth. With this new evidence, Setoppia paraquattuor n. sp. is morphologically most similar to Setoppia quattuor and S. tuberosa in having tripartite rostrum, very long bothridial seta, long notogastral setae la, lm, lp, and h2 located in two longitudinal rows and other notogastral setae short. Setoppia paraquattuor n. sp. differs from S. quattuor by the smaller lateral teeth and incision in the rostrum, very short seta h3 (versus longer), similar sized notogastral setae la, lm, lp, and h2 (114–135) (versus setae of different sizes; la, h2 \textasciitilde 88, lm \textasciitilde100, lp \textasciitilde130) and seta p1 longer than p2 and p3 (versus p1, p2 and p3 subequal in size). The new species differs from S. tuberosa by the smaller or reduced middle tooth and incisions in the rostrum, smaller body size (381–465 × 215–249 versus 475–533 × 270–303) and the presence of long interlamellar seta (versus very short) and two pairs of interbothridial tubercles (versus four or five pairs).
Key to known species of Setoppia
1. Notogastral seta h3 slightly shorter (3/4 of length) than setae la, lm, lp or similar to them in length
...... (2)
— Notogastral seta h3 distinctly shorter (less 1/2 of length) than setae la, lm, lp
...... (8)
2. Rostrum tripartite; body size: 367–453 × 198–200
...... S. toroki Balogh, 1982
— Rostrum rounded
...... (3)
3. Notogastral seta lm located posterior to la
...... (4)
— Notogastral seta lm located anteromedial or medial to la
...... (5)
4. Anterior notogastral seta c long, similar in length to setae la, lm and lp; notogastral seta la reaching insertion of seta lm; interlamellar seta of medium length, distinctly longer than diameter of bothridium; body size: 661 × 411
...... S. fortis (Balogh & Mahunka, 1966)
— Anterior notogastral seta c represented by alveolus, setae la, lm and lp of medium length; notogastral seta la clearly not reaching insertion of seta lm; interlamellar seta short, not longer than diameter of bothridium; body size: 359 × 195–204
...... S. toxotes Balogh, 1982
5. Notogastral seta lm located anteromedial to la; rostral setae straight, divergent distally; body size: 796–813 × 431–448
...... S. parrillarensis Ermilov, 2019
— Notogastral seta lm located medial to la; rostral setae arch-like, not divergent distally
...... (6)
6. Interlamellar seta long, similar in length to rostral and lamellar setae; notogastral seta lm reaching insertion of seta lp; interbothridial region with tubercles; body size: 623–632 × 380–384
...... S. vanga (Mahunka, 1994)
— Interlamellar seta short, distinctly shorter than rostral and lamellar setae; notogastral seta lm clearly not reaching insertion of seta lp; interbothridial region without tubercles
...... (7)
7. Rostral seta longer than lamellar seta; lamellar seta located closer to interlamellar seta than rostral seta; body size: 469–527 × 226–245
...... S. bornemisszai (Balogh, 1982)
— Rostral and lamellar setae similar in length; lamellar seta located closer to rostral seta than interlamellar seta; body length: 430
...... S. mahunkai (Hammer, 1968)
8. Rostrum incised medially
...... (9)
— Rostrum rounded
...... (16)
9. Rostrum with broad median indentation and two lateral tubercles; body size: 690–792 × 454–523
...... S. izinyosa Hugo-Coetzee, 2017
— Rostrum tripartite
...... (10)
10. Medial tooth of rostrum distinctly longer than lateral ones; body size: 336–380 × 192–212
...... S. clavimera (Mahunka, 1985)
— Medial tooth and lateral ones of rostrum slightly different in length
...... (11)
11. Interlamellar seta long, distinctly longer than exobothridial seta
...... (12)
— Interlamellar seta short, distinctly shorter than exobothridial seta
...... (15)
12. Lamellar seta not longer than interlamellar seta
...... (13)
— Lamellar seta longer than interlamellar seta
...... (14)
13. Notogastral seta c of medium length, longer than diameter of bothridium; body size: 431–475 × 254–275
...... S. antennata (Balogh & Mahunka, 1966)
— Notogastral seta c minute; body size: 408–438× 222–258
...... S. quattuor (Kok, 1967)
14. Notogastral seta h2 short, distinctly shorter than setae la, lm, lp; interbothridial region with three pairs of muscle sigillae; body size: 418–484 × 237–278
...... S. verrucosa (Mahunka, 1985)
— Notogastral seta h2 long, similar to la, lm, lp in length; interbothridial region with two pairs of muscle sigillae; body size: 381–465 × 215–249
...... S. paraquattuor n. sp.
15. Notogastral seta la reaching insertion of seta lp; interbothridial region with several pairs of tubercles; body size: 475–533 × 270–303
...... S. tuberosa (Mahunka, 1984)
— Notogastral seta la distinctly not reaching insertion of seta lp; interbothridial region without tubercles; body length: 700
...... S. angustopili (Hammer, 1962)
16. Notogastral seta la located anterolateral to lm; lamellar seta inserted on short costula; interlamellar seta shorter than exobothridial seta; body size: 587 × 306
...... S. strinovichi (Balogh, 1982)
— Notogastral seta la located anterior or anteromedial to lm; lamellar seta inserted on prodorsal surface; interlamellar seta longer than exobothridial seta
...... (17)
17. Interlamellar seta distinctly longer than rostral and lamellar setae; interbothridial region without tubercles
...... (18)
— Interlamellar seta shorter than rostral and lamellar setae; interbothridial region with tubercles
...... (19)
18. Notogastral seta la located anterior to lm; body size: 475–500 × 240–295
...... S. compressa (Balogh & Mahunka, 1975)
-\cledetermination{– Notogastral seta la located anteromedial to lm; body size: 611–625 × 325–340}{S. longisetosa (Balogh & Mahunka, 1975)}
19. Notogastral seta c of medium length, distinctly longer than diameter of bothridium; two pairs of interbothridial tubercles; body size: 470–551 × 265–298
...... S. karinae (Mahunka, 1973) (see also Hugo-Coetzee 2017)
— Notogastral seta c short, not longer than diameter of bothridium; numerous tubercles in interbothridial region; body size: 475–533 × 262–296
...... S. szaboi (Mahunka, 1988)
Distribution and habitat of Setoppia
At present, representatives of Setoppia have been recorded only in the Southern Hemisphere, in the Afrotropical, Neotropical and Australasian regions (Fig. 3). Except for two species (S. angustopili, S. karinae), the other 18 species have a highly circumscribed geographic distribution, i.e. are endemic, to a single country.
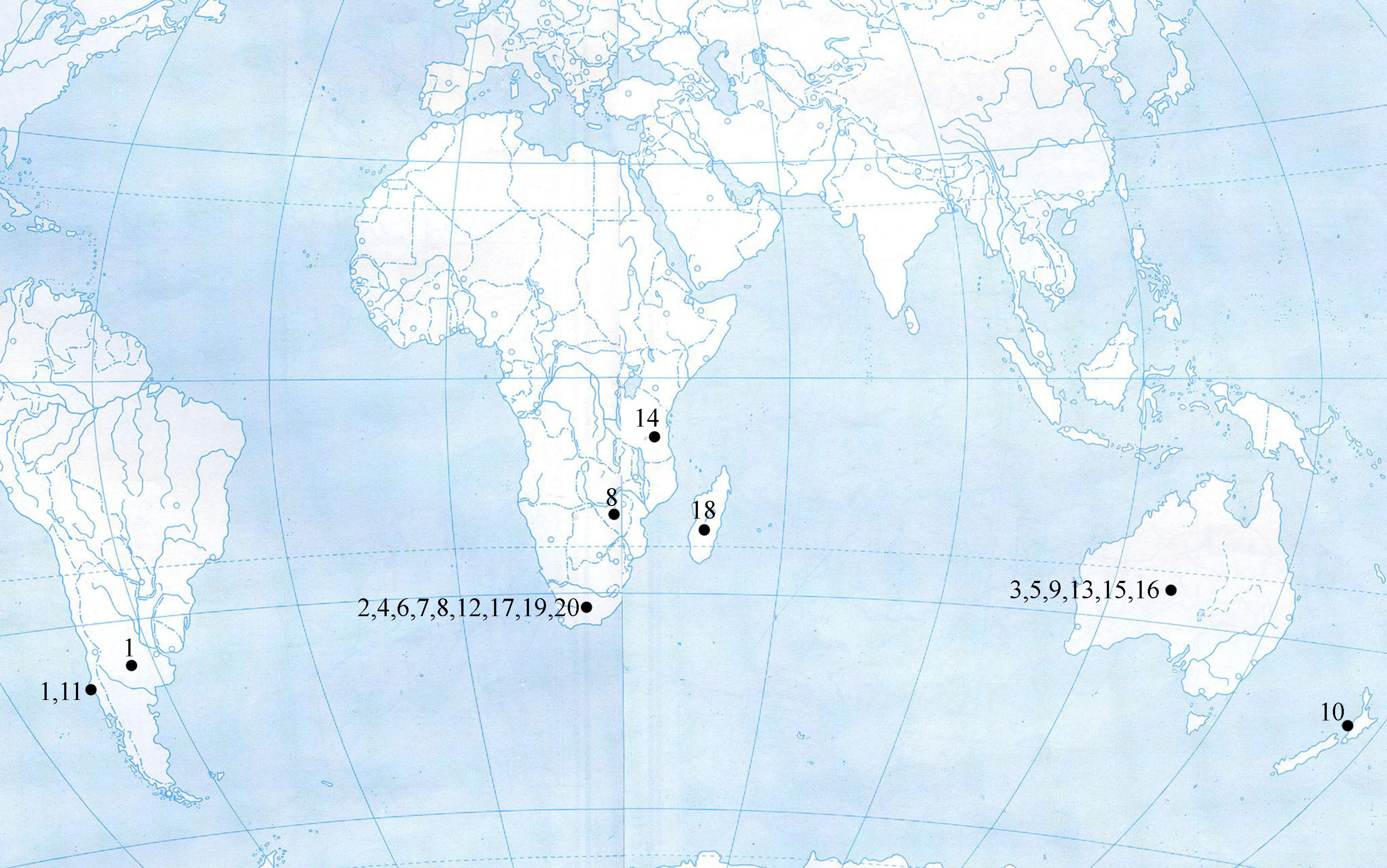


Afrotropical region (11 species). Eight species of Setoppia (S. antennata, S. clavimera, S. fortis, S. izinyosa, S. quattuor, S. tuberosa, S. verrucosa, and S. paraquattuor n. sp.) were found only in South Africa (see Fig. 4, for distribution). Some species are only known from their type locality: S. antennata was described from Table Mountain in the Table Mountain National Park, Cape Town in south-western South Africa, without designation of sample (Balogh & Mahunka 1966); S. clavimera from soil traps of Lottering Forest on Tsitsikamma mountains in Lottering Coast Reserve in southern South Africa (Mahunka 1985); S. fortis from KwaZulu-Natal Province in north-eastern South Africa, without designation of sample (Balogh & Mahunka 1966); S. tuberosa from Nature's Valley in Cape Province in south-eastern South Africa (Mahunka 1984); S. izinyosa from soil and leaves under shrubs of Vernon Crookes Nature Reserve in KwaZulu-Natal Province in north-eastern South Africa (Hugo-Coetzee 2017); and S. paraquattuor n. sp. from soil in Golden Gate Highlands National Park near Lesotho in central South Africa (this paper). Other South African species were recorded in more localities: S. verrucosa was described from Lottering Forest on Tsitsikamma mountains in Lottering Coast Reserve in southern South Africa (Mahunka 1985), but was also recorded in Wilderness National Park, southern South Africa, from leaf litter in forest (data from NMB collection); S. quattuor was described from soil and leaves under poplar trees and Leucosidia sp. at Fouriesburg near Lesotho in central South Africa (Kok 1967), but has a wide distribution (data from NMB collection) from central to north-eastern parts of South Africa (see Fig. 4) and has been recorded in various habitats, from moist to dry soils and leaves in forests, sugarcane and grasslands. Setoppia quattuor was also recorded in Lesotho (data from NMB collection).



Setoppia karinae was originally described from mountainous Chimanimani District (previous Melsetter) of Manicaland Province in eastern Zimbabwe, without designation of sample (Mahunka 1973). It was also recorded in KwaZulu-Natal Province, north-eastern South Africa, in Vernon Crookes Nature Reserve (Hugo-Coetzee 2017) and near Leisure Bay (data from NMB collection) from soil and leaves in coastal forest vegetation, and also near Harding from grass under Pine trees. The other two Afrotropical species, Setoppia szaboi was described from soil and litter in Kwamsambia Forest Reserve of Tanga region near Kwamkoro in north-eastern Tanzania (Mahunka 1988) and S. vanga from soil in primary forest in Nosy Boraha Island at the eastern coast of Madagascar (Mahunka 1994).
Neotropical region (two species). Setoppia angustopili was originally described from Chile and later recorded in Argentina. It was recorded from moss and fern on moist mouldering soil in forests of bamboo and tall trees in the Andes Mountains near Peulla in central Chile (Hammer 1962) and from soil, leaf litter and pitfall traps in Austrocedrus chilensis and Nothofagus forests of north-western Patagonia (Kun et al. 2010), and from an unknown collecting site in Argentina in the north-western Patagonia (Balogh & Csiszár 1963). Setoppia parrillarensis was described from swamp moss near Laguna Parrillar National Park in southern Chile (Ermilov 2019).
Australasian region (seven species). Six species of Setoppia (S. bornemisszai, S. compressa, S. longisetosa, S. strinovichi, S. toroki, and S. toxotes) were found and described in Australia: S. bornemisszai, S. strinovichi, S. toroki, and S. toxotes were registered from litter in rainforest near Urbenville village in eastern Australia (Balogh 1982); S. compressa from wet sclerophyllous rain forest on Mount Glorious in Queensland in north-eastern Australia, without designation of sample (Balogh & Mahunka 1975); and S. longisetosa from black rock, dry sclerophyllous country in Queensland in north-eastern Australia, without designation of sample (Balogh & Mahunka 1975). Setoppia mahunkai was recorded from Selaginella vegetation and dead leaves under tree-ferns of native forest on the west coast of the North Island near New Plymouth in New Zealand (Hammer 1968).
Acknowledgements
We are grateful to Jan Andries Neethling and Vladimir A. Khaustov for helping with fieldwork; and Dr. Julia Baumann (University of Graz, Graz, Austria) and two anonymous reviewers for valuable comments. Thank you to South African National Parks (SANParks) for allowing this study to be conducted in the Golden Gate Highlands National Park (Permit no. KHAUA1632). The study was funded by the Russian Foundation for Basic Research according to the research project № 18-04-00096A.
References
Balogh J. 1982. New oppioid mites from Australia (Acari: Oribatei). Acta Zool. Acad. Sci. Hung., 28(1-2): 3-14.
Balogh J. 1983. A partial revision of the Oppiidae Grandjean, 1954 (Acari: Oribatei). oppioid mites from Australia (Acari: Oribatei). Acta Zool. Acad. Sci. Hung., 29(1-3): 1-79.
Balogh J., Csiszár J. 1963. The zoological results of Gy. Topál's collectings in South Argentina. 5. Oribatei (Acarina). Ann. Hist.-Nat. Mus. Nat. Hung., 55: 463-485.
Balogh J., Mahunka S. 1966. New oribatids (Acari) from South Africa. Acta Zool. Acad. Sci. Hung., 12(1-2): 1-23.
Balogh J., Mahunka S. 1975. New oppioid mites (Acari: Oribatei) from Queensland. Acta Zool. Acad. Sci. Hung., 21(3-4): 241-256.
Ermilov S.G. 2019. New species of oribatid mites of the family Oppiidae (Acari, Oribatida) from Chile. Zootaxa, 4656(2): 274-286. doi:10.11646/zootaxa.4656.2.4
Hammer M. 1962. Investigations on the oribatid fauna of the Andes Mountains. III. Chile. Det Kong. Dansk. Vidensk. Selsk. Biol. Skr., 13(2): 1-96.
Hammer M. 1968. Investigations on the Oribatid fauna of New Zealand with a comparison between the oribatid fauna of New Zealand and that of the Andes Mountains, South America. Part III. Det Kong. Dansk. Vidensk. Selsk. Biol. Skr., 16(2): 1-96.
Hugo-Coetzee E.A. 2017. New Oppiidae (Acari: Oribatida) from Vernon Crookes Nature Reserve, South Africa. Zootaxa, 4311(2): 211-232. doi:10.11646/zootaxa.4311.2.3
Kok D.J. 1967. Studies on some South African Oppiidae Grandjean, 1953 (Acarina: Oribatei). J. Ent. Soc. Southern Afr., 30(1): 40-74.
Kun M.E., Martinez P.A., Gonzalez A. 2010. Oribatid mites (Acari: Oribatida) from Austrocedrus chilensis and Nothofagus forests of Northwestern Patagonia (Argentina). Zootaxa, 2548: 22-42. doi:10.11646/zootaxa.2548.1.2
Mahunka S. 1973. Neue und interessante Milben aus dem Genfer Museum XI. Neue und wenig bekannte Oribatiden aus Rhodesien (Acari). Arch. Sci., 26(3): 205-225.
Mahunka S. 1984. Oribatids of the Eastern Part of the Ethiopian Region (Acari). VI. Acta Zool. Hung., 30(3-4): 393-444.
Mahunka S. 1985. Oribatids from Africa (Acari: Oribatida) II. Folia Ent. Hung., 46(1): 73-113.
Mahunka S. 1988. The oribatid fauna of Tanzania (Acari), I. Acta Zool. Hung., 34(4): 345-378.
Mahunka S. 1994. Oribatids from Madagascar II. (Acari: Oribatida). (New and interesting mites from the Geneva Museum LXXIX). Rev. suisse Zool., 101(1): 47-88. doi:10.5962/bhl.part.79900
Norton R.A. 1977. A review of F. Grandjean's system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae. In: Dindal D.L. (Ed.). Biology of oribatid mites. Syracuse: SUNY College of Environmental Science and Forestry. pp. 33-61.
Norton R.A., Behan-Pelletier V.M. 2009. Oribatida. Chapter 15. In: Krantz G.W., Walter D.E. (Eds.). A Manual of Acarology. Lubbock: Texas Tech University Press. pp. 430-564.
Subías L.S. 2020. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles) (15ª actualización). Online version accessed in January 2020, 527 pp.; http://bba.bioucm.es/cont/docs/RO_1.pdf

Travé J., Vachon M. 1975. François Grandjean. 1882-1975 (Notice biographique et bibliographique). Acarologia, 17(1): 1-19.



2020-09-05
Date accepted:
2020-11-30
Date published:
2020-12-02
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Ermilov, Sergey G.; Hugo-Coetzee, Elizabeth A. and Khaustov, Alexander A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




