Two new species from the Hygrobates nigromaculatus-complex (Acariformes, Hydrachnidia, Hygrobatidae), based on morphological and molecular evidence
Pešić, Vladimir1 ; Jovanović, Milica2 ; Manović, Ana3 ; Zawal, Andrej4 ; Bańkowska, Aleksandra5 ; Broda, Łukasz6 ; Martin, Peter7 and Dabert, Miroslawa8
1✉ Department of Biology, University of Montenegro, Cetinjski put b.b., 81000 Podgorica, Montenegro.
2Department of Biology, University of Montenegro, Cetinjski put b.b., 81000 Podgorica, Montenegro.
3Department of Biology, University of Montenegro, Cetinjski put b.b., 81000 Podgorica, Montenegro.
4Institute of Marine and Environmental Sciences, Center of Molecular Biology and Biotechnology, University of Szczecin, Wąska 13, 71–415 Szczecin, Poland.
5Institute of Biology, University of Szczecin, Wąska 13, 71–415 Szczecin, Poland.
6Department of Animal Morphology, Institute of Environmental Biology, Adam Mickiewicz University, Umultowska 89, 61–614 Poznan, Poland.
7Zoological Institute: Limnology, Christian-Albrechts-Universität zu Kiel, Am Botanischen Garten 1-9, D-24118 Kiel, Germany.
8Molecular Biology Techniques Laboratory, Faculty of Biology, Adam Mickiewicz University in Poznan, Poznan, Poland.
2020 - Volume: 60 Issue: 4 pages: 753-768
https://doi.org/10.24349/acarologia/20204400ZooBank LSID: 754BE1B0-A316-409B-8008-556CE54ED5E4
Original research
Keywords
Abstract
Introduction
Water mites of the genus Hygrobates Koch, 1837 are often the most ubiquitous and usually the most abundant representatives of the group in different types of running and standing waters over the Palaearctic (Pešić et al. 2017). In terms of ecology, many species of this genus have been reported in literature to be present in both standing (lakes) and running (streams) water habitats. Such kind of bipolar habitat preference, for example has been reported for H. nigromaculatus Lebert, 1879 and H. longipalpis (Hermann, 1804), both often reported as common species in Europe living both in lakes and streams (see Martin et al. 2010 for a discussion). Recently, the status of these species has been questioned by the integrative studies using DNA barcodes, proving that lake and stream populations, indeed, represent morphologically and genetically distinct lineages (see Martin et al. 2010 for H. nigromaculatus; Pešić et al. 2019a for H. longipalpis).
Hygrobates nigromaculatus has been subject of controversial debate in taxonomy for a long time (see Martin et al. 2010 for an overview). The status of populations from Northern and Central Europe was resolved by a molecular study: using the DNA barcode region of the mitochondrial cytochrome c subunit I (COI) gene and the nuclear D2 region of 28S rRNA gene, the presence of two well-defined species could be revealed, H. setosus Besseling, 1942 living in streams, and H. nigromaculatus in lakes (Martin et al. 2010).
In this study we used morphological data and results of DNA-barcoding to analyse specimens of the H. nigromaculatus s.l. from the Balkans, with the aim to evaluate potentially cryptic species and establish the nigromaculatus species complex in the genus Hygrobates. As a result, two species new to science are described.
Materials and methods
Water mites were collected by hand netting, sorted live in the field, and immediately preserved in 96% ethanol. Specimens for molecular analysis were examined without dissecting under a compound microscope in ethanol, using a cavity well slide with a central depression. After DNA extraction, some specimens were dissected and slide mounted in Faure's medium.
Morphological nomenclature follows Pešić et al. (2017; for explanations concerning morphology and measurements of Hygrobates species see there Figs. 1B-D). The holotypes of the new species are deposited in Naturalis Biodiversity Center in Leiden (RMNH). DNA sequences prepared in the course of this study are published in BOLD with accession numbers indicated in Table 1.



All measurements are given in µm. The following abbreviations are used: Ac-1 = most anterior acetabulum; Cx-I = first coxae; dL = dorsal length; H = height; I-L-4-6 = fourth to sixth segments of first leg; L = length; mL = median length; n = number of specimens examined; P-1– P-5 = palp segments 1 to 5; W = width; χ = mean value.
Molecular analysis
Molecular analysis was conducted in the Department of Invertebrate Zoology and Hydrobiology, University of Łódź, Poland. For methods used for COI gene amplification and sequencing see Pešić et al. (2017). For this study, DNA was extracted from a total number of three specimens of genus Hygrobates from Montenegro and North Macedonia (Table 1).
For DNA-barcoding and phylogenetic analysis we used previously published COI sequence data from Martin et al. (2010). In total, we used 51 sequences representing COI haplotypes of Hygrobates lacrima sp. nov. (1), H. limnocrenicus sp. nov. (2), H. setosus (n=33), H. nigromaculatus (n=11), with H. trigonicus (2), H. prosiliens (1) and Limnesia undulata (1) as outgroup taxa; the latter ones were chosen following Martin et al. (2010).
Sequences were aligned by MUSCLE 3.8.425 algorithm as implemented in Geneious Prime 2020.1.1 (Biomatters Ltd.). Phylogenetic tree for species delimitation was constructed using FastTree 2.1.11 and MrBayes, both using GTR+G model, as implemented in Geneious Prime 2020.1. Statistical supports for branches were estimated by Shimodaira-Hasegawa test (SH) (Shimodaira and Hasegawa 1999) and Bayesian posterior probability (PP). Tree was edited in MEGA7 and further in Corel Draw X5. Pairwise distance calculations between nucleotide sequences were computed using Kimura's 2-parameter (K2P) distance model (Kimura, 1980) for all codon positions and transition/transversion ratio was calculated using MEGA7 (Kumar et al. 2016). Additionally, sequence data were analysed using the Automatic Barcode Gap Discovery (ABGD) method to delimit genetic clusters by detecting a significant gap in the pairwise distance distribution (Puillandre et al. 2012). We used the online ABGD version (http://wwwabi.snv.jussieu.fr/public/abgd/abgdweb.html) with default settings and K2P distance model.
Results
Species delimitation using DNA-barcodes
The final alignment for species delimitation using COI sequence data comprised 603 nucleotide positions (nps) for 51 specimens including outgroups. The nucleotide sequences could be translated into amino acid sequences without any stop codons. In the dataset, 243 nps out of 603 were variable, and average transition to transversion ratio for all variable sites was 1.7.
Molecular analysis shows that COI sequences from Hygrobates specimens collected in the Balkans form sister groups to the species previously known in the complex (Fig. 1). The sequence representing H. lacrima sp. nov. is reconstructed as a sister branch to the clade grouping COI sequences found in H. nigromaculatus, and two haplotypes found in H. limnocrenicus sp. nov. form a sister clade to the sequences found in H. setosus (Fig. 1); both relationships were recovered with strong support (0.90 PP, 88% SH and 0.99 PP, 96% SH, respectively).
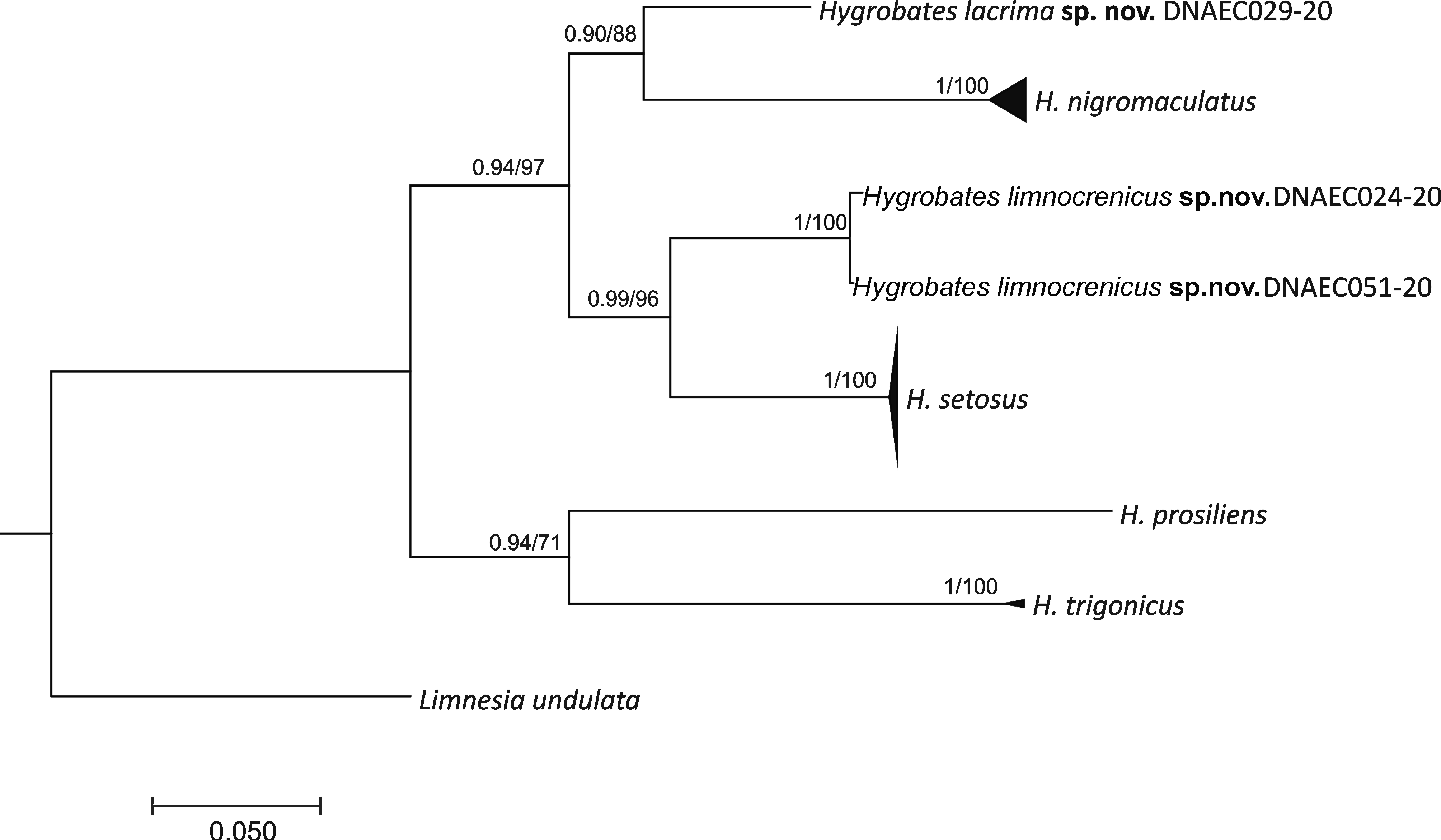


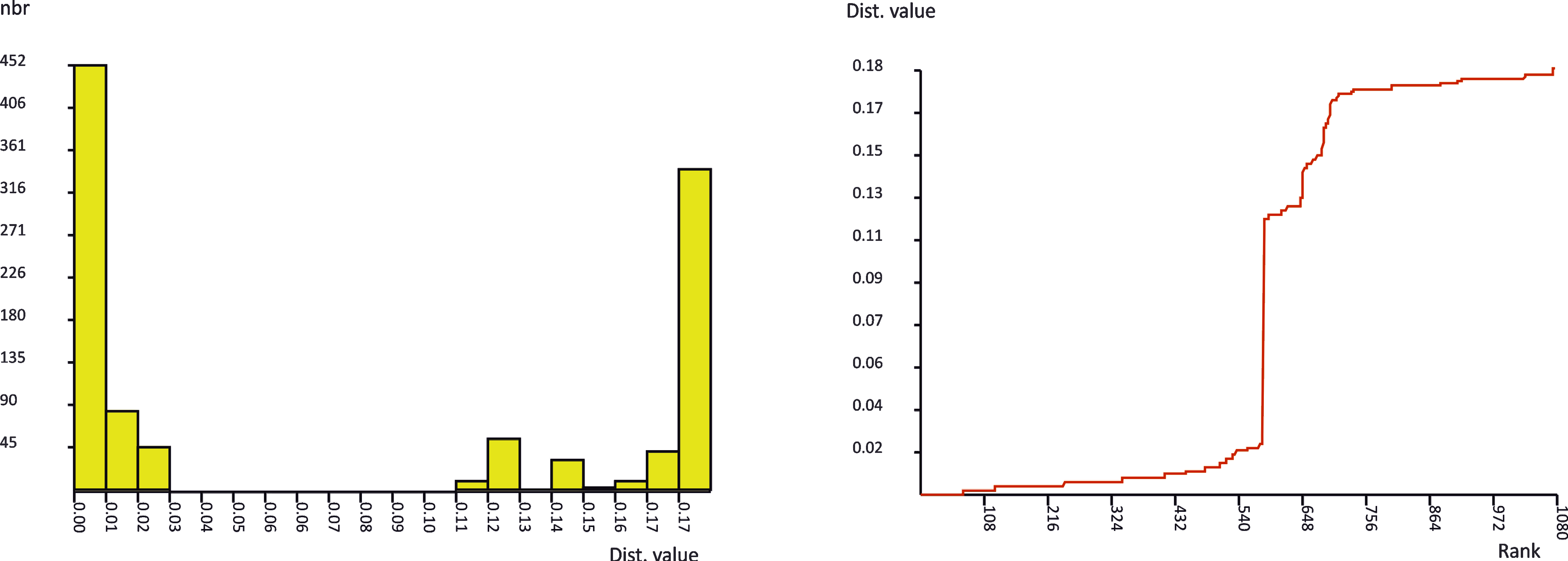
The results of genetic distance analysis strongly supported the species status of the Hygrobates specimens collected in the Balkans. The genetic distance between the COI sequence of H. lacrima sp. nov. and its closest relative, H. nigromaculatus, was 15.87% (SD = 1.74) K2P, whereas the distance between H. limnocrenicus sp. nov. and H. setosus amounted to 12.43% (SD = 1.47) K2P. These distances were higher than the barcoding gap found by the ABGD method (3 to 11%) in the distances among all species belonging to the H. nigromaculatus-complex (Fig. 2), which additionally supported the species-status of the two new clades.


Systematics
Family Hygrobatidae Koch, 1842
Genus Hygrobates Koch, 1837
Subgenus Hygrobates s.s.
Hygrobates lacrima Pešić sp. nov.
ZOOBANK: 107B544E-3B5E-41BA-96BB-4D2F2F967F5E ![]()
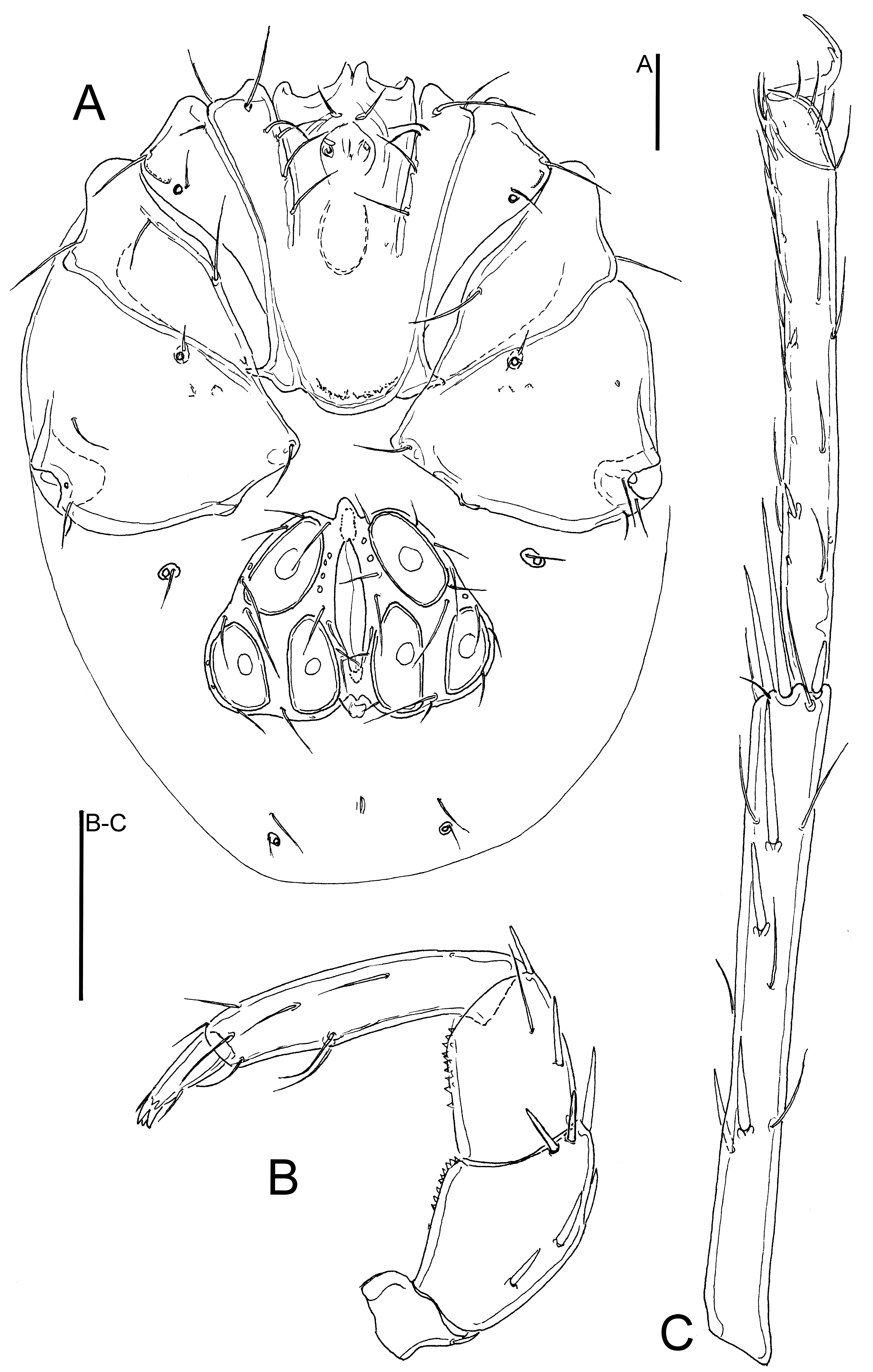
Figs. 3, 4, 5A-B







Material examined — Holotype ♀ [27. CG2020_3_C7], sequenced, dissected and slide mounted, Montenegro, River Tara near Mateševo, 42°47'18.78'' N, 19°32'20.71'' E, 26.10.2019, leg. Pešić. Paratypes: 1♂, 2♀♀ (1♂ and 1♀ partially dissected [palp, I-L and IV-L from one side slide mounted], River Tara near Trebaljevo, 42°51'47.68'' N, 19°31'37.30'' E, 1.9.2019, leg. Pešić.
Diagnosis — Large in size (mL of Cx-I + gnathosoma ˃ 340, L genital plate ˃ 210, P-4 ˃ 170 μm); Cx-I+II apodemes protruding in both sexes, in females mediocaudal margins of Cx-IV with well-developed apodemes; anterior margin of male genital field with a bluntly pointed medial projection; L of IV-L-6 proximoventral seta ♂ ˂ 20, ♀ ˂ 35 μm.
Description — General feature – Colour yellowish to brown. Integument finely striated. Posteromedial margin of Cx-I rounded, caudal apodemes of Cx-I+II well-developed (Figs. 3A, 4A); Cx-IV subtriangular, with a distinct nose-like protruding medial margin. Genital field (in female holotype slightly damaged during dissection and mounting): Ac in triangular arrangement (Figs. 5A-B). P-2 ventral margin straight, distally forming a right angle, denticles covering distal half of ventral margin; P-3 with denticles covering distal two thirds of ventral margin; P-4 ventral setae on the same level (Figs. 3B-C, 4B).
Male – Anterior margin of genital field convex, with a small bluntly pointed medial projection, posterior margin indented, with a small central protrusion not extending beyond posterior genital plate margin (Fig. 5A).
Female – Genital plates distinctly longer than gonopore (Fig. 5B); P-4 slenderer than in male, L/H ratio 4.2-4.5.
Measurements — Female (holotype; in parentheses measurements of paratype from Trebaljevo, Montenegro, n = 1)
Idiosoma – L (1025), W (944); coxal field: L 481 (506); Cx-II W 458 (485); Cx-III W 600 (653); mL of Cx-I + gnathosoma L 344 (361); distance between lateralmost ends of Cx-II apodemes, 191 (212); genital field L/W 219/294 (244/345); genital plate L 219 (241-248); gonopore L (203); L gonopore/genital plate ratio (0.82-0.84); L Ac 1-3: 119 (119-125), 128 (108-123), 95-113 (81-84).
Palp – total L 549 (623); dL/H, dL/H ratio: P-1, 44/50, 0.88 (52/53, 0.97); P-2, 147/89, 1.65 (161/98, 1.64); P-3, 99/77, 1.29 (122/82, 1.49); P-4, 192/45, 4.24 (217/48, 4.48); P-5, 67/23, 2.9 (71/29, 2.4); P-2/P-4 ratio 0.77 (0.74).
Legs – dL of I-L-1-6: 92 (95), 116 (141), 164 (184), 241 (259), 242 (272), 247 (266). dL of IV-L-1-6: 173 (188), 180 (200), 284 (328), 397 (438), 394 (441), 350 (364); L of IV-L-6 proximoventral seta 34 (23).
Male (paratype from Trebaljevo, Montenegro, n = 1)
Idiosoma – L 875, W 680; coxal field: L 477; Cx-II W 428; Cx-III W 577; mL of Cx-I + gnathosoma L 350; distance between lateralmost ends of Cx-II apodemes, 197; genital field L/W 231/300, ratio 0.77; L Ac 1-3: 106-119, 97-100, 106-115.
Palp – total L 511; dL/H, dL/H ratio: P-1, 41/47, 0.87; P-2, 134/86, 1.56; P-3, 95/70, 1.35; P-4, 177/42, 4.2; P-5, 64/22, 2.9; P-2/P-4 ratio 0.76.
Legs – dL of I-L-1-6: 78, 109, 147, 213, 231, 216. dL of IV-L-1-6: 150, 166, 263, 378, 362, 314; L of IV-L-6 proximoventral seta 18.
Etymology — ''lacrima'' (Latin): tear, in reference to the name of Tara River – The Tear of Europe.
Remarks — Based on molecular analysis, Hygrobates lacrima sp. nov. is closely related to H. nigromaculatus. The distance between these two species was 15.87% (SD = 1.74) K2P.
In comparison with the new species from Montenegro, H. nigromaculatus (data taken from Martin et al. 2010) generally is smaller in dimensions. In males of H. nigromaculatus, the length of genital plate is ˂ 160 (vs. ˃ 220 μm in H. lacrima), in females ˂ 175 (vs. ˃ 210 μm in H. lacrima); mL Cx-I + gnathosoma in males is ˂ 280 (vs. ˃ 340 μm in H. lacrima), in females ˂ 350 μm (vs. ˃ 340 μm in H. lacrima); the length of P-4 in males H. nigromaculatus is ˂ 130 (vs. ˃ 160 μm in H. lacrima), in females ˂ 165 μm (vs. ˃ 180 μm in H. lacrima). Moreover, the species live in different habitats: H. nigromaculatus in lakes, whereas H. lacrima inhabits pools and shallow eddies along faster flowing waters (see Figs. 9A-C). From H. setosus (in parentheses, based on material from Farver Au, Germany), male of new species differs in having the bluntly-pointed medial projection of the genital field and comparatively wider genital field (L/W ratio 0.83-0.9; compare Fig. 5A and 5G). Although Tuzovskij (2017) showed different types of male genital plates for H. nigromaculatus, we cannot exclude that he dealt with several species. Therefore, we think that the differences between the male genital plates of H. lacrima and other species as given in the key are justified.
Due to its larger size and habitat preference for running waters, the new species is similar to H. limnocrenicus sp. nov. (see there for further discussion).
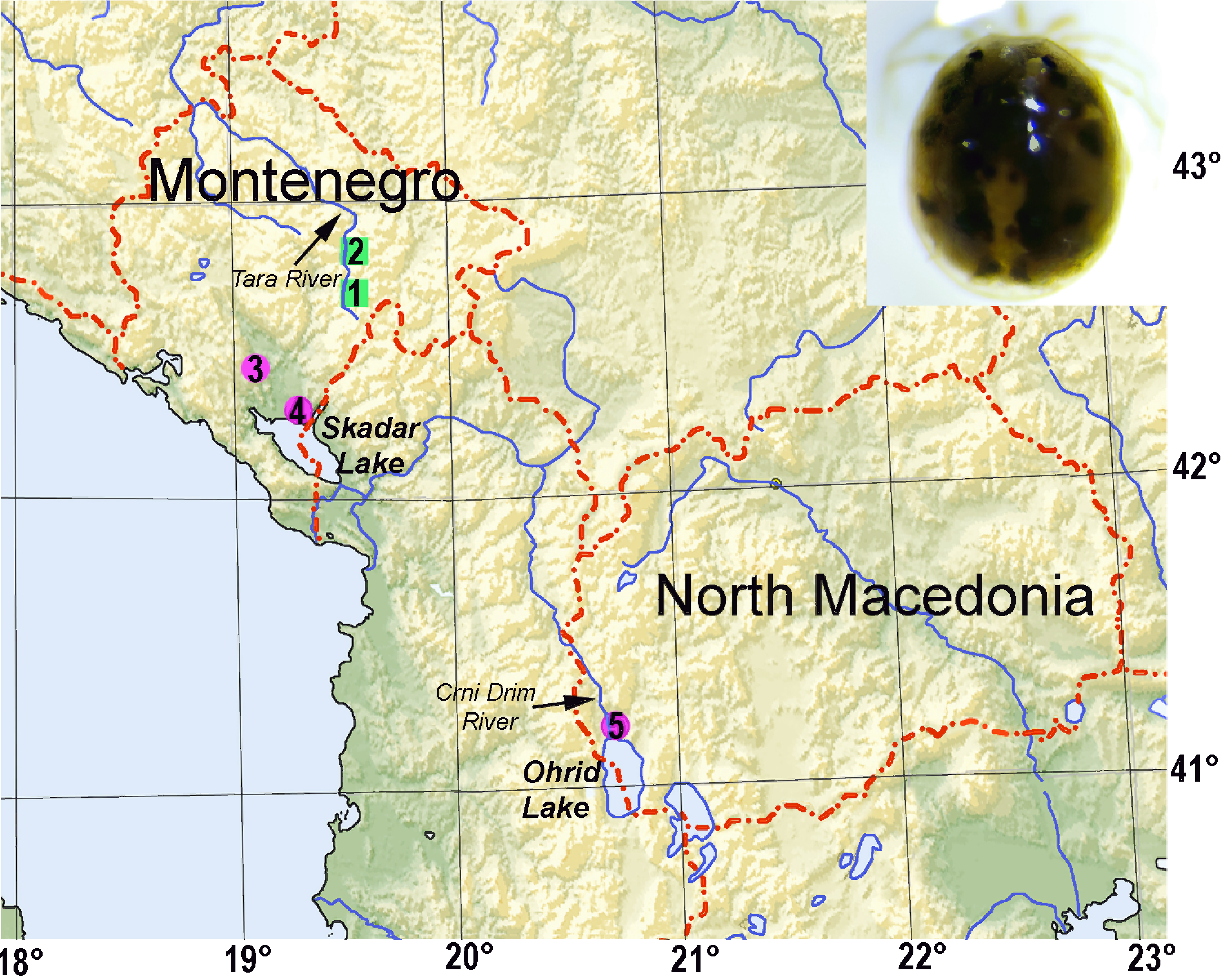
Distribution — Montenegro; know from two localities along the middle course of the Tara River (Figs. 9A-C). These two sites were subject of a monthly monitoring survey in 2019 on the river biota of the Tara River during the Bar-Boljare highway development activities (see Pešić et al. 2020a). At the Mataševo site, only one specimen (which was successfully barcoded) was collected, probably due to the negative ecological impact associated with highway development activities in the immediate vicinity (for the controversy surrounding highway construction over the Tara River see Pešić et al. 2020b). At the Trebaljevo site located downstream, the negative impact was less pronounced - here, three individuals were collected.
Hygrobates limnocrenicus Pešić sp. nov.
ZOOBANK: F010A08A-BFD9-4E57-9E22-E9CE9CDA740A ![]()
Figs. 5C-F, 6, 7



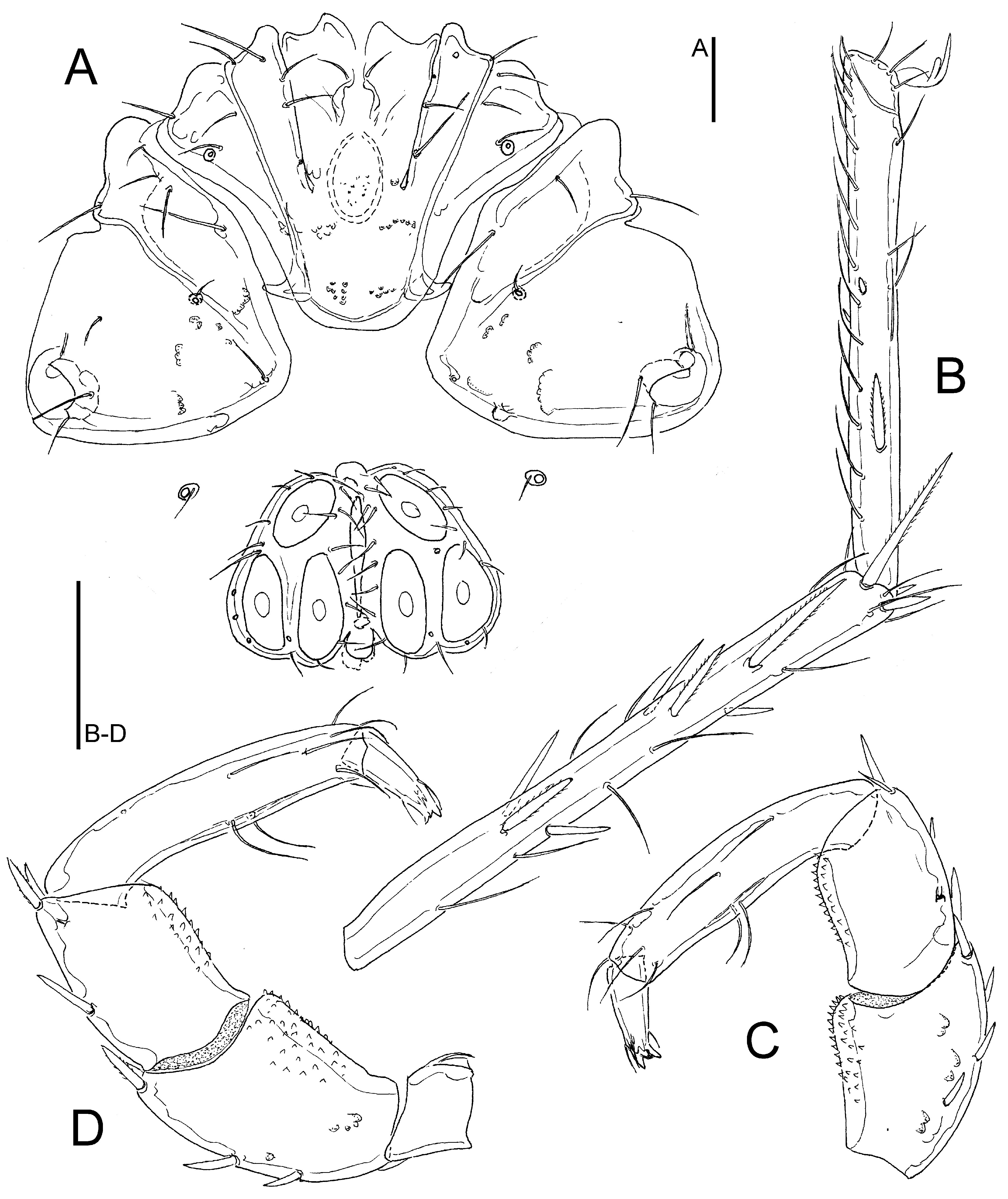


Synonym – Hygrobates setosus sensu Pešić et al. 2018a: 170; Bańkowska et al. 2016: 1028; Pešić et al. 2019c: 471.
Material examined — Holotype ♂ [19. M19_20_5_E4], sequenced, dissected and slide mounted, Montenegro, Podgorica, Mareza, outflow of limnocrene spring, 42°28'44.01'' N, 19°10'52.50'' E, 24.6.2019, leg. Pešić & Zawal. Paratypes: 1♀ (dissected and slide mounted), Podgorica, Daljam, limnocrene spring ''Kraljičino Oko'', springbrook, 42°29'9.61'' N, 19°10'25.28'' E, 31.5.2018, leg. Pešić & Zawal; North Macedonia, Struga, Crni Drim River-outlet of Ohrid Lake, 41°11'3.39'' N, 20°40'40.88'' E, 12.9.2019, leg. Pešić, 1♀ [19. MECD2019_3.2_C2], sequenced, conserved in Koenike fluid.
Other material — Montenegro: Podgorica, Vitoja springs near Skadar Lake, 42°19'31.25'' N, 19°21'45.93'' E, 5.8.2017, leg. Pešić, 6♂♂, 8♀♀ (1♂ and 1♀ dissected and slide mounted); ibid., 30.7.2014, leg. Pešić, 1♂, 1♀; ibid., 31.5.2018, leg. Pešić & Zawal, 142 ex. (115 ex. in ethanol; 5♂♂, 4♀♀ in Koenike fluid; 2♂♂, 2♀♀ dissected and slide mounted).
Compared material – Hygrobates setosus, Germany, Farver Au, 54°16'0.5556'' N, 10°48'9.0396'' E, small stream, 5.9.1992, leg. P. Martin, 2♂♂, 3♀♀ (2♂♂, 1♀ dissected and slide mounted).
Diagnosis — Large in size (mL of Cx-I + gnathosoma ˃ 350, L genital plate ˃ 200, P-4 ˃ 170 μm); Cx-I+II apodemes moderately protruding, mediocaudal margins of Cx-IV in females without prominent apodemes; anterior margin of male genital field with a knob-shaped medial projection; L of IV-L-6 proximoventral seta ♂ ˃ 40, ♀ ˃ 35; running waters.
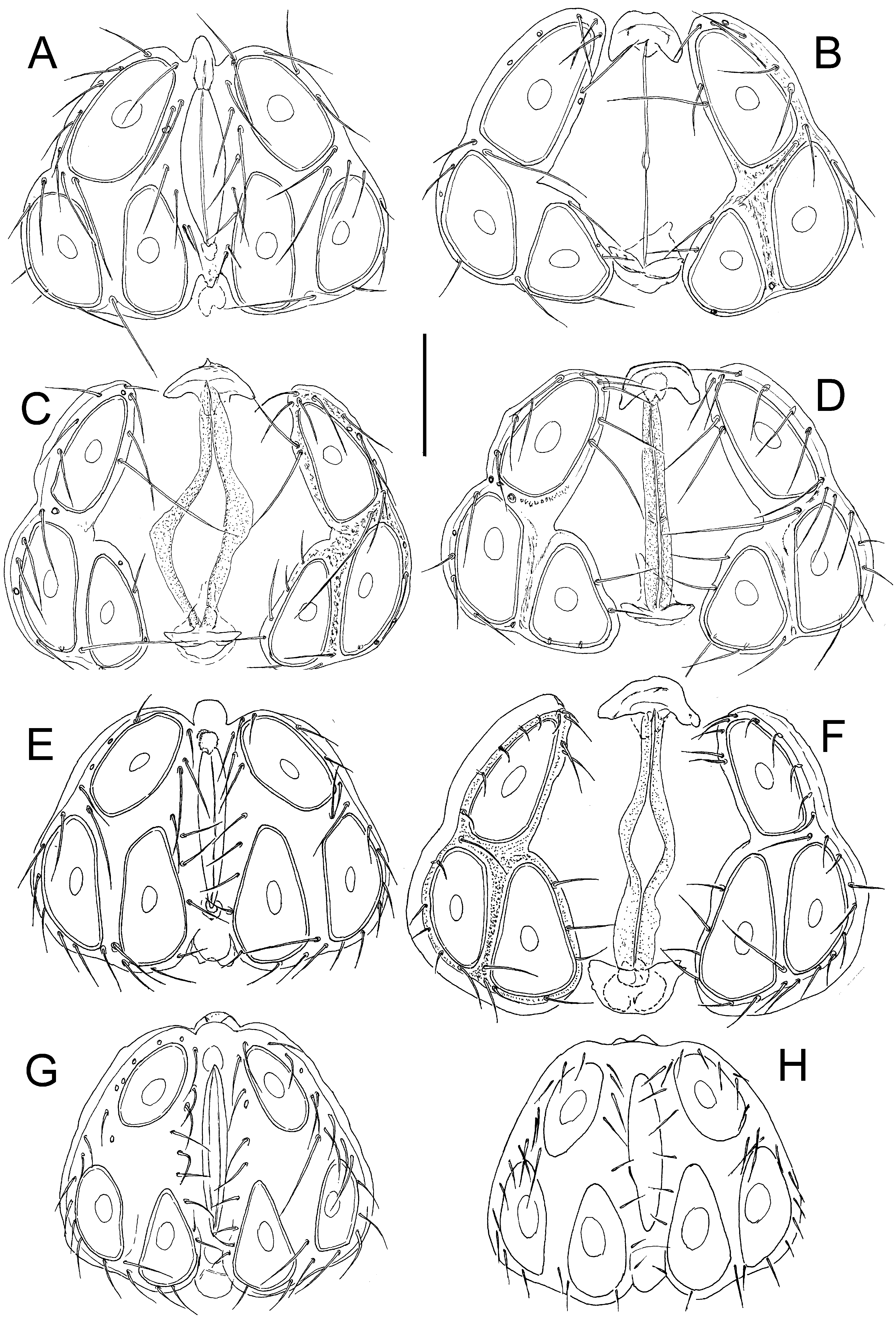
Description — General features – Colour yellowish to brown (Fig. 8, inset). Integument finely striated. Posteromedial margin of Cx-I rounded, caudal apodemes of Cx-I+II well-developed (Figs. 6A, 7A); Cx-IV subtriangular. Genital field: Ac in a triangular position. P-2 ventral margin straight, distally forming a right angle, denticles covering distal half of ventral margin; P-3 with denticles covering distal two thirds of ventral margin (Figs. 6B-C, 7C-D); P-4 ventral setae on the same level.
Male – Anterior margin of genital field convex, with a small knob-shaped medial projection, posterior margin indented, with a rounded central projection not extending beyond posterior genital plate margin (Figs. 5E).
Female – Genital plates distinctly longer than gonopore (Fig. 5C-D, F).


Measurements — Female (Holotype; in parentheses measurements of paratype from Crni Drim River, Macedonia; in square brackets measurements of specimens from Vitoja, given as range and mean, n = 3)
Idiosoma – L (1050), W (853) [1080-1210, χ = 1136]; coxal field: L 475 (525) [489-536, χ = 514]; Cx-II W 453 (484) [406-513, χ = 472]; Cx-III W 634 (627) [689-753, χ = 721]; mL of Cx-I + gnathosoma L 375 (403) [339-403, χ = 370]; distance between lateralmost ends of Cx-II apodemes, 203 (221) [197-213, χ = 202].
Genital field – L/W 241 (256) [259-281, χ = 271.7], W 353 (334) [356-386, χ = 371.3]; genital plate L 233-238 (236-241) [247-268, χ = 258]; gonopore L 194 (206) [209-238, χ = 224.3]; L gonopore/genital plate ratio 0.82-0.83 (0.85-0.87) [0.85-0.89, χ = 0.87]. L Ac 1-3: 109-116 (103) [109-116, χ = 112.7]; 108 (109-113) [112-117, χ = 114]; 84-86 (94) [106-124, χ = 112].
Palp – total L 537 [568-625, χ = 598]; dL: P-1, 41 [48-45, χ = 47]; P-2, 147 [157-172, χ = 165]; P-3, 94 [109-123, χ = 115]; P-4, 183 [194-209, χ = 203]; P-5, 72 [63-73, χ = 68.3]; H: P-1, 55 [53-57, χ = 55]; P-2, 97 [100-108, χ = 104.3]; P-3, 80 [82-86, χ = 84]; P-4, 52 [50-53, χ = 51]; P-5, 27 [23-25, χ = 23.7]; dL/H ratio: P-1, 0.74 [0.84-0.87, χ = 0.86]; P-2, 1.52 [1.54-1.64, χ = 1.59]; P-3, 1.18 [1.32-1.42, χ = 1.36]; P-4, 3.54 [3.88-4.15, χ = 3.99]; P-5, 2.7 [2.67-2.94, χ = 2.85]; P-2/P-4 ratio 0.8 [0.81-0.82, χ = 0.81]. Chelicera total L 434 [403-442, χ = 423.3], L basal segment 278 [269-291, χ = 281], claw 163 [153-172, χ = 160], L basal segment/claw ratio 1.7 [1.65-1.87, χ = 1.76].
Legs – dL of I-L-1-6: 84 (86) [84-89, χ = 86]; 103 (116) [116-136, χ = 126.7]; 144 (169) [160-172, χ = 168]; 203 (244) [225-256, χ = 242.7]; 222 (255) [245-272, χ = 260]; 216 (240) [238-256, χ = 248]. dL of IV-L-1-6: 163 [172-192, χ = 185], 167 [188-205, χ = 198], 266 [284-325, χ = 304], 353 (411) [413-466, χ = 440], 356 (414) [416-445, χ = 432], 309 (325) [338-366, χ = 356]. L of IV-L-6 proximoventral seta 41 [37-55, χ = 44].
Male (specimens from Vitoja springs, Montenegro, measurements given as range and mean, n = 3)
Idiosoma – L 1040-1140, χ = 1086; coxal field: L 487-511, χ = 500; Cx-II W 459-500, χ = 474; Cx-III W 631-700, χ = 656; mL of Cx-I + gnathosoma L 372-386, χ = 381; distance between very lateral ends of Cx-II apodemes, 190-216, χ = 206. Genital field L 227-258, χ = 243, W 300-322, χ = 311.7. L Ac 1-3: 100-122, χ = 110; 113-122, χ = 116; 116-134, χ = 126.
Palp – total L 546-585, χ = 561; dL: P-1, 46-50, χ = 48; P-2, 144-158, χ = 151.3; P-3, 105-113, χ = 108; P-4, 185-198, χ = 190.3; P-5, 61-66, χ = 63.3; H: P-1, 52-54, χ = 53; P-2, 94-98, χ = 95.3; P-3, 75-78, χ = 76; P-4, 42-50, 46.7; P-5, 23, χ = 23; dL/H ratio: P-1, 0.98-0.93, χ = 0.91; P-2, 1.53-1.68, χ = 1.58; P-3, 1.4-1.44, χ = 1.42; P-4, 3.96-4.38, χ = 4.1; P-5, 2.68-2.8, χ = 2.74; P-2/P-4 ratio 0.77-0.82, χ = 0.8. Chelicera total L 391-413, χ = 400.3, L basal segment 254-273, χ = 266, claw 147-153, χ = 150, L basal segment/claw ratio 1.66-1.86, χ = 1.77.
Legs– dL of I-L-1-6: 75-89, χ = 81.7; 113-121, χ = 117; 148-154, χ = 152; 213-228, χ = 220; 228-248, χ = 237; 225-239, χ = 232. dL of IV-L-1-6: 181-203, χ = 190; 181-188, χ = 183; 269-288, χ = 276; 396-411, χ = 404; 398-397, χ = 398; 331-341, χ = 335.3. L of IV-L-6 proximoventral seta 45-48, χ = 46.7.
Etymology — The species is named after its dominant occurrence in limnocrene springs.
Remarks — Due to its larger dimensions (and also regarding habitat preference), the new species is similar to H. lacrima sp. nov. Females of the latter species differ from H. limnocrenicus sp. nov. in having well-developed apodemes at mediocaudal margins of Cx-IV (compare Fig. 3A with Fig. 6A), males in the medial projection of the genital field bluntly-pointed (knob-shaped in H. limnocrenicus sp. nov.; compare Fig. 5A and 5E), both sexes bear a shorter proximoventral seta on IV-L-6 (L H. lacrima vs. H. limnocrenicus, ♂♂: ˂ 20 vs. ˃ 40, ♀♀: ˂ 35 vs. ˃ 35 μm).
Analysis of COI sequences suggests that H. limnocrenicus sp. nov. is most closely related to H. setosus Besseling, 1942, which has also a preference for running water habitats. However, the latter species, as well as H. lacrima sp. nov., was mostly found in pools of running waters, while H. limnocrenicus sp. nov. prefers deeper, fast flowing water, typically being found in the outflow of limnocrenic springs or lake outlets (Figs. 9D-F). The COI divergence between H. setosus and H. limnocrenicus sp. nov. was 12.43% (SD = 1.47). K2P indicating a long independent history of these two species. The studied specimens of H. setosus (in parentheses) differ in both sexes by a shorter proximoventral seta on IV-L-6 (L H. setosus vs. H. limnocrenicus, ♂♂: ˂ 30 vs. ˃ 40, ♀♀: ˂ 20 vs. ˃ 35 μm) and in males by the comparatively less wide genital field (L/W ratio ˃ 0.8), a smaller dimensions of acetabula (Ac-3 ˂ 105 μm), and the medial projection of the genital field less pronounced, typically with irregular margin of a secondary sclerotization (see Figs. 5G-H).



Biology — Morphological and genetical analysis of populations from the Vitoja springs situated at the northeastern shore of the Lake Skadar indicates that the oviposition data published by Bańkowska et al. (2016) for H. setosus in fact refers to H. limnocrenicus sp. nov. The highest number of females laying eggs was found in springs, with an average number of eggs per female of 58.2±30.6. A lower number of females laying eggs was noted in rivers, but here the average number of eggs per female (80±24.5) was higher (Bańkowska et al. 2016). A statistically significant difference in number of laid eggs between May and October was found. The average time of hatching was 14.1 ± 5.29 days.
Distribution — Montenegro and North Macedonia (Fig. 8). Details of distribution are unknown due to the previous confusion with H. setosus, but the species is obviously widespread in the Balkans. It is likely that the records of the latter species from the limnocrene springs of the Mediterranean region of the Balkan refers to H. limnocrenicus sp. nov. For example, Pozojević et al. (2019) reported occurence of H. setosus in the limnocrene spring Modro Oko in Southern Dalmatia, in a low abundance not exceeding 4 individuals per square meter.
Key to the European species of the Hygrobates nigromaculatus-complex
1. Median length of Cx-I + gnathosoma ˂ 340 μm. Males: P-4 L ˂ 130, genital plate L ˂ 160. Females: P-4 L ˂ 165, genital plate L ˂ 175 μm.
...... Hygrobates nigromaculatus Lebert, 1879; Central Europe (Preferably in standing waters)
— Median length of Cx-I + gnathosoma ˃ 340 μm. Males: P-4 L ˃ 140, genital plate L ˃ 170. Females: P-4 L ˃ 165, genital plate L ˃ 175 μm. (preferably in running waters)
...... 2
2. Male genital field with a narrow, bluntly-pointed anteromedial projection (Fig. 5A).
...... Hygrobates lacrima sp. nov.; Montenegro (in pools of running waters)
— Male genital field with a more broad-based, knob-shaped anteromedial projection (Figs. 5E, G)
...... 3
3. Male: genital field comparatively wider (L/W ratio ˂ 0.8), acetabula larger in dimension, Ac-3 ˃ 110 μm, anteromedial projection of genital field well pronounced (Fig. 5E); IV-L-6 proximoventral seta L: males, ˃ 40, female, ˃ 35 μm.
...... Hygrobates limnocrenicus sp. nov.; Montenegro, Macedonia (Preferably in deeper, fast flowing water)
— Male: genital field comparatively less wide (L/W ratio ˃ 0.8), acetabula smaller in dimension, Ac-3 ˂ 110 μm, anteromedial projection of genital field less pronounced, often with irregular border of secondary sclerotization (Figs. 5G-H); IV-L-6 proximoventral seta L: males, ˂ 30, female, ˂ 20 μm.
...... Hygrobates setosus Besseling, 1942; Central Europe (preferably in pools of running waters)
Discussion
The use of an integrative approach based on molecular data and on morphology allowed us to define a new species-complex in the genus Hygrobates, the H. nigromaculatus-complex, containing at least four species, H. nigromaculatus, H. setosus, H. lacrima sp. nov., and H. limnocrenicus sp. nov.
The study conducted by Martin et al. (2010) on H. nigromaculatus and H. setosus and our study on two new species from the Balkans reveal that these species substantially overlap in morphology. Without the results of molecular analyses, it would have been very difficult to circumscribe in this complex species boundaries, and to determine their similarities. For example, molecular data suggest that H. lacrima sp. nov. is closer to H. nigromaculatus than to H. setosus with which it shares a larger size and a preference for running waters.
From H. setosus and two new species described from the Balkans, H. nigromaculatsus differs in smaller size and its habitat preference for standing waters, often preferring surge shores of the littoral zone of lakes (Martin 1996, Martin et al. 2010). Hygrobates setosus is exclusively found in pools of streams with slow current (Martin et al. 2010). Similar to H. setosus, H. lacrima sp. nov. inhabits pools and shallow eddies along faster flowing waters such as the Tara River. Finally, H. limnocrenicus sp. nov. prefers deeper, fast flowing water, typically found in the outflow of a limnocrenic springs or a lake outlets.
During our survey, an abundant population of H. limnocrenicus sp. nov. was found in the Vitoja springs, located at the shore of Skadar Lake, and falling below the lake water level during high water periods in winter and early spring (Pešić et al. 2018b; see Fig. 9F). Probably, the proximity of the lake provides optimal conditions to sustain a large population of H. limnocrenicus sp. nov. compared with isolated water bodies where it was found in a low abundance only (regardless of the effort made to sample more material, only a few specimens were collected in the outflows of the Mareza and Kraljičino Oko (Queen's Eye) springs). It is worth mentioning that H. limnocrenicus sp. nov. was found also in the Crni Drim (Black Drin) River near the exit from the Ohrid Lake which shares the same watershed as the Skadar Lake. The latter finding supports the congruence of the distribution of this species in the Adriatic Sea watershed.
As demonstrated by Martin and Davids (2002), differences in life-cycle strategies may contribute to species discrimination within the H. nigromaculatus-complex. The stream-dwelling populations of H. setosus have a parasitic larva, whereas the lake-living populations of H. nigromaculatus have reduced larval parasitism (Martin and Davids 2002). The question if parasitism eventually went lost in a common ancestor of H. lacrima sp. nov. and the H. nigromaculatus merits particular attention. Martin et al. (2010) hypothesized that H. nigromaculatus secondarily lost parasitic larvae and probably originated from a stream-living Hygrobates species with a larval stage able to disperse. Both new species have a relatively large body size which may be a first hint to a parasitic life style. Often, non-parasitic lineages are able to afford to be smaller by producing less abundant, but larger eggs (Smith 1998).
Although our study is based on a relatively small number of individuals that could be sequenced, it resulted in the discovery of two new species and the definition of a H. nigromaculatus species complex. Our results suggest that efforts to investigates mites of this complex should be intensified all over Europe.
Acknowledgements
This study is part of the ''DNA-Eco'' scientific project supported by a grant of the Montenegrin Ministry of Science. AB is supported by Polish National Science Centre, Poland, grant no. 2017/27/N/NZ8/01568. Special thanks to Michał Grabowski and Aleksandra Jabłońska (Department of Invertebrate Zoology and Hydrobiology, University of Lodz, Łódź, Poland) for their help with laboratory work. We thank Reinhard Gerecke (Tübingen), Joanna Mąkol (Wrocław) and one anonymous reviewer, whose constructive comments greatly improved this work.
References
Bańkowska A., Kłosowska M., Gadawski P., Michoński G., Grabowski M., Pešić V., Zawal A. 2016. Oviposition by selected water mite (Hydrachnidia) species from Lake Skadar and its catchment. Biologia 71(9): 1027-1033. doi:10.1515/biolog-2016-0126
Besseling A.J. 1964. De Nederlandse Watermijten (Hydrachnellae Latreille 1802). Monogr. Nederl. Entomol. Ver., 1: 1-199.
Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol., 16: 111-120. doi:10.1007/BF01731581
Kumar S., Stecher G., Li M., Knyaz C., Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol., 35: 1547-1549. doi:10.1093/molbev/msy096
Martin P. 1996. Faunistisch-ökologische Benthosstudien an den Wassermilben (Hydrachnidia, Acari) zweier Bäche des Norddeutschen Tieflandes (Ostholsteinisches Hügelland, Schleswig-Holstein). Faun.-Ökol. Mitteil., 7: 153-167.
Martin P., Davids C. 2002. Life history strategies of Hygrobates nigromaculatus, a widespread palaearctic water mite (Acari, Hydrachnidia, Hygrobatidae). In: Bernini F., Nannelli G., Nuzzaci G., de Lillo E. (Eds). Acarid phylogeny and evolution. Adaptation in mites and ticks. Kluwer Academic, Dordrecht, pp. 101-110. doi:10.1007/978-94-017-0611-7_11
Martin P., Dabert M., Dabert J. 2010. Molecular evidence for species separation in the water mite Hygrobates nigromaculatus Lebert, 1879 (Acari, Hydrachnidia): evolutionary consequences of the loss of larval parasitism. Aquat. Sci., 72: 347-360. doi:10.1007/s00027-010-0135-x
Pešić V., Asadi M., Cimpean M., Dabert M., Esen Y., Gerecke R., Martin P., Savić A., Smit H., Stur E. 2017. Six species in one: Evidence of cryptic speciation in the Hygrobates fluviatilis complex (Acariformes, Hydrachnidia, Hygrobatidae). Syst. Appl. Acarol., 22: 1327-1377. doi:10.11158/saa.22.9.4
Pešić V., Bańkowska A., Goldschmidt T., Grabowski M., Michoński G., Zawal A. 2018a. Supplement to the checklist of water mites (Acari: Hydrachnidia) from the Balkan peninsula. Zootaxa, 4394(2): 151-184. doi:10.11646/zootaxa.4394.2.1
Pešić V., Karaman G.S., Kostianoy A.G., Vukašinović-Pešić V. 2018b. Conclusions: Recent Advances and the Future Prospects of the Lake Skadar/Shkodra Environment. In: Pešić V., Karaman G., Kostianoy A. (Eds). The Skadar/Shkodra Lake Environment. The Handbook of Environmental Chemistry, vol 80. Springer, Cham, pp. 481-500. doi:10.1007/698_2018_274
Pešić V., Broda Ł., Dabert M., Gerecke R., Martin P., Smit H. 2019a. Re-established after hundred years: Definition of Hygrobates prosiliens Koenike, 1915, based on molecular and morphological evidence, and redescription of H. longipalpis (Hermann, 1804) (Acariformes, Hydrachnidia, Hygrobatidae). Syst. Appl. Acarol., 24(8): 1490-1511. doi:10.11158/saa.24.8.10
Pešić V., Saboori A., Zawal A., Dabert M. 2019b. Hidden but not enough: DNA barcodes reveal two new species in Hygrobates fluviatilis complex from Iran (Acariformes, Hydrachnidia, Hygrobatidae). Syst. Appl. Acarol., 24(12): 2439-2459. doi:10.11158/saa.24.12.11
Pešić V., Savić A., Jabłońska A., Michoński G., Grabowski M., Bańkowska A., Zawal A. 2019c. Environmental factors affecting water mite assemblages along eucrenon-hypocrenon gradients in Mediterranean karstic springs. Exp. Appl. Acarol., 77 (4): 471-486. doi:10.1007/s10493-019-00360-w
Pešić V., Grabowski M., Hadžiablahović S., Marić D., Paunović M. 2020a. The biodiversity and biogeographical characteristics of the River basins of Montenegro. In: Pešić V., Paunović M., Kostianoy A. (Eds). The rivers of Montenegro. The handbook of environmental chemistry. Springer, Cham. doi:10.1007/698_2019_414
Pešić V., Paunović M., Kostianoy A., Pešić-Vukašinović V. 2020b. Rivers of Montenegro - from conflicts to science-based management. In: Pešić V., Paunović M., Kostianoy A. (Eds). The rivers of Montenegro. The handbook of environmental chemistry. Springer, Cham. doi:10.1007/698_2020_480
Pozojević I., Pešić V., Gottstein S. 2019. Two water mite species (Acari: Hydrachnidia) from karst springs new for the fauna of Croatia with notes on distribution and environmental preferences. Nat. Croat., 28(2): 417-424. doi:10.20302/NC.2019.28.27
Puillandre N., Lambert A., Brouillet S., Achaz G. 2012. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol., 21(8), 1864-1877. doi:10.1111/j.1365-294X.2011.05239.x
Shimodaira H., Hasegawa M. 1999. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol. Biol. Evol., 16(8), 1114-1116. doi:10.1093/oxfordjournals.molbev.a026201
Tuzovskij, P.V. 2017. On the systematic of the water mites Hygrobates nigromaculatus Lebert, 1879 and H. setosus Besseling, 1942 (Acari, Hydrachnidia, Hygrobatidae). Zootaxa, 4277 (1): 17-31. doi:10.11646/zootaxa.4277.1.2



2020-08-18
Date accepted:
2020-10-16
Date published:
2020-10-20
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Pešić, Vladimir; Jovanović, Milica; Manović, Ana; Zawal, Andrej; Bańkowska, Aleksandra; Broda, Łukasz; Martin, Peter and Dabert, Miroslawa
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



