Oribatid mites (Acari: Oribatida) from the Sella massif (Dolomites, Trentino, Italy) with description of Trichoribates valeriae n. sp. (Ceratozetidae)
Schatz, Heinrich1
1✉ c/o Institute of Zoology, Technikerstrasse 25, A-6020 Innsbruck, Austria
2020 - Volume: 60 Issue: 4 pages: 842-862
https://doi.org/10.24349/acarologia/20204405ZooBank LSID: 52425B43-57A2-40C7-A407-9425E7606898
Original research
Keywords
Abstract
Introduction
The Sella group is a massif in the Dolomites, Northern Italy. The highest elevation is Piz Boè (3152 m a.s.l., Fig. 1A) at the intersection of South Tyrol, Trentino, and Veneto (Prov. Belluno). The Sass Pordoi (2950 m a.s.l., Fig. 1B), a plateau-shaped summit, is situated next to and west of Piz Boè. Both mountains are very popular touristic destinations, a funicular from the Pordoi Pass reaches the summit of Sass Pordoi. The isolated mountain ranges are fossil coral reefs of a Middle Triassic carbonate platform from the Raibl formation. It preserves fossils dating back to the Norian Middle Triassic sub-period during the Mesozoic Era (Reithofer 1928, Moroder 2008). Only small vegetation patches are scattered on karstic limestone.
During two excursions in 2017 soil and litter samples were taken on Sass Pordoi and the Piz Boè massif. A commented list of the found oribatid mite species is presented, and a species new to science is described. Results of sampling at the nearby Sella Pass on the edge of the Langkofel group (Gruppo di Sassolungo) are already published (Schatz 2017).
Materials and methods
Material examined
Figure 1
The material was collected during two excursions of the author and Irene Schatz on Sass Pordoi (2932 – 2940 m a.s.l.) and Piz Boè (2980 – 3150 m a.s.l.). A total of 10 soil and litter samples (each ca. 10 x 10 cm, volume ca. 0,5 liter) were taken in vegetation patches within the cushion plant zone, keeping the environmental impact as low as possible. The samples were extracted for 12 days with moderate heating with light, preservation fluid was 75% ethanol. Morphological investigations were carried out in temporary slides in lactic acid.
List of samples
• TN 157: 20. June 2017: Sass Pordoi, near cable car station, water logged with snowmelt, wet moss cushion (46°30.218'N, 11°48.279'E, 2935 m a.s.l., Fig. 1B).
• TN 159: 20. June 2017: Sass Pordoi, ibid., dry Cerastium uniflorum cushion from preceding year (46°30.218'N, 11°48.279'E, 2935 m a.s.l., Fig. 1F).
• TN 160: 20. June 2017: Sass Pordoi, ibid., dry Cerastium uniflorum cushion from preceding year (46°30.218'N, 11°48.279'E, 2935 m a.s.l.).
• TN 161: 20. June 2017: Sass Pordoi, ibid., moist moss cushion between rocks (46°30.059'N, 11°48.386'E, 2935 m a.s.l.).
• TN 162: 19. July 2017: Piz Boè, southeast of summit, moist moss on rock (46°30.555'N, 11°49.697'E, 3149 m a.s.l., Fig. 1C).
• TN 163: 19. July 2017: Piz Boè, ibid., moist Cerastium uniflorum cushion (46°30.555'N, 11°49.697'E, 3150 m a.s.l., Fig. 1D).
• TN 164: 19. July 2017: Piz Boè, base of summit area, dry to moist Cerastium uniflorum cushion with humus and sand (46°30.383'N, 11°49.522'E, 2980 m a.s.l., Fig 1E).
• TN 165: 19. July 2017: Piz Boè, ibid., dry to moist Saxifraga oppositifolia cushion with moss and humus (46°30.383'N, 11°49.522'E, 2980 m a.s.l., Fig. 1E).
• TN 166: 19. July 2017: Sass Pordoi, near cable car station, below water logged (near TN157), moist to wet Carex grass cushion with humus (46°30.068'N, 11°48.455'E, 2932 m a.s.l., Fig. 1B).
• TN 167: 19. July 2017: Sass Pordoi, ibid., 2940 m, dry to moist Cerastium uniflorum cushion with humus (46°30.059'N, 11°48.386'E, 2940 m a.s.l.).



Identification
Measurement of each parameter was done from the particular optimal parallax-free perspective. The total body length was measured in lateral view, from tip of the rostrum to the posterior edge of the notogaster, body width as maximal width of notogaster from dorsal view (without pteromorphs). Indicated are means, range in brackets. All measurements are given in micrometers (µm). The systematic arrangement follows Schatz et al. (2011), the terminology of morphological features that of F. Grandjean (summarized by Travé et al. 1996, Norton and Behan-Pelletier 2009), in the descriptive part of the Trichoribates species also that of Behan-Pelletier and Ermilov (2019).
Following abbreviations are used: L—length, W—width, no—nose-like protuberance on rostrum, ro, le, il, bo, ex—rostral, lamellar, interlamellar, bothridial, exobothridial seta, tu—tutorium, gt—genal tooth, pt I, pt II—pedotectum I, II, Ad—dorsal porose area, Am, Ah, Al—anterior dorsolateral porose areas, Dp—dorsophragma, Pp—pleurophragma, len—lenticulus, c1-3, d1-3, e1, e2, f1, f2, la, lm, lp, da, dm, dp, h1-3, p1-3—notogastral seta, Aa, A1, A2, A3—notogastral porose areas, ap—postanal porose area, ia, im, ih, ip, ips—notogastral lyrifissures, gla—opithonotal gland opening, opisthonotal gland region in immatures, cus—custodium, dis—discidium, cp—circumpedal carina, 1a-c, 2a, 3a-c, 4a-c—epimeral setae, ad1-3—adanal setae, po—preanal organ, iad—adanal lyrifissure, cha, chb— cheliceral setae, σ, φ, φ1, φ2, ω, ω1, ω2—solenidia on genu, tibia, tarsus, pa—porose area on leg, PY—pygidial sclerite, ho—humeral organ.
Results
Species list
Family Brachychthoniidae Thor, 1934
Taxonomical notes — The three Liochthonius species found on Biz Boè are part of the ''Lapponicus-group'' (Moritz 1976) with common features as double-pointed sensillus, small velum on relatively short dorsal setae, and within this group they are separated from other members in having setae c1 – c1 slightly more distant than d1 – d1. They were distinguished by following characters:
• L. strenzkei: with small transverse crest anteriorly of setae le, notogastral setae f1, h1 on separate tubercles (not always easily visible).
• L. lapponicus: without tubercles on pygidium, without transverse crests near setae le.
• L. sellnicki: with transverse crests anteriorly and between setae le, with transverse crests posterior of setae f1, h1.
Liochthonius lapponicus (Trägårdh, 1910)
Moritz 1976: p. 69, fig. 15a, b. Schatz 2004: p. 347. Weigmann 2006: p. 78, fig. 41e, f.
Piz Boè — TN 164: 4 adults, TN 165: numerous specimens.
General distribution — Holarctic. Recorded also at nearby Sella Pass (Schatz 2017).
Liochthonius sellnicki (Thor, 1930)
Moritz 1976: p. 76, fig. 17 a, b. Schatz 2004: p. 48. Weigmann 2006: p. 78, fig. 41 c, d.
Piz Boè — TN 165: numerous specimens.
General distribution — Holarctic, Oriental (China).
Liochthonius strenzkei Forsslund, 1963
Moritz 1976: p. 80, fig. 18 a, b. Weigmann 2006: p. 78, fig. 41 a, b.
Sass Pordoi — TN 159: 3 adults, TN 160, TN 161: numerous specimens.
Piz Boè — TN 162, TN 163, TN 164, TN 165: numerous specimens in each sample.
General distribution — Holarctic, Oriental (China). Recorded also at nearby Sella Pass (Schatz 2017).
Family Crotoniidae Thorell, 1876
Camisia foveolata Hammer, 1955
Hammer 1955: p. 8, fig. 1. Colloff 1993: p. 1368, figs 22-24. Seniczak 1991b: p. 334, figs 3, 4, 6, 8.
Sass Pordoi — TN 157: 7 protonymphs, 1 tritonymph. TN 161: 9 adults, 11 larvae, 40 protonymphs, 20 deutonymphs, 13 tritonymphs.
Taxonomical notes — Setae le on short apophyses in all instars, in larva weakly spinose, 15 – 17, in other instars thick, spinose, legs monodactylous in all juvenile instars, tridactylous in adult; larva – size 340 – 360 x 160 – 170, le 15- 17; protonymph – size 440 – 510 x 220 – 250, le 20 – 25, 5 – 6 pairs of genital setae; deutonymph – size 530 – 550 x 280 – 300, le 25 – 30, 14 pairs of genital setae; tritonymph – size 670 – 685 x 345 – 370, le 30 – 40, 17 – 18 pairs of genital setae; adult – size 670 – 750 x 310 – 370, le 30 – 40, 17 – 18 pairs of genital setae.
Remarks — The specimens from Sass Pordoi correspond widely to the redescription by Colloff (1993) and are considered conspecific. The juvenile instars correspond to the morphological description by Seniczak (1991b). Some variabilities are noteworthy:
• shape of setae le: frequently bent mediad in different intensity, but also almost straight in some specimens, length 30-40 (vs. Colloff 1993 "not curved into arch", and Hammer 1955). Seniczak (1991b) illustrated setae le also bent mediad.
• thick and continuous transversal line between the apophyses of setae le as in Hammer (1955) (vs. weak or interrupted in Colloff 1993 and Seniczak 1991b).
• microsculpture between prodorsal ridges fine, punctate, forming small irregular lines (vs. areolate/scalloped in other descriptions).
• The asymmetrical number of genital setae as mentioned from Seniczak (1991b) could also be observed in some specimens of protonymphs, tritonymphs and adults.
This mosaic of differences of morphological characters in far distant populations do not justify the status of a separate species for the population in the Dolomites.
General distribution — Northern Holarctic (Alaska, Yukon, Greenland, Svalbard, Scandinavia), Chilenian Highland. New record for Central Europe, the Alps and Italy.
Camisia horrida (Hermann, 1804)
Colloff 1993: p. 1381, figs 30-32. Seniczak 1991a: pp. 270 ff., figs 3, 4, 6, 11, 12. Weigmann 2006: p. 153, fig. 80a.
Piz Boè — TN 164: 2 adults, 1 protonymph, 2 deutonymphs, TN 165: 3 adult specimens.
Taxonomical notes — The juvenile instars correspond to the description by Seniczak (1991a) and were allocated to the respective instars according to body size and number of genital setae (protonymph – size 470 – 360, 1 pair of genital setae, deutonymph – size 540 – 550 x 310 – 330, 4 pairs of genital setae).
General distribution — Holarctic, Oriental, Ethiopian, Neotropical (Central America).
Remarks — Schweizer (1956) reported C. horrida frequently from the alpine zone in Switzerland up to 3109 m a.s.l. and considered it as a boreo-alpine species. In South Tyrol it was already found in the Dolomites in the nearby Schlern/Sciliar massif at 2200–2560 m a.s.l. (Schatz 2008a), and in the North-Tyrolean Central Alps (Austria) in the alpine grassland up to 2650 m a.s.l. (Schatz 1979). Camisia horrida was also reported from higher altitudes of Central America (Costa Rica: Volcán Irazú, 3400 m, Volcán Chirripó, 3800 m; Panama: Volcán Barú, 3400–3475 m a.s.l., Schatz 2006).
Family Damaeidae Berlese, 1896
Kunstidamaeus lengersdorfi (Willmann, 1932)
Belba lengersdorfi Willmann 1932: p. 5, figs 4 – 6. Kunstidamaeus lengersdorfi Weigmann 2006, p. 191, fig. 100a. Kunstidamaeus lengersdorfi Miko and Mourek 2008: p. 6, figs 1 – 12.
Piz Boè — TN 165: 3 adult specimens.
Taxonomical notes — Measurements L 740 – 770, W 450, c1 85, c2 60-65, la 60-65, lm 43, lp 42, h1-3 \textasciitilde45-48, p1-3 40-45 (thinner than others). leg IV 1150-1200. Spinae adnatae bent laterad 90-100°, distal part shorter than proximal stem.
Remarks — The specimens from Piz Boè correspond to the redescription by Miko and Mourek (2008) and are considered conspecific. The relatively short distal parts of spinae adnatae vary. Willmann (1932, fig. 4) drew spinae adnatae with long distal part, apex slightly bent anteriad (''lange, geschweifte Spitze''). In Weigmann (2006, fig. 100a) the spinae adnatae also show a long distal part which is curved outward and directed more than 100° posteriad. In specimens from Harz (Miko and Mourek 2008, fig. 1A) the spinae adnatae are similarly bent as in the figure of Weigmann, in specimens from Slovak Karst) Miko and Mourek (op. cit.), fig. 2A) the spinae adnatae show a short distal part and are bent laterad to different degrees.
General distribution — Central Europe, frequently in caves. New record for the Dolomites and Italy.
Family Tectocepheidae Grandjean, 1954
Tectocepheus sarekensis (Trägårdh, 1910)
Pérez-Íñigo 1997: pp. 276f., fig. 110 C, D. Weigmann 2002: pp. 141 ff., figs 1, 2. Weigmann 2006: p. 255, fig. 137d, e, f. Laumann et al. 2007: pp. 113 ff., fig. 1c1-c4.
Sass Pordoi — TN160: 1 adult, TN 166: 1 protonymph.
Piz Boè — TN 163: 1 adult, 11 juvenile instars, most larvae, TN 164: \textgreater170 adults, numerous juvenile instars, TN 165: \textgreater50 adults, numerous juvenile instars.
Taxonomical and nomenclatorial notes — The specimens from the different samples on Sass Pordoi and Piz Boè show a mosaic of morphological characters between T. velatus and T. sarekensis. But they all are considered to be T. sarekensis based on characters given by Weigmann (2002, 2006) and Laumann et al. (2007) in having mainly broad and rounded cusp tip (with some exceptions), longitudinal striae on the interlamellar field (all specimens), and 2 – 4 pairs of notogastral depressions, the latter sometimes fainter (Table 1). The body length is slightly larger than in most other investigations (250 – 390 vs. 302 – 349 – Laumann et al. 2007, 312 – 374 – Pérez-Íñigo 1997, 295 – 362 – Weigmann 2002). The small granules of the specimen in TN 160 remind of T. alatus Berlese, 1913, but in that species the granules are larger, and small granules on the cerotegument can be found in other Tectocepheus species too. Weigmann (2002, 2006), Subías (2004) and other authors list T. sarekensis as subspecies of T. velatus (Michael, 1880); I follow Laumann et al. (2007) in considering it a distinct species.



General distribution — cosmopolitan excl. Antarctic. Recorded also at nearby Sella Pass (Schatz 2017).
Family Achipteriidae Thor, 1929
Anachipteria shtanchaevae Subías, 2009
Anachipteria alpina Weigmann 2006: p. 353, fig. 188e. Anachipteria shtanchaevae Subías 2009: 79.
Piz Boè — TN 164 26 adults, TN 165: 12 adults, juvenile instars in both samples.
Nomenclatorial notes — New name for Oribata tecta alpina Schweizer, 1922 nec Halbert.
General distribution — Alps, Central, South, Southeast Europe. Mainly in montane to alpine habitats.
Family Oribatulidae Thor, 1929
Oribatula interrupta (Willmann, 1939)
Zygoribatula interrupta Willmann 1939: p. 450, fig. 179. Oribatula interrupta Seniczak et al. 2012: p. 2, figs 1, 2, 3A, 4 – 8. Weigmann 2006: p. 434, fig. 232f, g.
Piz Boè — TN 164: 46 adults, TN 165: 340 adults, numerous juvenile instars in both samples.
General distribution — Holarctic, Ethiopian. In the Alps frequently in montane to alpine habitats. Recorded also at nearby Sella Pass (Schatz 2017).
Family Ceratozetidae Jacot, 1925
Trichoribates valeriae n. sp.
ZOOBANK: 5E466591-43EA-4CCA-806C-225A81E7608F ![]()
Sass Pordoi — TN 159: 52 adults, numerous immatures, TN 160: 152 adults, numerous immatures, TN 161: 1 adult, TN 167: 2 adults.
Piz Boè — TN 162: 2 adults, numerous immatures, TN 163: 18 adults, numerous immatures, TN 164: \textgreater300 adults, numerous immatures, TN 165: 52 adults, numerous immatures.
Diagnosis
Trichoribates valeriae n. sp. differs morphologically from other Trichoribates species by the following combination of characters: Total length of males 550-620 µm, females 595-670 µm, rostrum rounded with nose-like protuberance, lamella and translamella small ridges, cusps relatively wide apart, teeth on cusp minute or absent, lamellar and interlamellar setae long, setiform with small bristles, bothridial seta with small club-shaped head, tutorium large, anterior notogastral tectum present, lenticulus indistinct, triangular, 10 pairs of medium long notogastral setae, setiform with small bristles, four pairs of round or oval notogastral porose areas, A1 divided in two parts, epimeral setal formula: 3–1–3–3. Small porose areas present on tibiae and tarsi I–IV. Nymphs with large pygidial sclerite, humeral organ absent in larva, present in nymphs. Larva with 12 pairs of gastronotal setae, nymphs with 15 pairs each.
Description
Adult
Figures 2–7
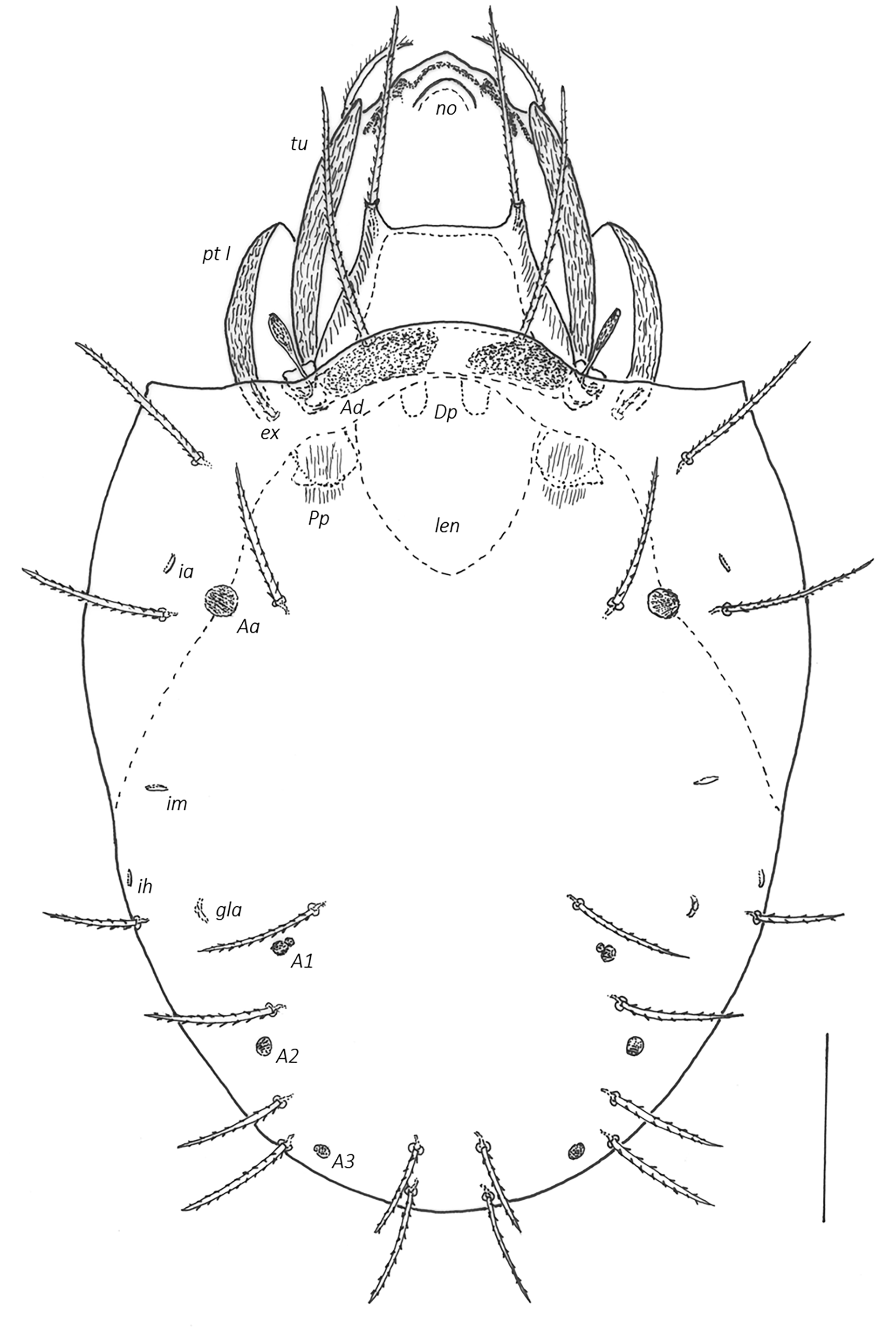





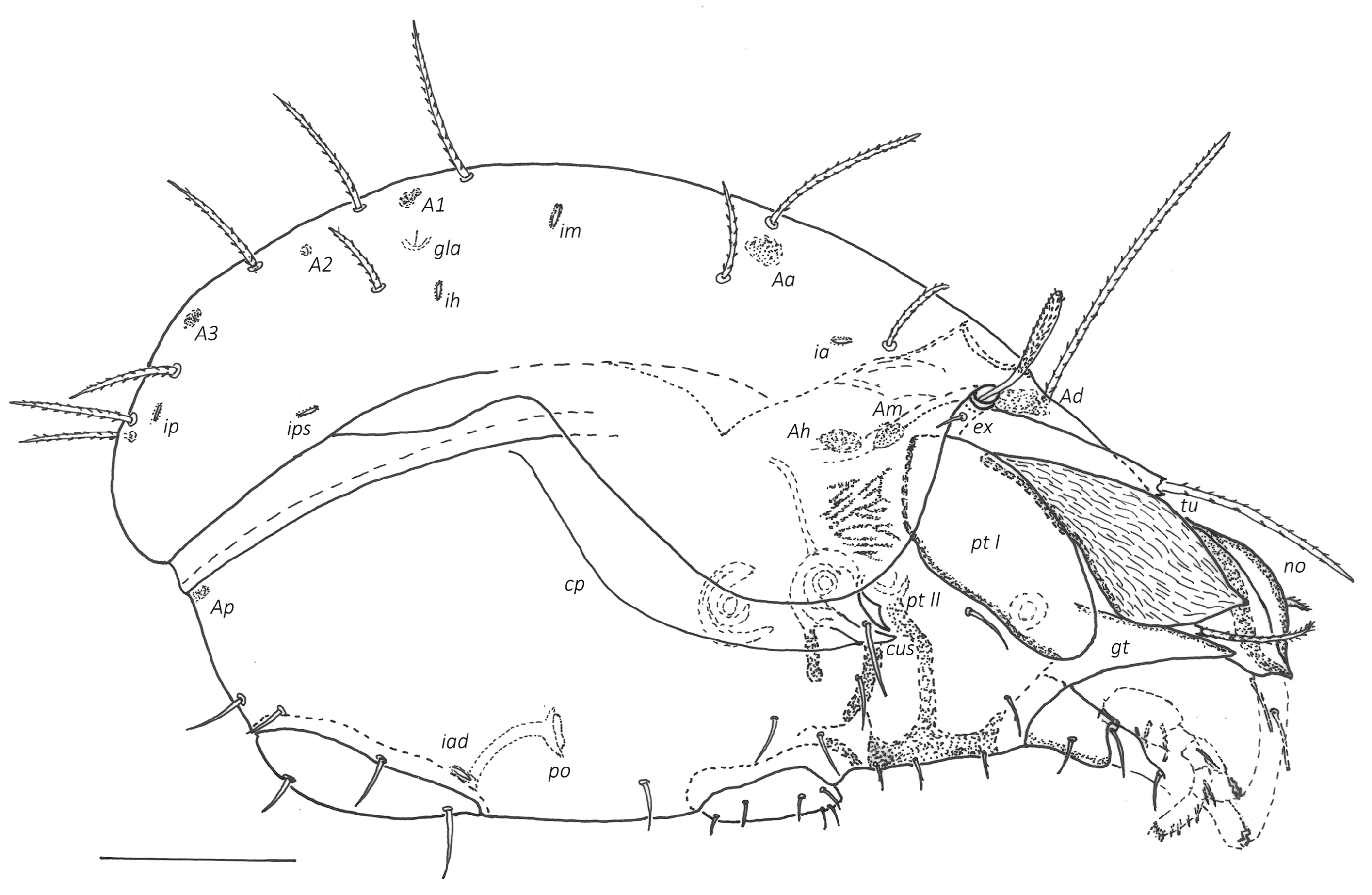


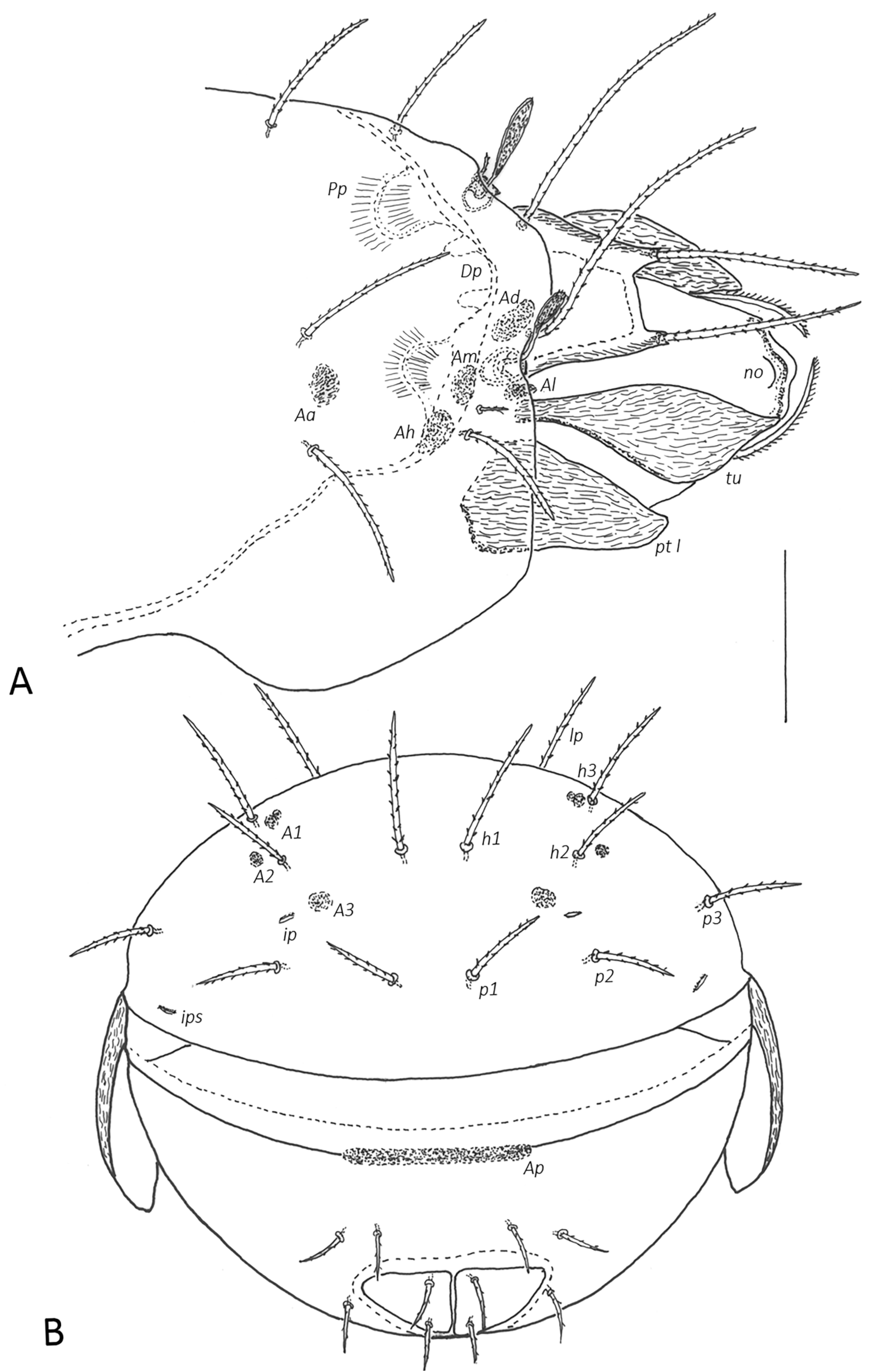


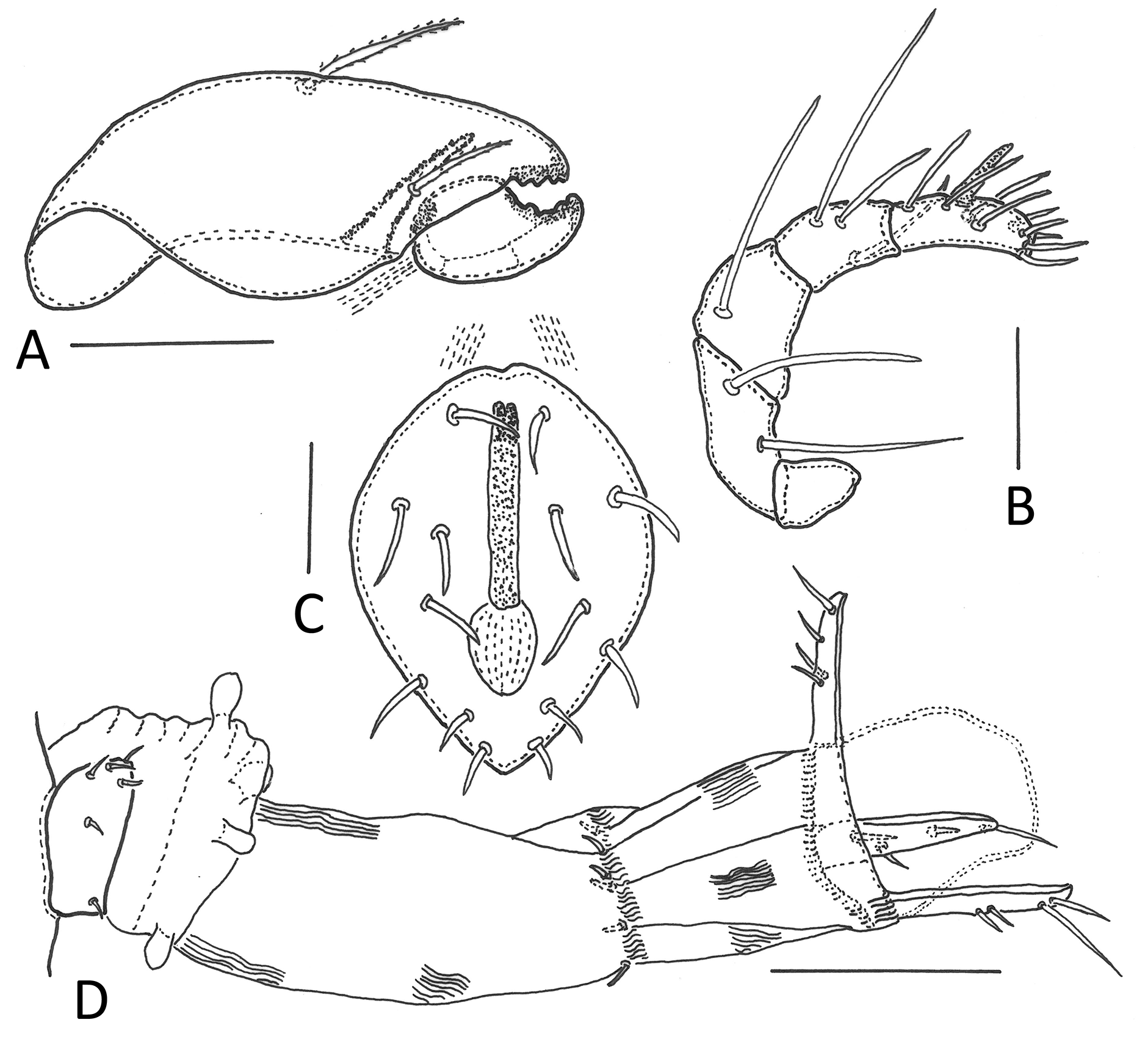


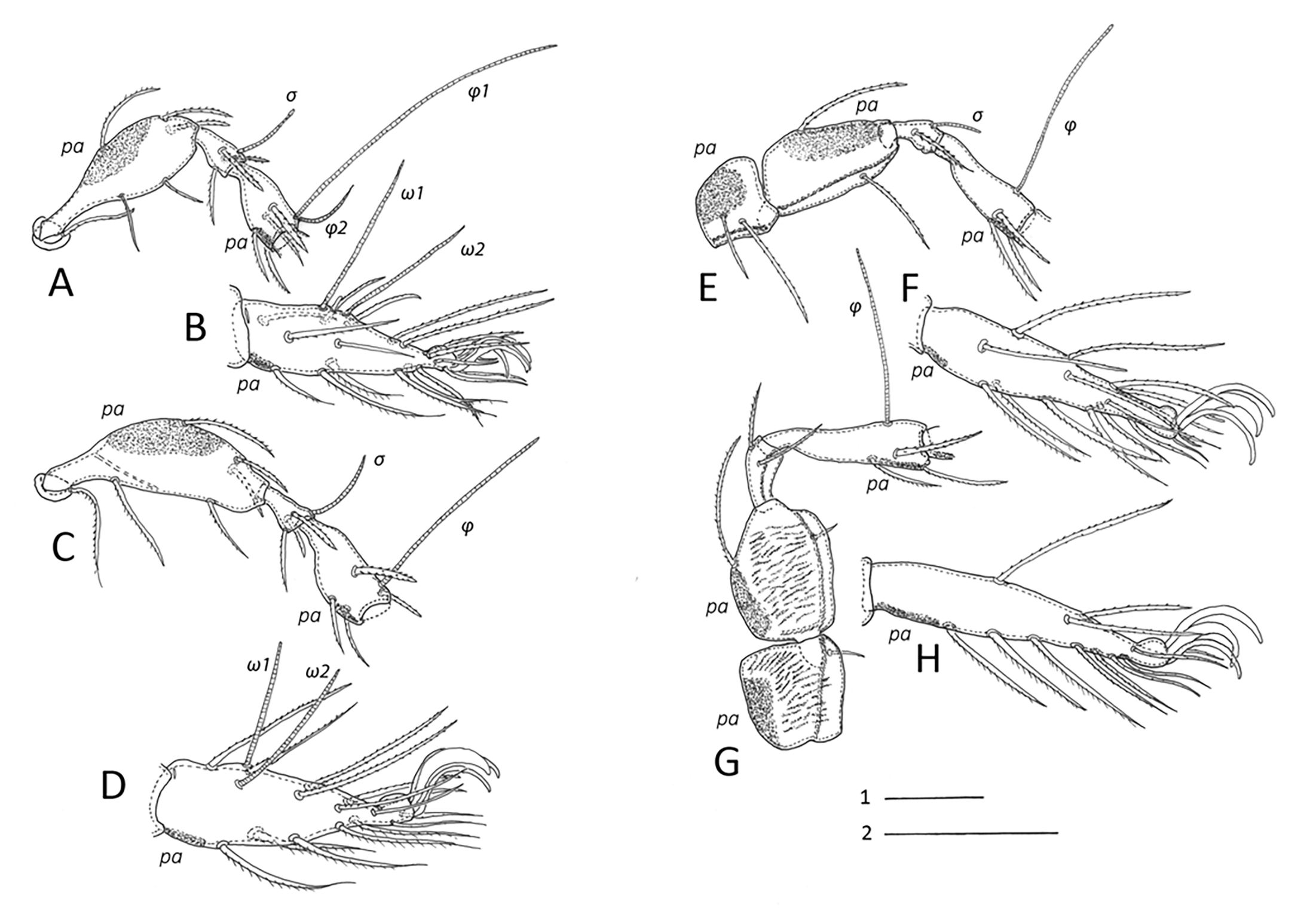


Measurements — females (n=42): L 631 (595–670), W 379 (350–410), males (n=24): L 594 (550–620), W 342 (310–360).
Integument — Body colour light brown. Dorsal and ventral part of body finely punctured.
Prodorsum (Figs 2–5A) — Rostrum broadly rounded in dorsal view, rarely with slightly anteriad projecting central part, with nose-like protuberance dorsally (no, about 18–22 elevated above prodorsal shield). Below front edge of rostral tectum a transverse trilobate thickening, lateral lobes with round or hornlike protuberances. Lamella small ridge, translamella small, about twice length (\textasciitilde50) as cusp (\textasciitilde25), lamellar cusp shorter than lamella, small, teeth beside root of le minute or absent. Rostral (80–100), lamellar (120–135) and interlamellar (150–180) setae setiform with small bristles, bristles on antiaxial part of rostral seta slightly longer (up to 3), basal part of rostral seta covered by tutorium in dorsal view. Tutorium (tu) large, broad, anterior tip pointed (rarely with two small teeth), antiaxial side of tutorium with rough longitudinally striae. Bothridium partially or completely covered by anterior notogastral margin, without medial or lateral scales. Bothridial seta with short stalk (25–30) and small spoon-shaped head (length 25–30, width \textasciitilde15), with pointed apex and short spines, directed anterolaterad. Exobothridial seta (25–30) setiform with small bristles. Dorsosejugal porose area Ad large, medial to bothridium under anterior notogastral tectum. Internal apodemes dorsophragmata (Dp) and pleurophragmata (Pp) distinct, dorsophragmata small and short, close to each other, pleurophragmata larger, posterior to bothridia, muscle fibers visible through integument.
Notogaster (Figs 2, 4, 5) — Anterior margin bent anteriad forming broad notogastral tectum between pteromorphs. Lenticulus indistinct, triangular. Pteromorphs without hinge, immovable, broadly rounded laterally. Ten pairs of notogastral setae, all setiform with small bristles, series c, l, h longer (80–100), p1-3 slightly shorter (60–80). Four pairs of porose areas, round to oval in shape, Aa largest (15–17 x 20–23), A1–A3 smaller (6–8 x 10–12), A1 divided in two parts, lateral area larger, medial smaller. Lyrifissures (ia, im, ip, ih, ips) small, distinct, best visible in lateral view. Opisthonotal gland (gla) opening located laterad to seta lp.
Gnathosoma (Figs 6A, 6B) — Subcapitulum diarthric. Mentum scutiform (85–90 x 85–90). Genal tooth narrow, anteriorly pointed. Subcapitular setae setiform with small bristles, seta h straight (25–30), m curved (\textasciitilde30), a almost smooth (19–22). Adoral setae slightly ciliate (18–22). Palp (\textasciitilde80), setal formula 0-2-1-3-9(+ω). Axillary saccule at base of rutellum present, small. Chelicera (145–155) with two setae, both setiform with bristles, cha (47–50) longer than chb (28–33), movable digit robust, length 40–50, with 4 strong teeth. Trägårdh's organ on paraxial face, tapering anteriad (45–50).
Lateral aspect of podosoma, epimeral region (Figs. 3, 4, 5A) — Pedotectum I (Pt I) large scale, dorsally convex, anteriorly rounded, with small longitudinal striae. Pedotectum II (Pt II) small shell-shaped scale. Horizontal folds present in integument dorsal of acetabula II and III. Discidium (dis) well developed, triangular, projecting laterally. Circumpedal carina (cp) merging into custodium. Custodium (cus) short, pointed anteriad. Humeral porose areas Am, Ah, Al indistinct (latter only visible in dorsolateral view, see Fig. 5A, or in dissected specimens). Epimeral setae setiform with small bristles, epimeral setal formula 3–1–3–3. length 1a, 2a, 3a, 4c (originating on base of discidium) 18–23, 1b, 3b, 4a, 4b 25–30, 1c, 3c (distal of custodium) 40–45. Apodemes blade-like, well sclerotized.
Anogenital region (Figs 3, 4, 5B, 6C, 6D) — All genito-anal setae setiform with small bristles, genital plates with 6 pairs (14–18), 2 anterior pairs situated side by side on anterior thickening of genital plate, 1 pair of aggenital (27–30), 2 pairs of anal (20–23), 3 pairs of adanal setae (ad1 35, ad2, ad3 20–23). Adanal lyrifissures (iad) short, adjacent and parallel to anal plates, level with their anterior half. Preanal organ (po) cup-shaped.
Legs (Fig. 7) — Legs of moderate length (42 to 50 % of body size), leg I (including claws) \textasciitilde260, leg II \textasciitilde270, leg III \textasciitilde260, leg IV \textasciitilde310. All legs tridactylous, lateral claws thinner than medial claw. All femora with ventral thickening, trochanter and femur IV with transverse wrinkles and strong bladelike ventral keel each. Genua I and II distally with ventral spine. Solenidia ω1 and ω2 on tarsi I almost equal in length (95–100), φ1 on tibia I longest (140–160), φ2 shorter (30–40), σ on genu I about same length (35–40). Setae l'' on genua and tibiae I and II considerably thickened (40–45). Almost all setae with short bristles, setae (p) and (u) with very short bristles, seta v on trochanter IV smooth. Setal formula of legs (trochanter to tarsus, solenidia in parentheses): leg I 1 – 5 – 3(1) – 4(2) – 20(2), leg II 1 – 5 – 3(1) – 4(1) – 15(2), leg III 2 – 2 – 1(1) – 3(1) – 15, leg IV 1 – 2 – 2 – 3(1) – 12. Position and length of setae as in Fig. 7. Large porose areas (pa) on all femora and on trochanters III, IV. Porose areas on legs proximoventrally on tarsi I–IV and distoventrally on tibiae I–IV, all small and roundish to elliptical in shape.
Immatures
Figures 8 and 9
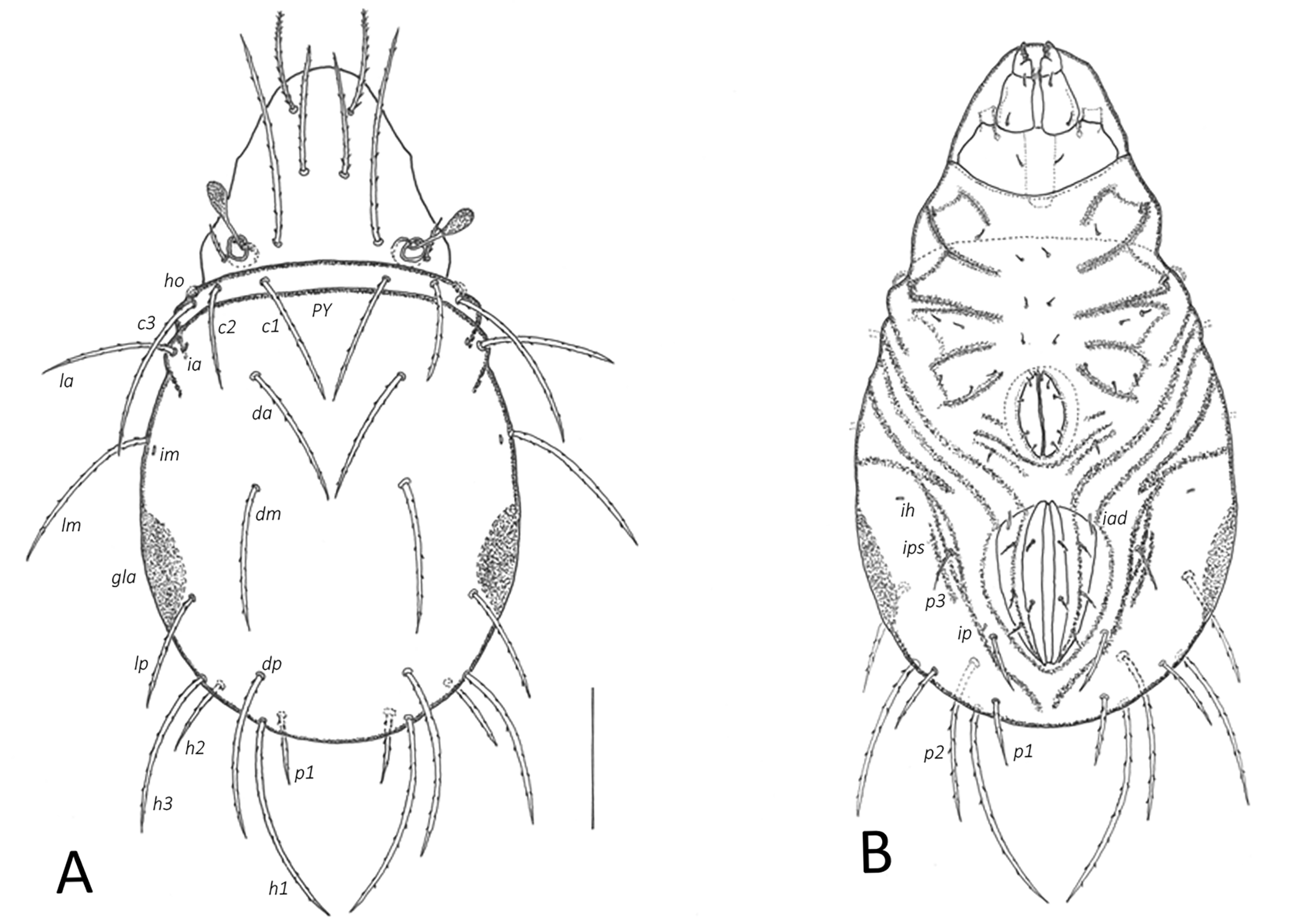


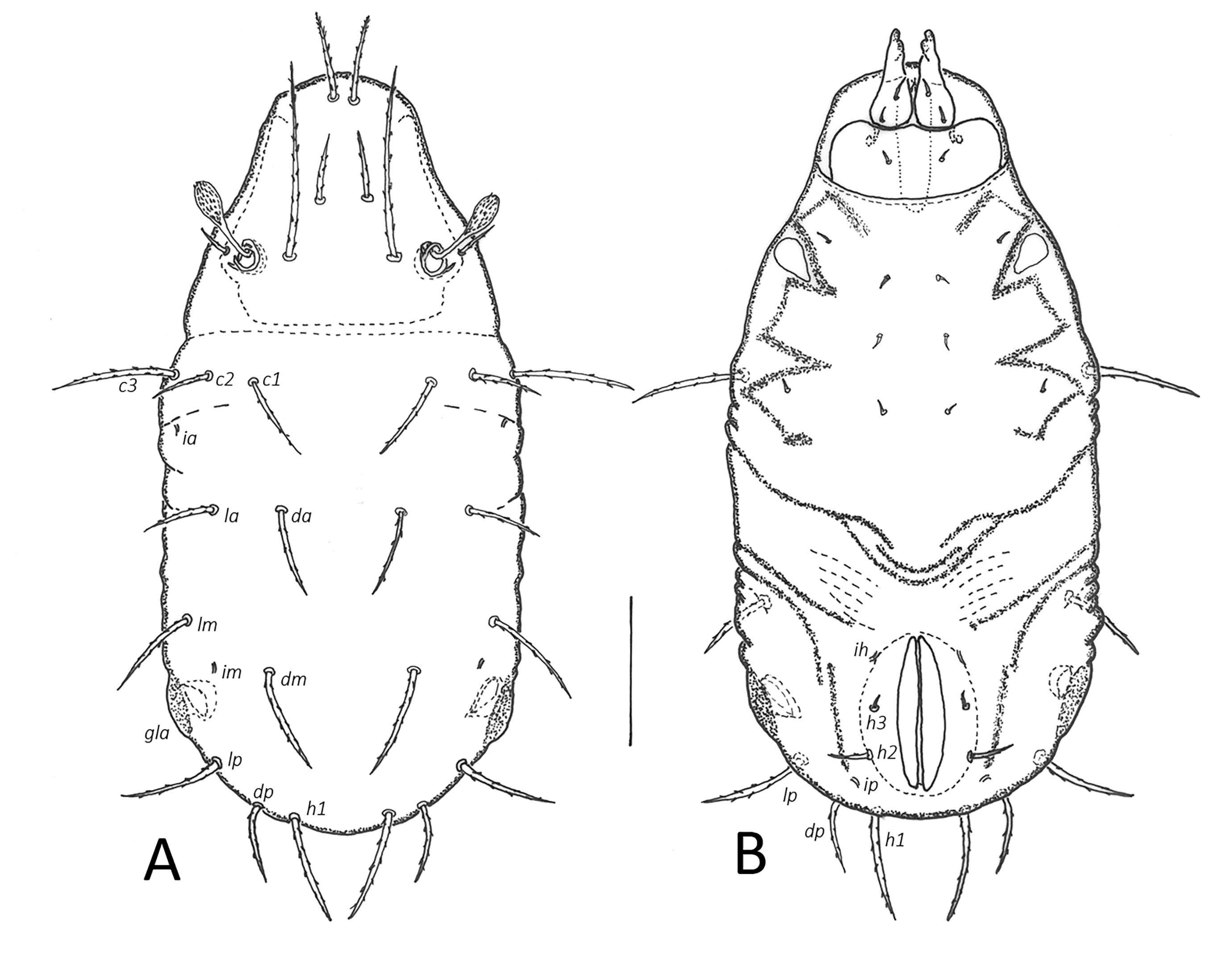


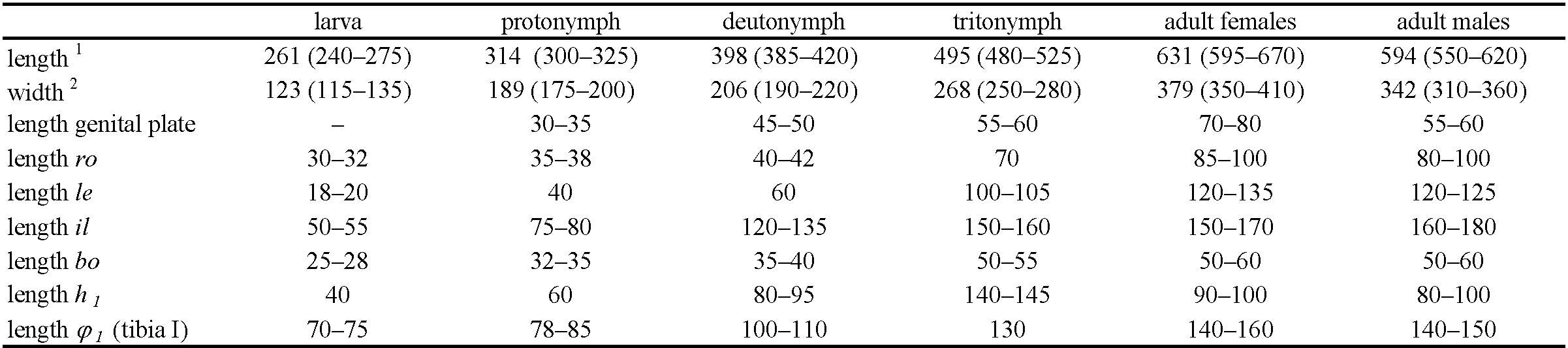
Measurements — Table 2.
Integument — Cuticle pale yellowish in larva, darker greyish-yellow in nymphs. Pygidial sclerite (PY) micropunctate in all nymphal instars. Opisthonotal gland region dark brown. Dorsal part of gastronotum without wrinkles, laterally with small furrows, ventrally with some furrows leading from position of setae la and lm posteriad around anal plates in all immature instars, and around genital plates in nymphs.
Prodorsum — Rostrum broadly rounded. Prodorsal setae setiform with short bristles. Interlamellar setae longest in all instars. Bothridial seta (bo) with club-shaped head and short spines in all instars, size according to the size of the instar (Table 2).
Gastronotic region — Generally weakly sclerotized. Setae of series c without small sclerites in larva, setae of series c, ps2, ps3 on small sclerites in nymphs. Pygidial sclerite in larva indicated by lateral furrows posterior to seta c3. Nymphs with large pygidial sclerite bearing setae of l, d, h series and p1 (latter on edge of the sclerite), anterior region with c series and opisthonotal regions separated. Humeral organ (ho) absent in larva, in nymphs present as protruding papilliform cupule. Larva with 12 pairs of gastronotal setae (c, l, d, h series, seta h3 lateral to medial part of anal plates), nymphs with 15 pairs each (including p series), all setiform with short bristles, most setae of similar length except shorter h2, h3 in larva as well as h2 and p series shorter in nymphs. Lyrifissures expressed as small cupules, ia, im visible on dorsal side, others on ventral side. Lateral sclerite surrounding opisthonotal gland opening dark colored.
Ventral region — Ontogenetic development of epimeral / anogenital setae: larva 3–1–2 / 0–0–0, protonymph 3–1–2–1 / 1–0–0–0, deutonymph 3–1–2–2 / 3–1–0–3, tritonymph 3–1–3–3 / 5–1–2–3. Setae setiform, short, slightly barbed. In larva large scale in place of Claparède's organ instead of epimeral seta 1c. Length of genital plate in different nymphal instars see Table 2.
Legs — All legs monodactylous with strong claws. Setal formula of legs (trochanter to tarsus, solenidia in parentheses): larva I 0–2–2(1)–3(1)–16(1), II 0–2–2(1)–2(1)–13(1), III 0–2– 1(1)–1(1)–13, protonymph I 0–2–2(1)–3(1)–16(2), II 0–2–2(1)–2(1)–13(1), III 0–2–1(1)–1(1)–13, IV 0–0–0–0–7, deutonymph I 0–4–2(1)–3(2)–16(2), II 0–4–2(1)–3(1)–13(2), III 1–2–1(1)–2(1)–13, IV 0–2–2–1(1)–12, tritonymph I 1–4–3(1)–4(2)–18(2), II 1–4–3(1)–4(1)–15(2), III 2–2– 1(1)–3(1)–15, IV 1–2–2–3(1)–12). Lengths of solenidion φ1 in different nymphal instars see Table 2.


Sexual dimorphism, eggs
Adult females slightly larger than males, ranges overlapping. Genital plates slightly larger in females (length 70-80) than in males (length 55-60). Apart from these size differences, no external sexual dimorphism could be observed. Most females bearing 1–5 eggs, frequently arranged diagonally or transverse in the posterior part of the body. Shape of eggs oval to elliptical, frequently kidney-shaped, surface pustulate. Dimensions (n=12) length 165 (140–185), width 72 (60–80). Among the 42 investigated females 17 specimens (40%) do not bear eggs, 4 (10%) bear one egg, 5 (12%) two, 5 (12%) three, 10 (24%) four, and 1 (2%) bears five eggs.
Variation
Some specimens (including holotype) have seta 4a doubled on one side.
Type deposition
The holotype (adult male, TN-163) and six paratypes (TN-163), preserved in ethanol, are deposited in the collection of the Senckenberg Museum, Görlitz, Germany (SMNG). Additional material is deposited in the collection of the author which will finally also be placed in the Senckenberg Museum, Görlitz. Specimens are preserved in ethanol.
Etymology
This species is named in honour of my friend and colleague Dr. Valerie M. Behan-Pelletier, who has extensively contributed to our knowledge of oribatid mites. She supported my work in many ways.
Remarks
1 ● Systematic position. The broad tectum on the anterior margin of the notogaster of the new species and the small banded lamellar complex remind of Jugatala Ewing, 1913. The generic diagnosis of Jugatala (Ewing 1913) underlines the ''broad shelf-like expansion'' (anterior notogastral tectum) as the main trait distinguishing Jugatala species from other genera ''except some of the species of the genus Pelops C. L. Koch'' (Ewing 1913, p. 131). Sellnick (1923) noted that this character also occurs in other genera as Punctoribates or Trichoribates, and, according to him, the genus Jugatala is generally very close to Trichoribates. A main difference between Trichoribates and Jugatala species is the shape of the pedotectum I which is concave dorsally in Jugatala, but convex in Trichoribates (Behan-Pelletier 2000, pers. comm.). In Jugatala angulata (C.L. Koch, 1839) the pedotectum I is straight to slightly concave dorsally (Bayartogtokh and Schatz 2008a, additional observations on own material).
The revised generic diagnosis of Trichoribates given by Behan-Pelletier and Ermilov (2019) lists a number of specific apomorphies for the genus which also pertain to T. valeriae n. sp. This concerns mainly: anterior of notogaster forming tectum, usually covering at least base of bothridium; pedotectum I convex dorsally; pteromorphs without unsclerotized band; octotaxic system with four pairs of porose areas: A1 is divided in two parts of different size; humeral porose areas Ad, Am, Ah, Al present; axillary saccule on mentum present; lateral horizontal folds present dorsal of acetabula I and II; femur III with 2 setae (seta l' absent); humeral organ absent in larva, present in nymphs; gastronotal region of nymphs with large pygidial sclerite; larva with 12 pairs of gastronotal setae, nymphs with 15 pairs, adult with ten pairs of notogastral setae (in adult setae c1, c3, da, dm, dp lost).
2 ● Trichoribates valeriae n. sp. shows morphological similarities to Trichoribates biarea Gjelstrup and Solhøy, 1994 in having lamellar cusps relatively wide apart, bothridium without sharp projection, anterior notogastral tectum, 10 pairs of notogastral setae, porose area A1 divided in two parts. They differ in the shape of rostrum (two apices laterally, without nose-like protuberance in T. biarea), lamellar structure (lamella, cusp and translamella wider, well-developed teeth on cusp of T. biarea), tutorium (4–6 dentations in T. biarea), porose area Aa (larger in T. biarea). Trichoribates biarea was described from Iceland (Gjelstrup and Solhøy 1994) and was also found in montane and subalpine habitats of the Allgäu Alps in Southern Germany (Beck et al. 2018).
The new species is also morphologically similar to Trichoribates scilierensis Bayartogtokh and Schatz, 2008 in having a nose-like protuberance on prodorsum, lamellar cusps relatively wide apart, bothridium without sharp projection, bothridial setae relatively small, anterior margin of notogaster arched anteriad, 10 pairs of notogastral setae, porose area Aa large, A1 divided in two parts. The species differ in lamellar structure (lamella and cusp wider, well-developed teeth on cusp of T. scilierensis), shape of tutorium (part of T. scilierensis specimens with 4–5 small dentations at the dorsodistal end, others without teeth and broader end), epimeral setal formula (3–1–3–2 in T. scilierensis). Trichoribates scilierensis was described from the nearby Sciliar massif in the Italian Dolomites (Bayartogtokh and Schatz 2008a) and later found in further localities in the Italian and Austrian Alps (Fischer and Schatz 2013, Schatz 2018, 2020), mainly in alpine and high alpine altitudes.
Among all Trichoribates species only T. rausensis Aoki, 1982 has a comparable small lamellar structure. This species differs from T. valeriae n. sp. primarily in having longer cusp with developed outer tooth, shorter notogastral setae, and undivided porose area A1. Trichoribates rausensis was described from mountainous areas in Japan (Aoki 1982) and was also found at the foot of Himalaya in West Bengal, India (Mondal and Kundu 1999).
3 ● The fine muscle fibers on pleurophragmata are hitherto only reported in T. biarea (Fig. 3, Gjelstrup and Solhøy 1994) and commented by Beck et al. (2018, p. 161, fig. 17) (''A conspicuous Strahlenfigur [radiating pattern] exists inside behind the front of the notogaster, presumably consisting of fibrillar muscle attachments on apodemal arches''). By checking other species of Trichoribates from my collection I found these muscle fibers invisible or only very weakly visible (e.g. in T. scilierensis).
4 ● The frontal view of the retracted male spermatopositor (Fig. 6C) resembles that of Damaeus onustus (Fig. 2 in Grandjean 1956). Only few studies on the male genital morphology of oribatid mites have been conducted (e.g. Alberti and Coons 1999, Grandjean 1955, 1956, Warren 1947, Woodring 1970), a comparison with other species was not possible in this context. The ovipositor (Fig. 6D) matches other oribatid species. Among the numerous specimens of this species I found only one female with an everted ovipositor, just releasing an egg.
5 ● The presence of porose areas on tibiae and tarsi has not been studied in most Trichoribates species. Beside T. valeriae n. sp. only T. sidorchukae Behan-Pelletier and Ermilov, 2019 is known to have porose areas distoventrally on all tibiae. Porose areas on tarsi are also known from T. novus Sellnick, 1928, T. sidorchukae, T. striatus (Behan-Pelletier, 1986) (in these species proximoventrally on tarsi I – IV), and T. zingerlei Bayartogtokh and Schatz, 2008 (on tarsi II, III, see Bayartogtokh and Schatz, 2008b). As Behan-Pelletier and Ermilov (2019) stated these characters may be more widespread.
A reexamination of Trichoribates scilierensis Bayartogtokh and Schatz, 2008 (specimens from the Dolomites and different places in the Alps) revealed the presence of porose areas on tarsi I – IV, all small and roundish, posterior to seta v' on tarsus I, posterior on seta pv'' on tarsi I – IV. In that species no porose areas exist on tibiae I – IV.
6 ● Trichoribates valeriae n. sp. shares the absence of the humeral organ in the larva and its presence in nymphs with other Trichoribates species examined in this context (T. polaris Hammer, 1953, T. tepetlensis Palacios-Vargas and Norton, 1985, T. ocotlicus Palacios-Vargas and Norton, 1985, see Behan-Pelletier and Ermilov 2019).
7 ● The setation of legs of immature Trichoribates valeriae n. sp. coincides with T. berlesei (Seniczak 1980, sub T. trimaculatus) and T. polaris (Behan-Pelletier 1985). Differences with Diapterobates brevidentatus, a well-studied species regarding immatures (Bayartogtokh and Ermilov 2016), are in tibia II of protonymph (3(1), including l''), tibia I of deutonymph (4(2), including v''), tibia II of deutonymph (4(1), including v'').
8 ● Trichoribates valeriae n. sp. is the most frequently recorded species on both mountains of this investigation. It was found in large numbers in Cerastium uniflorum cushions and in moist moss on the plateau of Sass Pordoi and the Piz Boè mountain up to the summit (3150 m a.s.l.). The latter site was chosen as type locality.
9 ● Several Trichoribates species and other Ceratozetoidea were found in the high mountains of Europe, Asia, Central and South America or in the Arctic zone. Many seem to be restricted to the harsh environment with short vegetation periods and show adaptations such as cold hardiness (Schatz and Sømme 1981) and prolongation of life cycle (e.g. Schatz 1985, Grishina 1997).
Discussion
A total of ten oribatid species from seven families were found (Table 3). The samples were generally very poor in species, on Sass Pordoi four species were recorded, on Piz Boè nine species. The richest spot in species number was a vegetation patch at the foot of Piz Boè (TN 164, TN 165, 2980 m a.s.l.) with nine species, among them six species found only in this site (Anachipteria shtanchaevae, Camisia horrida, Kunstidamaeus lengersdorfi, Liochthonius lapponicus, L. sellnicki, Oribatula interrupta). Three species were recorded on both mountains (Trichoribates valeriae, Liochthonius strenzkei, Tectocepheus sarekensis), all in more than one sample and most in relatively high abundances. Camisia foveolata was only found in moss on Sass Pordoi.
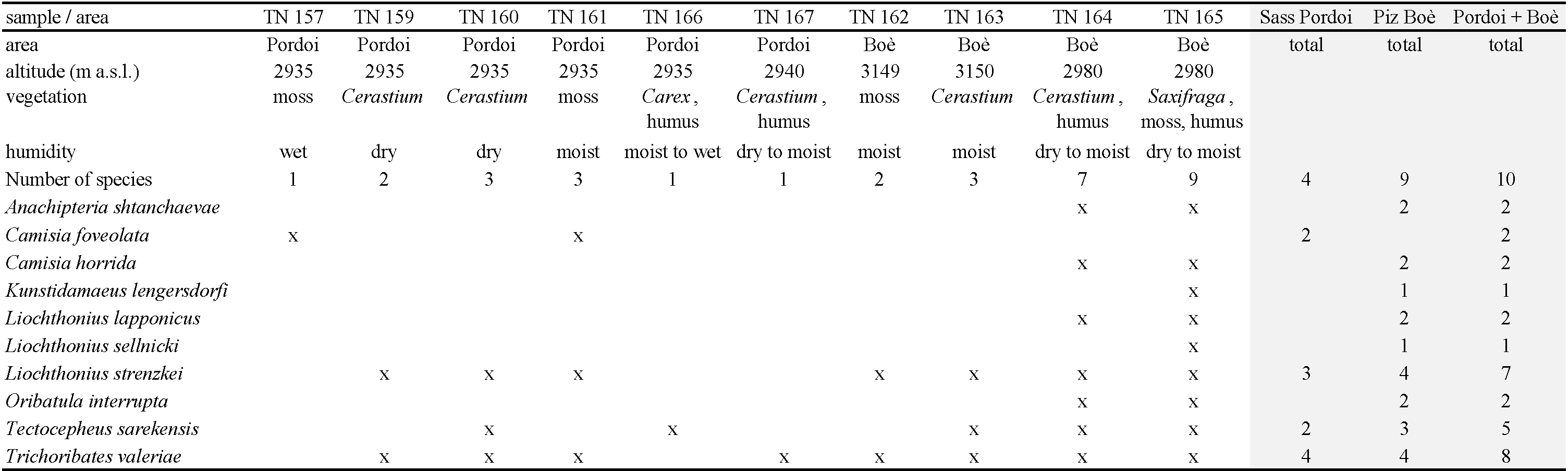


The individual numbers were not estimated in all samples, especially not in the samples which were rich in individuals, but the results indicate an extreme aggregation of species and individuals wherever a suitable microhabitat exists. Especially the small section at the foot of Piz Boè with cushions of Saxifraga oppositifolia, Cerastium uniflorum, moist moss and a dry to moist underlying humus layer (samples TN 164, TN 165) shows a surprising diversity of species with high abundances. This reflects a new assessment of a possible minimum area for mesofaunal elements (Schatz and Schatz 1991).
The majority of the oribatid species from this study have a wide general distribution (holarctic or semicosmopolitan – 7 spp.), one species is known from the Alps, Central, South, Southeast Europe (Anachipteria shtanchaevae), one species is hitherto only known from some sites in Central Europe (Kunstidamaeus lengersdorfi). Trichoribates valeriae is new to science, and until now its known distribution is restricted to the investigation area. Four species were also recorded at the nearby Sella Pass (Liochthonius lapponicus, L. strenzkei, Tectocepheus sarekensis, Oribatula interrupta, Schatz 2017). Three species are new records for the Dolomites and Italy (Camisia foveolata, Kunstidamaeus lengersdorfi; the newly discovered Trichoribates valeriae enlarges the new records for Italy).
These data are snapshots of the investigated vegetation patches. Additional species might possibly occur in the area, but due to conservation considerations only few samples were taken in the scattered vegetation. Despite the small yield, the species spectrum gives an interesting insight into the faunal distribution of high summits. Beside some ubiquitous species three species are frequently or mainly found in the montane to alpine zone (Camisia horrida, Anachipteria shtanchaevae, Oribatula interrupta). Trichoribates valeriae might be added to this ''alpine'' species group.
Camisia foveolata was hitherto only known from the boreal climate zone in the Northern Holarctic (Subías 2004) and from the Chilean Andean highlands (Covarrubias 2004). The finding of this species in the Dolomites lines up with species of disjunct boreo-alpine or arcto-alpine distribution and restriction to high altitudes in the Alps (some remarkable examples are Ceratozetes spitsbergensis Thor, 1934 – cf. Fischer et al. 2016, Mycobates sarekensis (Trägårdh, 1910) – cf. Schatz 2020). These species are considered as preglacial relicts with a wider geographical extension previous to the last glaciation. They survived in certain habitats and retreats such as unglaciated alpine summits (nunataks), in subterranean niches, or in ice-free massifs de refuge along the margins of the Alps, with special adaptations to the extreme conditions in life cycle and development of cold-hardiness (Schatz 2008a, b, Fischer et al. 2016). A corresponding explanation is assumed for high alpine endemic species which could have evolved in recent isolation (e.g. Trichoribates valeriae – present study, Kunstidamaeus granulatus (Willmann, 1951), Mycobates alpinus (Willmann, 1951), Oppiella obscura (Mahunka and Mahunka-Papp, 2000), Trichoribates scilierensis Bayartogtokh and Schatz, 2008, T. zingerlei Bayartogtokh and Schatz, 2008).
Another remarkable finding is Kunstidamaeus lengersdorfi which marks the highest recorded altitude for this species. Kunstidamaeus lengersdorfi is known to be troglobiontic or troglophilic (Miko and Mourek 2008), most records were reported from caves or cave entrances in different parts of Central Europe (Austria, Belgium, Czechia, Germany, Hungary, Slovakia). The Piz Boè massif is a carbonate platform with many smaller and larger synclines, sinkholes, overhangs, and caves. The locality of K. lengersdorfi is situated on a small flat and plant covered section beside an overhanging rock with temporary trickle joining the rank of similar known habitats of that species.
Acknowledgements
Benno Baumgarten, Thomas Wilhalm, Museum of Nature South Tyrol, Bolzano, and the former director of the museum, Vito Zingerle, as well as Maria-Luise Kiem, department for nature, landscape and spatial development of the Autonomous Province Bolzano, South Tyrol provided valuable information on geology, vegetation, history and the area. The institutes of Ecology and Zoology, University of Innsbruck gave logistic support. I appreciate the excellent study of Valerie M. Behan-Pelletier and Sergey G. Ermilov on Trichoribates sidorchukae which was very helpful for understanding the morphology and systematic position of T. valeriae, Val also gave me important hints. Special thank deserves my wife Irene Schatz who accompanied all steps on the mountains and of this work, from assisting in the field until proofreading of an earlier version of this manuscript.
References
Alberti G., Coons B. 1999: Acari - Mites. In: Harrison F.W., Foelix R.F. (Eds). Microscopic anatomy of invertebrates. Volume 8C: Chelicerate Arthropoda. New York: Wiley-Liss. p. 515-1265.
Aoki J. 1982. The Japanese species of the genera Trichoribates and Diapterobates (Acari: Oribatida). Bulletin of the Institute of Environmental Science and Technology, Yokohama, 8(1): 189-205.
Bayartogtokh B., Ermilov S.G. 2016. Ontogenetic instars of Diapterobates brevidentatus, with remarks on morphology of the immatures of Trichoribatinae (Acari: Oribatida: Ceratozetidae). Systematic and Applied Acarology, 21(10): 1413-1435. doi:10.11158/saa.21.10.11
Bayartogtokh B., Schatz H. 2008a. Trichoribates and Jugatala (Acari: Oribatida: Ceratozetidae) from the Central and Southern Alps, with notes on their distribution. - Zootaxa, 1948(1): 1-35. doi:10.11646/zootaxa.1948.1.1
Bayartogtokh B., Schatz H. 2008b. Trichoribates zingerlei, a new species of oribatid mite from the Southern Alps, Italy (Acari: Oribatida: Ceratozetidae). International Journal of Acarology, 34(4): 367-372. doi:10.1080/17088180809434779
Beck L., Horak F., Woas S. 2018. Südwestdeutsche Oribatiden (Acari: Oribatida) - Arten, Taxonomie, Vorkommen. Andrias, Karlsruhe, 21: 1-196.
Behan-Pelletier V.M. 1985. Ceratozetidae of the Western North American Arctic. The Canadian Entomologist, 117: 1287-1366. doi:10.4039/Ent1171287-11
Behan-Pelletier V.M. 2000. Ceratozetidae (Acari: Oribatida) of arboreal habitats. The Canadian Entomologist, 132: 153-182. doi:10.4039/Ent132153-2
Behan-Pelletier V.M., Ermilov S.G. 2019. Trichoribates sidorchukae sp. nov. (Acari, Oribatida, Ceratozetidae) from tropical montane Ecuador, with revised generic diagnosis. Zootaxa, 4647(1): 348-361. doi:10.11646/zootaxa.4647.1.21
Colloff M.J. 1993. A taxonomic revision of the oribatid mite genus Camisia (Acari: Oribatida). Journal of Natural History, 27: 1325-1408. doi:10.1080/00222939300770761
Covarrubias R. 2004. Ácaros oribátidos (Acari: Oribatida) de la Región Altiplánica de Chile. Acta Entomológica Chilena, 28(1): 33-39. doi:10.25085/rsea.790203
Ewing H.E. 1913. Some new and curious Acarina from Oregon. Pomona Journal of Entomology and Zoology, 5(3): 123-136.
Fischer B.M., Schatz H. 2013. Biodiversity of oribatid mites (Acari: Oribatida) along an altitudinal gradient in the Central Alps. Zootaxa, 3626(4): 429-454. doi:10.11646/zootaxa.3626.4.2
Fischer B.M., Schatz H., Querner P., Pauli H. 2016. Ceratozetes spitsbergensis Thor, 1934: an arctic mite new to Continental Europe (Acari: Oribatida). International Journal of Acarology, 42(2): 135-139. doi:10.1080/01647954.2015.1133702
Grandjean F. 1955. Sur un Acarien des iles Kerguelen. Podacarus Auberti (Oribate). Mémoires du Muséum national d'histoire naturelle Nouvelle série, série A. Zoologie, 8: 109-150.
Grandjean F. 1956. Observations sur les Oribates (34e série). Bulletin du Museum d'Histoire Naturelle (2), 28: 205-212.
Gjelstrup P., Solhøy T. 1994. The oribatid mites (Acari) of Iceland. In: The Zoology of Iceland. Steenstrupia. Zoological Museum of Copenhagen, vol. 3, Part 57e. pp. 1-78.
Grishina L.G. 1997. Population dynamics of Oribatid mites in different parts of species areas. Abhandlungen und Berichte des Naturkundemuseums Görlitz, 69: 53‐56.
Hammer M. 1955. Alaskan oribatids. Acta Arctica, 7: 5-36.
Laumann M., Norton R.A., Weigmann G., Scheu S., Maraun M., Heethoff M. 2007. Speciation in the parthenogenetic oribatid mite genus Tectocepheus (Acari, Oribatida) as indicated by molecular phylogeny. Pedobiologia, 51: 111-122. doi:10.1016/j.pedobi.2007.02.001
Miko L., Mourek J. 2008. Taxonomy of European Damaeidae (Acari: Oribatida) I. Kunstidamaeus Miko, 2006, with comments on Damaeus sensu lato. Zootaxa, 1820: 1-26. doi:10.11646/zootaxa.1820.1.1
Mondal B.K., Kundu B.G. 1999. On a collection of oribatid fauna (Acari: Oribatei) from forest and tea soils in Jalpaiguri District, West Bengal, India. Records of the Zoological Survey of India, 97(2): 79-86.
Moritz M. 1976. Revision der europäischen Gattungen und Arten der Familie Brachychthoniidae (Acari, Oribatei). Teil 1. Allgemeiner Teil: Brachychthoniidae Thor, 1934. Spezieller Teil: Liochthonius v.d. Hammen, 1959, Verachthonius nov. gen. und Paraliochthonius nov. gen. Mitteilungen aus dem Zoologischen Museum in Berlin, 52(1): 27-136. doi:10.1002/mmnz.19760520104
Moroder E. (2008): Monografie der Sellagruppe. - Alpenvereinsjahrbuch 2008: 260-267.
Norton R.A., Behan-Pelletier V. 2009. Chapter 15, Oribatida. In: Krantz G.W., Walter D.E. (Eds). A Manual of Acarology 3rd Edition. Lubbock. Texas Tech University Press. p. 421-564. doi:10.1653/024.092.0323
Pérez-Íñigo C. 1997. Acari. Oribatei. Gymnonota I. In: Ramos A. et al. (Eds). Fauna Iberica. Museo de Ciencias Naturales. Madrid, vol. 9. pp. 373.
Reithofer O. (1928): Geologie der Sellagruppe (Südtiroler Dolomiten). - Jahrbuch der Geologischen Bundesanstalt Wien, 78: 529-580.
Schatz H. 1979. Ökologische Untersuchungen an Wirbellosen des zentralalpinen Hochgebirges (Obergurgl, Tirol). II. Phänologie und Zönotik von Oribatiden (Acari). Veröffentlichungen der Universität Innsbruck (Alpin-biologische Studien 10), Innsbruck, 117: 15-120.
Schatz H. 1985. The life cycle of an alpine oribatid mite, Oromurcia sudetica Willmann. Acarologia, 26: 95‐100.
Schatz H. 2004. Die Hornmilbenfamilie Brachychthoniidae (Acari, Oribatida) in Tirol (Österreich). Denisia, Linz, 12: 343-355.
Schatz H. 2006. Catalogue of known oribatid mite species (Acari: Oribatida) from the Central American landbridge (First part). Tropical Zoology, 19(2): 209-288.
Schatz H. 2008a. Hornmilben (Acari: Oribatida) im Naturpark Schlern - Rosengarten (Südtirol, Italien). Gredleriana, 8: 219-254.
Schatz H. 2008b. The Schlern/Sciliar Massif (Southern Alps, Italy) - a biodiversity hotspot for oribatid mites (Acari, Oribatida). In: Bertrand M. et al. (Eds). Integrative Acarology. Proceedings of the 6th European Congress. Montpellier, France: European Association of Acarologists. p. 24-31.
Schatz H. 2017. A collection of oribatid mites (Acari, Oribatida) from the Sella Pass (Dolomites, South Tyrol, Italy). Gredleriana, 17: 55-61.
Schatz H. 2018. Catalogue of oribatid mites (Acari: Oribatida) from South Tyrol (Prov. Bolzano, Italy). Zootaxa, 4435(1): 1-89. doi:10.11646/zootaxa.4435.1.1
Schatz H. 2020. Catalogue of oribatid mites (Acari: Oribatida) from Vorarlberg (Austria). Zootaxa, 4783(1): 1-106. doi:10.11646/zootaxa.4783.1.1
Schatz H., Behan-Pelletier V.M., OConnor B.M., Norton R.A. 2011. Suborder Oribatida van der Hammen, 1968. In: Zhang Z.-Q. (Ed). Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148: 141-148. doi:10.11646/zootaxa.3148.1.26
Schatz H., Schatz I. 1991. Populationsminimalareale endemischer, alpiner Wirbelloser als Grundlage für die Entwicklung von Schutzstrategien. Laufener Seminarbeiträge, Akademie für Naturschutz und Landschaftspflege (ANL), Laufen/Salzach, 3/91: 86-93.
Schatz H., Sømme L. 1981. Cold‐hardiness of some oribatid mites from the Alps. Cryo‐Letters 2: 207‐216.
Schweizer J. 1956. Die Landmilben des Schweizerischen Nationalparkes. 3. Teil, Sarcoptiformes Reuter 1909. Ergebnisse der wissenschaftlichen Untersuchungen des schweizerischen Nationalparks, Neue Folge, Liestal, 5(34): 213-377.
Sellnick M. 1923. Eine alte und eine neue Oribatidenart. Acari - Blätter für Milbenkunde, Lötzen, 1: 1-2. (Reprint in Abhandlungen und Berichte des Naturkundemuseums Görlitz, 43(1) (1968): 27-28).
Seniczak S. 1980. The morphology of the juvenile stages of moss mites of the subfamily Trichoribatinae (Acari, Oribatei), I. Annales Zoologici, Polska Akademia Nauk, Warszawa, 35(8): 83-92.
Seniczak S. 1991a. The morphology of juvenile stages of moss mites of the family Camisiidae (Acari, Oribatida). IV. Zoologischer Anzeiger, 226(5-6): 267-279.
Seniczak S. 1991b. The morphology of juvenile stages of moss mites of the family Camisiidae (Acari, Oribatida), VI. Zoologischer Anzeiger, 227(5-6): 331-342.
Seniczak S., Seniczak A., Kaczmarek S., Żelazna E. 2012. Systematic status of Oribatula Berlese, 1895 (Acari: Oribatida: Oribatulidae) in the light of the ontogeny of three species. International Journal of Acarology, 38(8): 664-680. doi:10.1080/01647954.2012.719030
Subías L.S. 2004. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (1758-2002). Graellsia, 60 (número extraordinario): 3-305. doi:10.3989/graellsia.2004.v60.iExtra.218
Subías L.S. 2009. Nuevo nombre y nueva cita del Cáucaso para una especie de Anachipteria Grandjean, 1932 (Acari, Oribatida, Achipteriidae). Graellsia, 65(1): 79-80. doi:10.3989/graellsia.2009.v65.i1
Travé J., André H.M., Taberly G., Bernini F. 1996. Les Acariens Oribates. Wavre, Belgium: AGAR Publishers. pp. 110.
Warren E. 1947. On the genital system and gut of the oribatid mite, Cepheus tegeocranus (Herm.), and the reaction of these organs to a ray-fungus parasite. Annals of the Natal Museum, 11(1): 1-36.
Weigmann G. 2002. Morphological variability between and within populations of Tectocepheus (Acari, Oribatida, Tectocepheidae) from the velatus-complex in Europe. In: Bernini F. et al. (Eds). Acarid Phylogeny and Evolution: Adapation in Mites and Ticks. Proceedings of the IV Symposium of the European Association of Acarologists, Siena 2000. Dordrecht - Boston - London: Kluwer Academic Publishers. p. 141-152. doi:10.1007/978-94-017-0611-7_15
Weigmann G. 2006. Hornmilben (Oribatida). Die Tierwelt Deutschlands, 76. Teil. Keltern: Goecke & Evers. pp. 520.
Willmann C. 1932. Milben aus Harzer Höhlen. Mitteilungen über Höhlen- und Karstforschung, 1932(3): 3-7.
Willmann C. 1939. Die Moorfauna des Glatzer Schneeberges. 3. Die Milben der Schneebergmoore. Beiträge zur Biologie des Glatzer Schneeberges, Breslau, 5: 427-458.
Woodring J.P. 1970. Comparative morphology, homologies and functions of the male systems in oribatid mites (Arachnida: Acari). Journal of Morphology, 132(4): 425-451. doi:10.1002/jmor.1051320405



2020-10-27
Date accepted:
2020-11-23
Date published:
2020-11-27
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Schatz, Heinrich
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



