A new Euseius species on citrus and wild lime, Zanthoxylum fagara (Rutaceae), in Florida and an updated key to Euseius species from the state
Ueckermann, Edward A.1 ; Moraes, Gilberto J. de2 and Childers, Carl C.3
1✉ Unit for Environmental Sciences and Management, Potchefstroom Campus, North-West University, Private Bag X6001, Potchefstroom, 2520, South Africa.
2Departamento de Entomologia e Acarologia, Escola Superior de Agricultura “Luiz de Queiroz”, Universidade de São Paulo, Piracicaba, Brazil.
3University of Florida, Citrus Research and Education Center, Lake Alfred, Florida, USA.
2020 - Volume: 60 Issue: 4 pages: 863-871
https://doi.org/10.24349/acarologia/20204408ZooBank LSID: 986EFD99-3E90-498E-9002-2A40F6E9B8BC
Original research
Keywords
Abstract
Introduction
In several crops, including citrus, mites in the family Phytoseiidae offer potential in suppressing below economic injury levels pest mite species of the families Diptilomiopidae, Eriophyidae, Tarsonemidae, Tenuipalpidae and Tetranychidae (Childers and Denmark 2011; Carrillo et al. 2012; Carrillo and Pena 2012; McMurtry et al. 2015). The phytoseiids are well represented on citrus and other plants in Florida, with numerous species identified (Muma 1975; Denmark and Evans 2011; Childers and Denmark 2011).
A major effort has been dedicated to update information about the phytoseiid fauna on Florida dooryard, experimental and commercial citrus plantations in the state, based on an extensive survey conducted in that state. During this survey, a new species in the genus Euseius Wainstein was found. This genus is presently composed of about 230 valid species, found in all continents, but predominantly in the tropics; altogether, six Euseius species have been reported from Florida (Muma et al. 1970; Demite et al. 2019). A large number of studies have been conducted about the biology and ecology of these mites, which have been classified as pollen feeding generalist predators, showing a preference for plants with glabrous leaves (such as citrus) as hosts (McMurtry et al. 2013). Different Euseius species are among the dominant phytoseiids on citrus in different countries, as for example E. mesembrinus (Dean) and E. hibisci (Chant) in North America (Denmark and Evans 2011), Euseius concordis (Chant) in Brazil, Euseius scutalis (Athias-Henriot) and S. stipulatus (Athias-Henriot) in the Mediterranean area (Ferragut et al. 2010), E. victoriensis in Australia (Schicha 1987) and E. citri (Van der Merwe and Ryke) in South Africa (Van der Merwe and Ryke 1964).
The objective of this publication is to describe the new Euseius species collected in Florida, and to present a key to separate the phytoseiid species so far reported from citrus in that state. The holotype and some paratypes will be deposited in the Acarology collections at the Florida Department of Agriculture & Consumer Services, Division of Plant Industry in Gainesville, Florida, USA and rest of paratypes at the National Collection of Arachnida, ARC-Plant Health and Protection (NCA-PPRI), Pretoria, South – Africa.
Material and Methods
A survey of dooryard, experimental and commercial plantations throughout Florida was conducted between 2009 and 2014 to determine the diversity of the family Phytoseiidae.
Samples of leaves, twigs and fruits were taken from the inner and outer areas of the tree canopy and washed in 80% ethanol, as descried by Childers and Denmark (2011) and Childers et al. (2017). In this process, plant parts were vigorously agitated in the solution and then removed to extract the mites. All the material was collected by the third author. Identification of the citrus species was done according to Hodgson (1967).
The mites were slide-mounted in Hoyer's medium (Walter and Krantz 2009). The slides were dried at 45-50 °C for at least two weeks, and then examined under phase contrast microscope (Zeiss AxioskopTM Research). Line drawings were made with the aid of photographs of the specimens taken with a Zen Soft Imaging System. All illustrations were edited using Adobe Illustrator C5. Measurements were taken with a Zen Soft Im., and are given in micrometers as a range (paratype measurements) followed by the holotype measurements in square brackets.
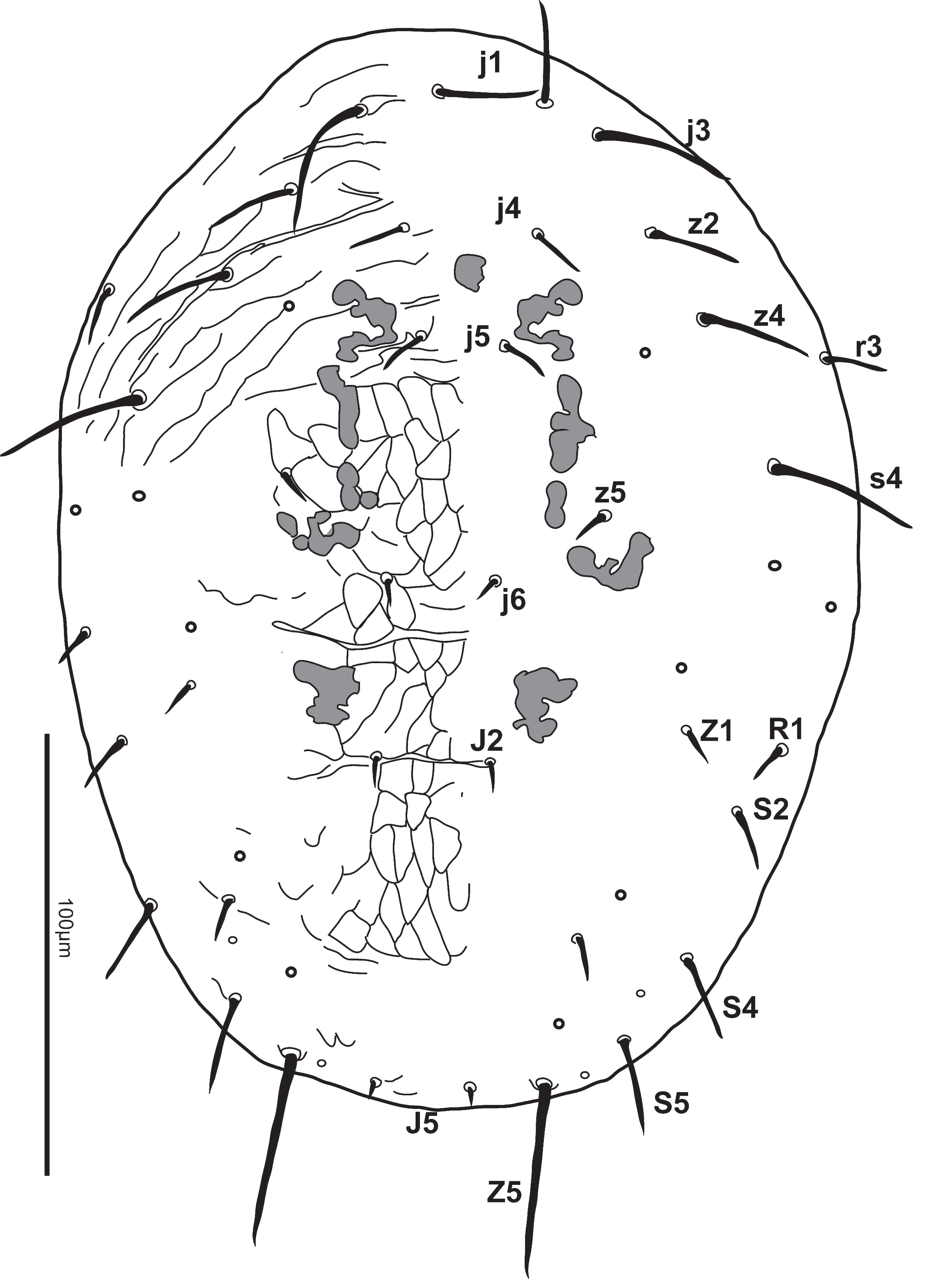
Idiosomal setal notation used in this paper is that of Lindquist and Evans (1965), as applied to the phytoseiids by Rowell et al. (1978) and Chant and Yoshida-Shaul (1989) for the dorsal surface, and by Chant and Yoshida-Shaul (1991) for the ventral surface. Macrosetal notation (Sge, genual macroseta; Sti, tibial macroseta; St, tarsal macroseta) are that of Muma et al. (1970). The system of classification follows that of Chant and McMurtry (2007). Pore (= solenostome) and poroid (= lyrifissure) notation are that of Athias-Henriot (1975).
Results and Discussion
Subfamily Amblyseiinae Muma
Tribe Euseiini Chant and McMurtry
Genus Euseius Wainstein
Euseius ennsi sp. n. (Figs. 1–4)
ZOOBANK: 7C513FDB-9D08-4D6A-967F-6157DF355BD7 ![]()
Type material — Holotype female, 3 paratype females and 2 paratype males, on Citrus aurantium L., 5620 SW 3rd Place, Margate, 26.22660°N, 80.20377°W, 18 June 2012; 3 paratype females on C. reticulata x C. paradise, 2817 NE 20 Court, Fort Lauderdale, 26.15238°N, 80.11113°W,18 June 2012; one paratype female on Citrus sinensis (L.), 2756 18th Street NE, Fort Lauderdale, 26.14980°N, 80.11139°W, 18 June 2012; one female on wild lime, Zanthoxylum fagara (L.) Sarg., Secret Woods Park, Fort Lauderdale, 26.08839°N, 80.18028°W, 25 January 2012; one paratype female on C. sinensis, 600 SW 9th Street, Cape Coral, 26.63397°N, 81.9869°W; 17 June 2013; one paratype female and one paratype male, on Citrus tangelo J.W. Ingram & H.E. Moore, 401 SE 1st Terr, Pompano Beach, 26.22495°N, 80.12369°W, 18 June 2018; one paratype female, on Citrus limon (L.) Burm. Fil., 617 NW 13th Terr, Cape Coral, 26.67776°N, 81.98931°W, 12 June 2013; 2 paratype females, on C. tangelo, 11773 85th Avenue N, Seminole, 27.85024°N, 82.80457°W, 19 June 2013; one paratype female, on Citrus paradise Macfad, 1288 Olympic Circle, Green Acres, 26.65660°N, 80.15261°W, 20 June 2012; ; one paratype female on C. sinensis, 14642 168 Avenue, Indiantown, 27.02817°N, 80.48491°W, 15 June 2012; one paratype female on Citrus limon, 507 Warwick Drive, Venice, 27.06157°N, 82.36867°W, 17 June 2013; one paratype female on Citrus limon, 27595 Tarpon Way, Bonita Springs, 26.33568°N, 81.82864°W, 11 June 2013.
Female (n = 8)



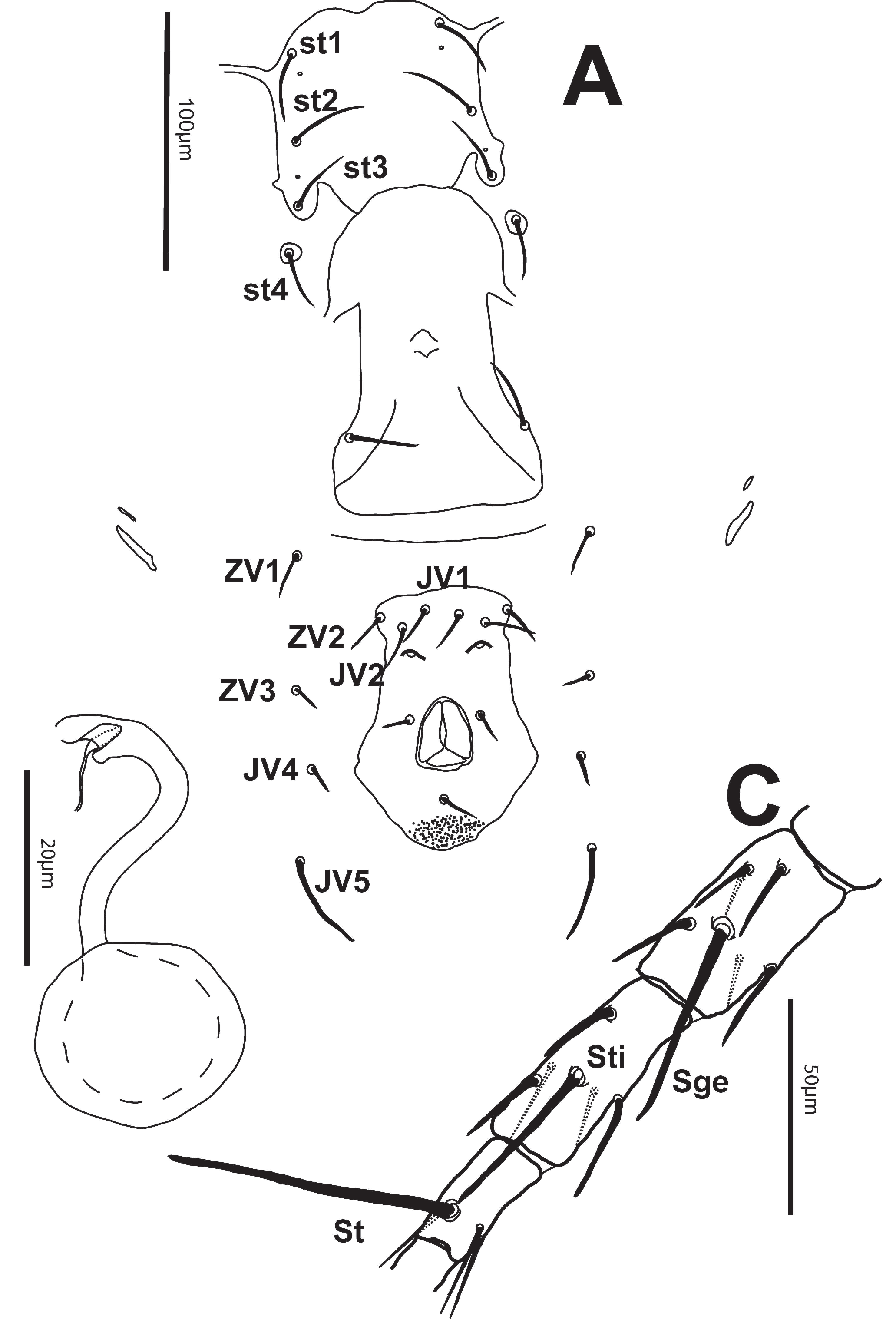


Dorsum — Shield strongly reticulated. Length of shield (305-329) [310], width (214-233) [217]. Setae j1 (28-33) [30], j3 (32-41) [31], j4 (9-14) [11], j5 (9-12) [11], j6 (8-12) [10], J2 (10-12) [12], J5 (4-6) [5], z2 (17-25) [19], z4 (23-34) [25], z5 (8-11) [10], Z1 (9-13) [13], Z4 (9-13) [13], Z5 (61-73) [64], s4 (36-52) [36], S2 (13-24) [17], S4 (17-26) [21], S5 (20-30) [24], r3 (12-17) [15], R1 (9-13) [11]. All dorsal setae smooth except for Z5, which are slightly serrate. Dorsal shield with 5 pairs of solenostomes (gd2, gd4, gd6, gd8, gd9) and 11 pairs of poroids. Sigilla present on prodorsum. Peritreme reaching to or just beyond setae z2 (130-153) [153] long (Fig. 1).
Venter — All ventral shields smooth. Distances between St1-St3 (58-65) [56], St2-St2 (65-72) [67], St5-St5 (64-72) [68]. Sternal shield with3 pairs of setae and 2 pairs of pores. Posterior margin of sternal shield lobed. Two pairs of metapodal shields present, primary shield (16-21) [20] long and secondary shield (9-120 [9] long. Ventrianal shield (95-105) [98] long, width at level of setae ZV2 (46-56) [49], width at level of anal opening (66-74) [69]. With 3 pairs of preanal setae almost transversely aligned and 2 pores. Setae JV5 smooth, (34-42) [37] (Fig. 2A).
Chelicera — The position of the chelicerae renders an illustration impossible. Fixed digit (20-26) [24], with apparently 4 small teeth and a pilus dentilis; movable digit (20-25) [24], with only one tooth.
Spermatheca — Calyx tubular with atrium bifid, (25-32) [27] long (Fig. 2B).
Legs — Macrosetae acute distally: Sge II (20-26) [24], Sge III (20-32) [28], St IV (66-76) [68], Sti IV (37-44) [38], Sge IV (47-55) [49]. Genu I 2-2/1-2/1-2, genu II 2-2/0-2/0-1, genu III 1-2/1-2/1-0, genu IV 1-2/1-2/1-0 (Fig. 2C).
Male (n = 2)


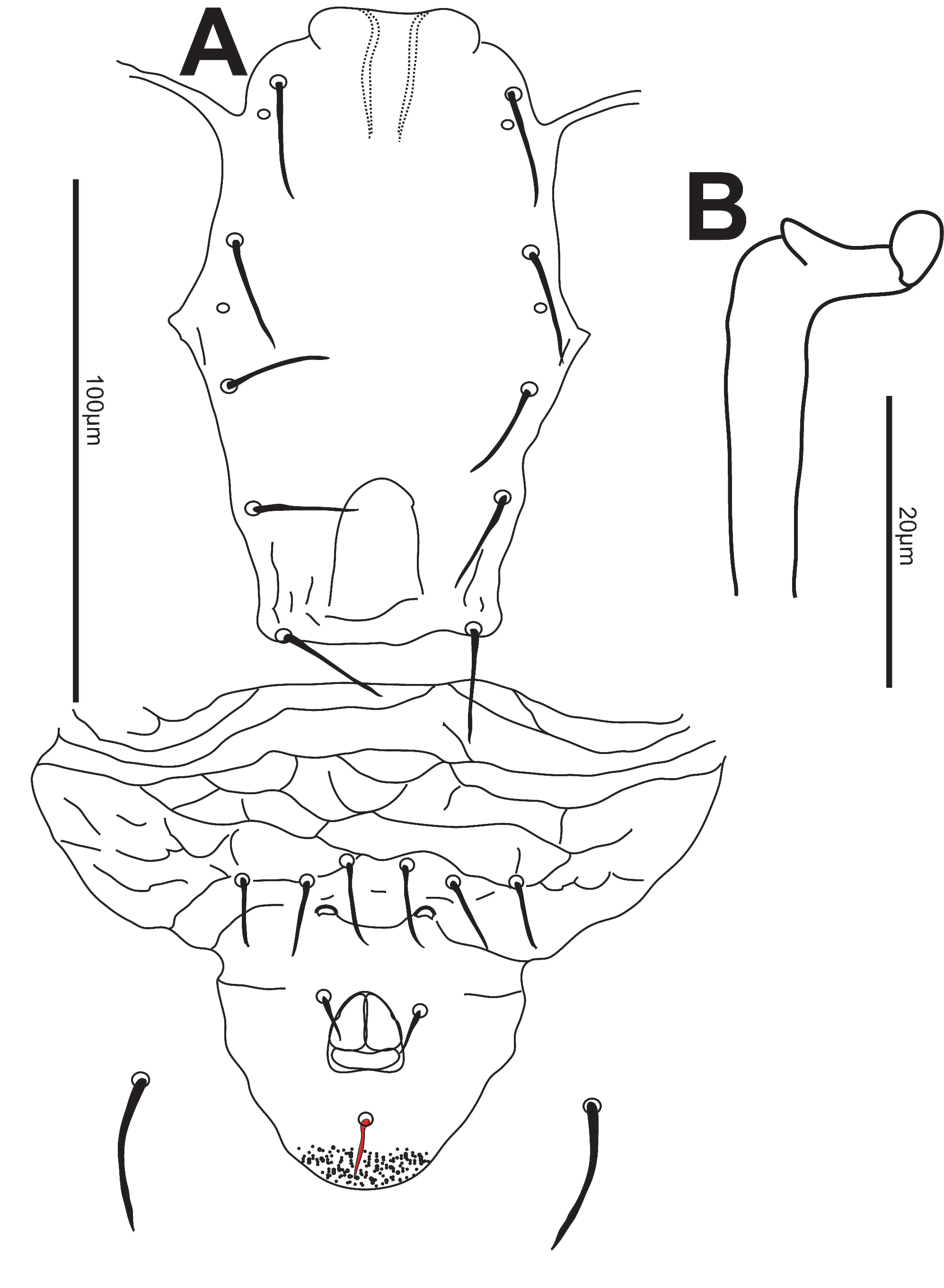


Dorsum — Similar to that of female, except for setae r3 and R1 which are on the dorsal shield (Fig 3). Length of shield 248-261, width 178-182. Setae j1 20-23, j3 28-32, j4 7-11, j5 8-10, j6 8-10, J2 10-11, J5 5-7, z2 19-22, z4 25, z5 7-10, Z1 9-10, Z4 11-12, Z5 52-53, s4 34-35, S2 15-16, S4 19-22, S5 17-26, r3 14-16, R1 9-10. All dorsal setae smooth except for setae Z5 which are slightly serrate. Peritreme reaching level of setae z4.
Venter — Sternogenital shield smooth, 123-134 long and 84-87 wide at level between setae st2 and st3 with 5 pairs of setae and 2 pairs of poroids. Ventrianal shield reticulate-striate, 104-105 long, width at level of anterior margin 143-149, with 3 pairs of transversely aligned preanal setae and 2 pores. Setae JV5 smooth, 22-23 (Fig. 4A).
Chelicera — Spermatodactyl L-shaped and 26-27 long (Fig. 4B). Fixed digit, with 2 teeth and a pilus dentilis; movable digit with only one tooth.
Legs — Macrosetae acute distally: Sge II 21, Sge III 22-23, St IV 53-54, Sti IV 29-31, Sge IV 36-38. Genu I 2-2/1-2/1-2, genu II 2-2/0-2/0-1, genu III 1-2/1-2/1-0, genu IV A 1-2/1-2/1-0.
Etymology
This species is named after the late Dr Wilbur R. Enns in recognition of his roles as teacher and advisor in the Department of Entomology, University Missouri-Columbia.
Remarks
This species closely resembles Euseius quetzali McMurtry et al., 1985, a species described from Guatemala and also reported from Mexico and California (Demite et al., 2019). Congdon & McMurtry (1986) compared the morphology of populations from California and Guatemala identified as E. quetzali. They were very similar, except for the larger ratios j1/ j3 and z4/ z2, and shorter peritreme in the Californian population; these characteristics of the Californian population are comparable with those of the specimens collected in this study. Results of crossing experiments conducted by Congdon & McMurtry (1986) led them to conclude that the Californian population belonged to the same species as populations they collected in Mexico and Guatemala. In the first part of that study, no offspring were obtained when crossing Californian E. quetzali and Mexican Euseius hibisci (Chant), indicating that they belonged to different species. However, the results obtained when crossing Californian populations of those species were not considerably different from the results obtained when crossing Californian and Guatemalan populations of E. quetzali. In both cases, females were never produced in the crossings. In the first case, males and females were produced only by E. hibisci females, while in the second case, males and females were produced by females from both areas. Hence, we consider that their decision about the identity of the species was based on the lack of sufficient evidence that they belonged to different species rather on the availability of evidence showing that they belonged to the same species. Phytoseiids are known to reproduce mainly by a process designated as pseudoarrhenotoky (Hoy, 2011), in which offspring are only produced after mating and fertilization of the eggs.
According to our examination of paratypes of E. quetzali, from data provided in the original description of the species (McMurtry et al., 1985) and in the redescription by Congdon & McMurtry (1986), this new species differs from E. quetzali by the ornamentation of the dorsal shield (smooth in the central area of the podonotal region of the dorsal shield in E. quetzali), and by the much shorter j4–j6, J2, z5, Z1 and Z4, which appear to be the shortest dorsal shield setae of the new species but also shorter than that of E. quetzali. In our examination of the paratype females, the peritreme extends only up to z2, instead of up to j3 as shown in the illustration of the original description.
Euseius ennsi n. sp. is also similar to E. obispoensis Aponte & McMurtry, 1997 especially for the strong reticulation of the dorsal shield. The latter differs for: having r3 always and R1 occasionally on dorsal shield; most dorsal shield setae as long as to up to 20% longer except z4 and s4, about 20% shorter; and ventrianal shield strongly reticulate in preanal region and light reticulation in anal region. The new species also resembles E. citri van der Merwe & Ryke, 1964 and E. citrifolius Denmark & Muma, 1970; however, E. citri has calyx of spermatheca slightly bulged near atrium and flaring near vesicle, whereas E. citrifolius has dorsal shield only lightly reticulate and macroseta of basitarsus IV distinctly bent. As the latter species, E. relictus Chaudhri, Akbar & Rasool differs from the new species by having macroseta of basitarsus IV (as well as macrosetae of genu and tibia IV) distally bent. Euseius vulgaris Liang & Ke, 1983 differs from the new species for having peritreme longer (reaching midway between insertions of j3 and z2, and ventrianal shield almost twice as wide at anus level than at level of Zv2.
This new species is also very closely related to E. vivax (Chant & Baker) and E. fructicolus (Gonzalez & Schuster) but differs from both in that the dorsal shield is completely reticulated and not smooth as in E. fructicolus and lightly reticulated as in E. vivax. They also differ from the new species in the shape of the tubelike calyx of the spermatheca, which is almost straight in the former two but bent or coiled in the new species. The macrosetae on leg IV of E. fructicolus are blunt distally but acute in the new species. Setae Z5 are serrated in the new species but smooth in both E. vivax and E. fructicolus. In the new species setae Z5 is about six times as long as j6 and J2 but about five times or less in E. vivax and E. fructicolus, respectively (Lopes et al., 2015).
Key to the Euseius species from citrus reported from Florida, USA
Euseius ho De Leon and E. brazilli (El-Banhawy) were synonymized with E. mesembrinus (Dean) (Lopes, et al., 2015). Additional information about the Euseius species from Florida can be found in Muma et al. (1970), Childers & Denmark (2011) and Denmark & Evans (2011). One of the species included in the key (E. ovalis (Evans, 1953)) represents a new record for Florida, collected on citrus trees in Fort Myers, Jupiter, Naples, Palm City and West Palm Beach between June 2012 and June 2013.
1. Setae r3 and R1 on dorsal shield; macrosetae only present on leg IV
...... Euseius sibelius (De Leon 1962)
— Setae r3 and R1 off dorsal shield (if on dorsal shield, with macrosetae on legs I–IV); macrosetae present at least on legs III and IV
...... 2
2. Setae r3 and R1 on dorsal shield; calyx of spermatheca short-tubular, less than three times as long as its largest diameter
...... E. urceus (De Leon 1962)
— Setae r3 and R1 off dorsal shield; calyx of spermatheca distinctly more than three times as long as its largest diameter
...... 3
3. Seta j3 less than half as long as j1; dorsal shield smooth over most of its extent, except anterolaterally (reticulate)
...... E. ovalis (Evans 1953)
— Seta j3 distinctly more than half as long as j1; ornamentation of dorsal shield variable
...... 4
4. Dorsal shield strongly reticulated; macrosetae of leg IV acute distally
...... E. ennsi n. sp.
— Dorsal shield not strongly reticulated; macrosetae of leg IV variable
...... 5
5. Macrosetae of leg IV acute distally
...... E. hibisci (Chant 1959)
— Macrosetae of leg IV blunt to spatulate
...... 6
6. Seta Zv1 well anteriad of Jv1.
...... E. victoriensis (Womersley 1954)
— Seta Zv1 about in transverse line with Jv1
...... E. mesembrinus (Dean 1957)
Acknowledgements
To Samuel Bolton, Florida Department of Agriculture and Consumer Services
Division of Plant Industry, for helping with the examination of the type specimens of E. quetzali. This work is based on the research supported wholly / in part by the National Research Foundation of South Africa (Grant Numbers 126938). Any opinion, findings and conclusions or recommendations expressed in the material are those of the authors and therefore the NRF does not accept any liability in regard thereto.
References
Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini II. Le relevé organotaxique de la face dorsale adulte (Gamasides Protoadénique, Phytoseiidae). Acarologia 17:20-29.
Carrillo D., De Coss M.E., Hoy M.A., Pena J.E. 2012. Variability in response of four populations of Amblyseius largoensis (Acari: Phytoseiidae) to Raoiella indica (Acari: Tenuipalpidae) and Tetranychus gloveri (Acari: Tetranychidae) eggs and larvae. BioControl, 60:39-45. doi:10.1016/j.biocontrol.2011.09.002
Carrillo D., Pena J.E. 2012. Prey-stage preferences and functional and numerical responses of Amblyseius largoensis (Acari: Phytoseiidae) to Raoiella indica (Acari: Tenuipalpidae). Exp. App. Acarol., 57:361-372. doi:10.1007/s10493-011-9488-7
Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidae). Part II. A Taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. Can. Entomol., 61(12): 1-166. doi:10.4039/entm9112fv
Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, Michigan, 219 pp.
Chant D.A., Yoshida-Shaul E. 1989. Adult dorsal setal patterns in the family Phytoseiidae (Acari: Gamasina). International Journal of Acarology, 15, 219-233. doi:10.1080/01647958908683852
Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). International Journal of Acarology, 17: 187-199. doi:10.1080/01647959108683906
Childers C.C., Denmark H.A. 2011. Phytoseiidae (Acari: Mesostigmata) within citrus orchards in Florida. Species distribution, relative and seasonal abundance within trees, associated vines and ground cover plants. Exp. App. Acarol., 54: 331-371. doi:10.1007/s10493-011-9449-1
Childers C.C., Rogers M.E., Ebert T.A., Achor D.S. 2017. Diptilomiopus floridanus (Acari: Eriophyoidea: Diptilomiopidae): its distribution and relative abundance with other eriophyoid species on dooryard, varietal block and commercial citrus in Florida. Fla. Entomol., 100 (2): 325-333. doi:10.1653/024.100.0230
Congdon B.D., McMurtry J.A. 1986. The distribution and taxonomic relationships of Euseius quetzali McMurtry in California (Acari: Phytoseiidae). Intern. J. Acarol., 12(1): 7-11. doi:10.1080/01647958608683433
Dean H.A. 1957. Predators of Oligonychus pratensis (Banks) (Tetranychidae). Ann. Entomol. Soc. Amer., 50: 164-165. doi:10.1093/aesa/50.2.164
Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R. C. 2020. Phytoseiidae Database. Available from: www.lea.esalq.usp.br/phytoseiidae
 (accessed 11/09/2019).
(accessed 11/09/2019).Denmark H.A., Evans G. 2011. Phytoseiidae of North America and Hawaii (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, Michigan, USA. 451 pp.
Evans G.O. 1953. On some mites of the genus Typhlodromus Scheuten, 1857, from S.E. Asia. Ann. Mag. Nat. Hist., 6: 447-467. doi:10.1080/00222935308654444
Hodgson R.W. 1967. Horticultural varieties of citrus. Chapter 4. In: Reuter W., Webber H.T. and Batchelor L.D. (eds). The citrus Industry. Vol. 1. History, World Distribution, Botany, and Varieties. University of California, Press, 614 pp.
Hoy M.A. 2011. Agricultural Acarology. Introduction to Integrated Mite Management. CRC Press, Boca Raton, 410 pp.
Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entomol. Soc. Can., 47: 1-64. doi:10.4039/entm9747fv
Lopes P.C., McMurtry J.A., Moraes G.J. de. 2015. Definition of the concordis species group of the genus Euseius (Acari: Phytoseiidae), with a morphological reassessment of the species included. Zootaxa, 4048(2): 174-190. doi:10.11646/zootaxa.4048.2.2
McMurtry J.A., Badii M.H., Congdon B.D. 1985. Studies on Euseius species complex on avocado in Mexico and Central America, with a description of a new species (Acari: Phytoseiidae). Acarologia, 26(2): 107-116.
McMurtry J.A., Sourassou N.F., Demite P.R. 2015. The Phytoseiidae (Acari: Mesostigmata) as biological control agents. In: Carrillo D., Moraes G. J. and Pena F. J. (Eds.). Prospects for biological control of plant feeding mites and other harmful organisms. Springer International Publishing.: 133-149. doi:10.1007/978-3-319-15042-0_5
Muma M.H., Denmark H.A., De Leon D. 1970. Phytoseiidae of Florida. Arthropods of Florida and neighbouring land areas, 6. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville, 150 pp.
Muma M.H. 1975. Mites associated with citrus in Florida. Univ. Fla. Bull., 640A: 92 pp.
Rowell H.J., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). The Can. Entomol., 110, 859-876. doi:10.4039/Ent110859-8
Schicha E. 1987. Phytoseiidae of Australia and neighbouring areas. Indira Publishing House, West Boomfield, Michigan, USA, 187 pp.
Van der Merwe, G.G., Ryke, P.A.J. 1964. The subgenus Typhlodromalus Muma of the genus Amblyseius Berlese in South Africa (Acarina: Phytoseiidae). Journal Ent. Soc. S. Africa. 26(2): 263-289.
Walter D.E., Krantz J.W. 2009. Collecting, rearing, and preparing specimens. In: Krantz J.W. and Walter D.E. (eds.). A Manual of Acarology. Third Edition. Texas Tech University Press, 807 pp.



2020-07-29
Date accepted:
2020-11-25
Date published:
2020-11-27
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Ueckermann, Edward A.; Moraes, Gilberto J. de and Childers, Carl C.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



