Two unusual new species of Caleremaeus (Acari: Oribatida) from eastern North America, with redescription of C. retractus and reevaluation of the genus
Norton, Roy A.1 and Behan-Pelletier, Valerie M.2
1✉ State University of New York, College of Environmental Science and Forestry, Syracuse, New York, 13210, USA.
2Invertebrate Biodiversity Program, Research Branch, Agriculture and Agri-Food Canada, K.W. Neatby Bldg., Ottawa, Ontario, Canada.
2020 - Volume: 60 Issue: 2 pages: 398-448
https://doi.org/10.24349/acarologia/20204375ZooBank LSID: 393A73A9-253B-4A24-8635-C54C22326D10
Original research
Keywords
Abstract
`... Damaeus monilipes: the remarkable part of this creature is the form of the legs, particularly the first pair, where the tibia is a globular mass which appears altogether too large for the Arachnid, and gives it the effect of carrying a mace on each side' (Michael 1882)
Introduction
Caleremaeus Berlese, 1910 (Caleremaeidae) is a distinctive genus of middle-derivative, brachypyline oribatid mites with adult traits that make identification rather easy, even at modest magnification: enlarged first tibiae (see epigram) and a notogaster with a T-shaped pattern of two conspicuous dorsal bulges separated by a foveate sulcus. Only three extant and one fossil-based Caleremaeus species have been named, collectively distributed in the western Palaearctic and eastern North America.
The extant type species, Caleremaeus monilipes (Michael, 1882), is widely distributed in Europe but it seems absent east of the Ural Mountains (Krivolutsky et al. 1995). We discount the unexplained listing of this species from tropical Mexico (Vázquez and Prieto Trueba 1999) as an identification error; we have not encountered this species in the western hemisphere, despite much sampling. By contrast, little is known of the other extant species, both of which are similar in many respects to C. monilipes. Caleremaeus divisus Mihelčič, 1952 is known only from the original collection (arboricolous moss in Tirol, Austria) and the incomplete, poorly illustrated original description. Supposedly it differs from C. monilipes in features of prodorsal sculpturing and the size of the notogastral humeral projection, but it has been considered a species inquirendum (Schatz and Schuster 2009; Krisper et al. 2017). The North American species, C. retractus (Banks, 1947) also is known only from its relatively poor original description.
The other named species, Caleremaeus gleso Sellnick, 1931, is known only from Baltic amber. The original specimens are not among the material preserved from Sellnick's amber research (Ezhova and Kostyashova 1997) and appear to have been lost. Since its brief description (Sellnick 1931) the species has received no attention in the literature, other than in literature reviews and checklists (e.g. Krivolutsky et al. 1990; Labandeira et al. 1997).
While Caleremaeus seems to have little diversity, there are certainly more than three extant species. For example, based on an integrative analysis including morphometrics and DNA data, Krisper et al. (2017 and G. Krisper, personal communication, 2017) indicated that what authors have considered C. monilipes is instead a species-complex. This seems consistent with some rather striking differences in size and form, as reported and illustrated in the various published descriptions (cf. Michael 1882, 1888; Sellnick 1928; Willmann 1931; Kunst 1971; Bulanova-Zachvatkina 1975; Miko and Travé 1996; Subías and Arillo 2001; Weigmann 2006; Seniczak and Seniczak 2019).
During general studies on Nearctic oribatid mites we have accumulated numerous specimens of the genus. Many had been tentatively identified as C. retractus, but others represent three undescribed species, one from western and two from eastern North America, that have features not previously known in the genus. Our main purpose is to describe the latter two new species and to redescribe C. retractus, based on studies of adults and nymphs. We precede this with a new diagnosis and description of Caleremaeus that incorporates information from all species known to us.
Another goal is to reexamine the family-group classification of Caleremaeus. Prior to the proposal of Caleremaeidae by Grandjean (1965b), the principal works on oribatid mites had grouped Caleremaeus in Damaeosomidae (in part, modern Oppiidae; Sellnick 1928), Oribatidae (Vitzthum 1931), Eremaeidae (Willmann 1931, Vitzthum 1943, Radford 1950, Baker and Wharton 1952) or Oppiidae (Balogh 1961, 1963). Since 1965, we know of only one different classification: Vázques and Prieto-Trueba 1999 listed Caleremaeus as a genus of Anderemaeidae, but without explanation; it may have been a mistaken inversion, since Anderemaeus has been included in Caleremaeidae in some recent literature (see Norton and Ermilov 2019). Both the composition of Caleremaeidae and their position among eupheredermous superfamilies are discussed.
Materials and methods
Specimens examined — Examined Caleremaeus specimens and their provenance are detailed below, under the respective species. Adults and juveniles were sorted from stored samples of Berlese-funnel extracts. Juveniles were associated with adults from the same sample using criteria explained by Norton and Ermilov (2014): size and body-leg proportions were consistent with adults; features of the gnathosoma were identical, except for size; a single Caleremaeus species was represented by adults in each sample; and adults of no other oribatid mite species with similar size, proportions or gnathosoma were represented. An additional guide was the illustrated habitus of a C. monilipes nymph by Michael (1882), whose observations were based on laboratory-reared specimens, and the ontogenetic study of Seniczak and Seniczak (2019). For comparison and preparation of the generic redescription, we studied European specimens of C. monilipes in the first author's collection, including adults from Germany (near Berlin), Spain (Barcelona) and Sweden (Bohuslän), and a single deutonymph accompanying the adults from Sweden. Several specimens of Hungarobelba visnyai Balogh, 1938 from Poland were examined for comparison.
Sources and depositories for specimens include the following: CNC – the Canadian National Collection of Insects, Arachnids and Nematodes, Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada; MCZ – the Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, USA; RNC – the personal collection of Roy A. Norton, Syracuse, New York, USA; USNM – the National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (currently housed with the U.S. Department of Agriculture collections in Beltsville, Maryland).
Preparation and documentation — Most observations and data are from specimens temporarily mounted in cavity slides in a medium of lactic acid diluted with water (2:1; Grandjean 1949). Dissected mouthparts, legs and fragments of body regions requiring close study were permanently mounted in Hoyer's medium for observation with oil-immersion lenses. Compound microscopy employed bright-field, polarized, and Nomarski (DIC) illumination using a Nikon Eclipse E800 compound microscope; line drawings were made with the aid of a drawing tube. Light micrographs were obtained, usually as image stacks, with an AmScope MU800 digital camera mounted on the compound microscope. Image stacks were combined using the Helicon Focus Pro (v. 5.0) suite; the stacks varied widely in number of individual images, usually only several for highly magnified (1000 x) images and 15-30 for lower magnifications. As needed, images were adjusted with Adobe Photoshop (CS3) for contrast and color balance.
Specimens for scanning electron microscopy (SEM Quanta 600 FEI Company TM, Brno, Czech Republic) were removed from alcohol and cleaned by soaking in Terg-a-zyme® solution for 6–12 h, followed by brief (1–2 s) submersion in an ultrasonic bath. Specimens were critical-point dried using the EM CPD300 (Leica Microsystems, Vienna, Austria), mounted on Al-stubs with double sided sticky tape, and gold-coated in a Hummer sputter apparatus.
Taxonomic context — The general context is the classification used by Norton and Behan-Pelletier (2009) and Schatz et al. (2011), except as noted. A principal digression relates to our use of the superfamily names Eremaeoidea and Zetorchestoidea: these names are fully or partly synonymous according to different concepts in the literature and we use them both below, according to context. Herein, Eremaeoidea is used in the sense of Balogh and Balogh (1992)—including only Eremaeidae and Megeremaeidae—which is how it also seems to have been perceived by Franklin and Woas (1992). Recent classifications (e.g. Schatz et al. 2011) have grouped these families with Zetorchestidae under the older synonym Zetorchestoidea, but the molecular study of Lienhard et al. (2013) casts doubt on the monophyly of such a taxon. Author and date for species-group taxa are given at the first use of the name; those of supraspecific taxa can be found in Subías (2004).
Terminology and conventions — Morphological terminology is mostly that of F. Grandjean (see Travé and Vachon 1975 for references, Norton 1977 for leg setal nomenclature and Travé et al. 1996 or Norton and Behan-Pelletier 2009 for overview). Terms are translated from French (Hammen 1980) but Grandjean's original abbreviations and figure notations are often retained. Paired structures are described in the singular unless noted otherwise. Throughout, there are references to numbered `Remarks on morphology' that conclude the text; each reference is parenthetic, in the form `(R1, R2, etc.).' However, several specific terminology issues are explained here.
Surface sculpturing. Impressed surface-sculpture is referred to as foveate if circular depressions are relatively large and separated by less than their diameter; foveolate sculpture refers to circular depressions that are relatively small and separated by more than their diameter; scrobiculate refers to closely-spaced, parallel elliptical depressions (Harris 1979). Projecting structures are referred to as knots if relatively small, simple, dome-shaped, or tubercles if relatively large and conspicuous, especially if conical, triangular or tooth-like.
Lamella and tutorium. Like Grandjean (1965b), we interpret the two pairs of longitudinal ridge-like structures that are variously developed on the prodorsum of Caleremaeus species as homologues of the lamella and tutorium. We refrain from referring to the middle pair of ridges in Caleremaeus as `costulae'. The latter term appears to have originated in the first of several important synopses by Balogh (1961); he introduced a simplistic dichotomy between a costula (rib-like) and a lamella (blade-like) that still troubles the descriptive literature (Norton and Behan-Pelletier 2009). Clearly, both lamella and tutorium originated as longitudinal ridges, then hypertrophied to flattened, projecting blades in derived groups. The plesiomorphic form is retained in Caleremaeus and Megeremaeidae (see R1). When applied to the prodorsum, we believe the term costula is best restricted to describing simple, secondary supporting ridges in taxa that plesiomorphically lack lamellae, as in Oppioidea, for example.
Measurements and counts — Body length was measured in dorsoventral aspect, from the tip of the rostrum to the posterior edge of the hysterosoma; width refers to the maximum hysterosomal width in dorsal aspect. Measurements of specific structures or distances are given either as a single number meant to be representative of an average-sized individual, or an estimated range taken from a small sample of several individuals; setae were measured when oriented in a single observational plane (e.g., perpendicular to the surface). Setal and solenidial formulas represent counts per segment for appendages (from leg I to IV; famulus included for tarsus I); epimeral setation is given as number of pairs per podosomal segment (I-IV).
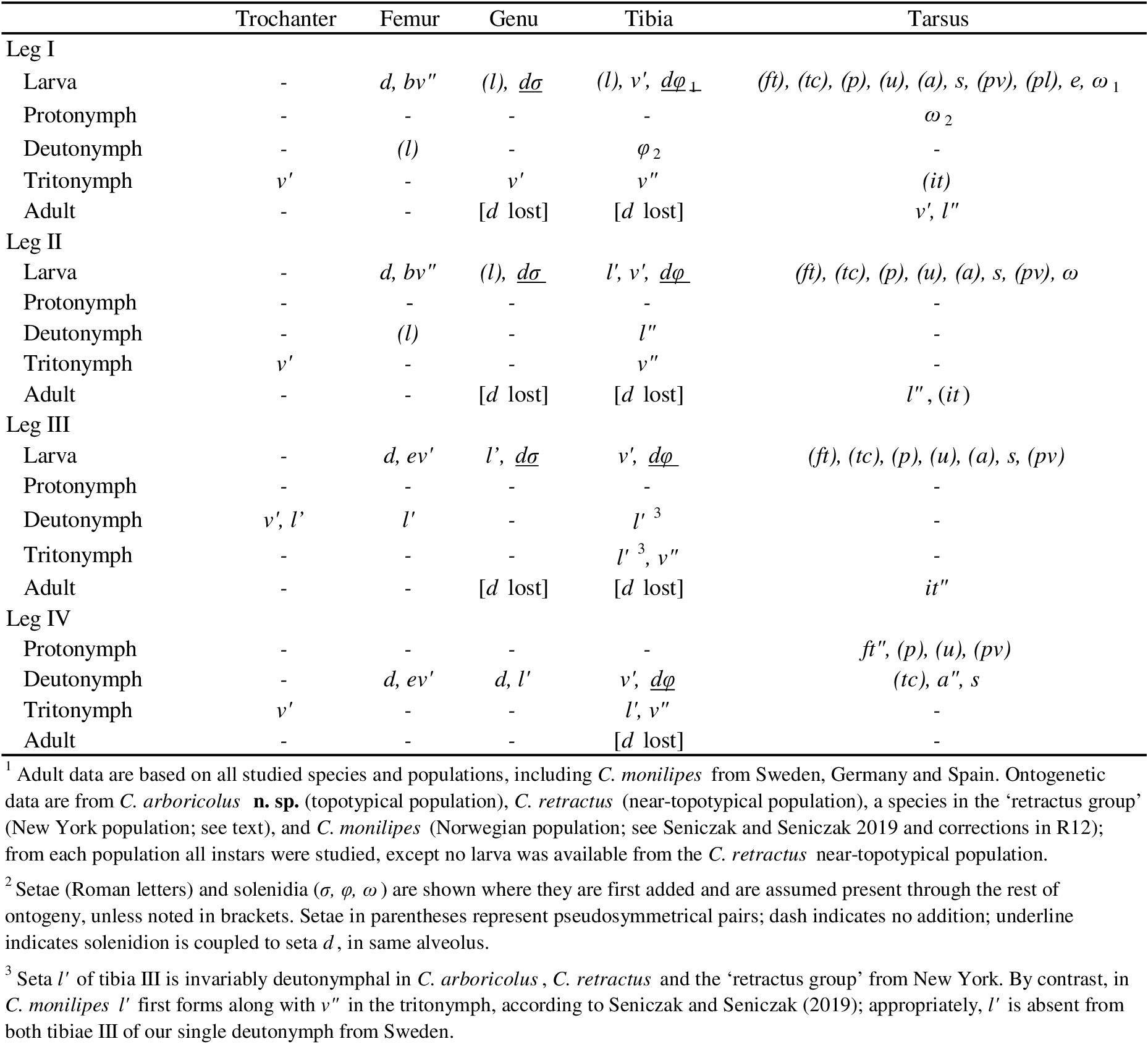
Abbreviations and notations — Prodorsum. Setae: ro, le, in, bs, ex – rostral, lamellar, interlamellar, bothridial and exobothridial setae, respectively. Other structures: apl – anterior prodorsal lobe; bo – bothridium; cu – lamellar cusp; dt – dorsosejugal tubercle; eA – prodorsal enantiophysis; exv – alveolar vestige of second exobothridial seta; lam – lamella; mu.gn – gnathosomal muscles; not –anterolateral bothridial notch; PD – prodorsum; rb – rostral bulge; rm – rostral margin; rph – rostrophragma; sej – dorsosejugal groove; smc – submarginal crest; tu – tutorium.
Notogaster, gastronotum. Setae: c (or c-row, c1, c2, c3 in juveniles); da, dm, dp (centrodorsal setae); la, lm, lp (laterodorsal setae); h-row (h1, h2, h3); p-row (p1, p2, p3). Other structures: ab – transverse anterior bulge; cgs – circumgastric scissure; eH – humeral enantiophysis; fov – foveae of transverse sulcus; gla – opening of opisthonotal gland; hpr – humeral process; ia, im, ip – anterior, middle, posterior lyrifissures (or cupules in juveniles), respectively; ih, ips – same, associated with setal rows, h and p, respectively; k – exuvial attachment cornicle of nymph; kp – coaptive pocket in larval exuvium to receive protonymph cornicle; NG – notogaster; pb – longitudinal posterior bulge of adult; pyb – pygidial bulge of nymphs; ts – transverse sulcus.
Coxisternum and lateral podosoma. Setae: 1a, 1b, 1c, 2a, 3a, 3b, 3c, 4a, 4b, 4c – setae of epimeres I–IV. Structures: bo.1, bo.2, bo.3, bo.4 – internally-defined borders of epimeres I–IV, respectively; bo.sj – sternal border; dis – discidium; dir – discidial ridge; e4 – aggenital enantiophysis, across groove running along bo.4; eL, eS – enantiophyses across sejugal groove (lateral and parastigmatic, respectively); mf – medial fossa of coxisternum – PdI – pedotectum I.
Anogenital region. Setae: ag – aggenital seta; ad1, ad2, ad3 – adanal setae;\down an1, an2 – anal setae. Other structures: AN – anal plate; GEN – genital plate (or aperture); iad, ian – adanal, anal lyrifissure (or cupules in juveniles), respectively; mu.gen – genital plate muscle; mu.ps – postanal suspensor muscle; ovp – ovipositor; po.st – postanal strut; pr.o – preanal organ; spr – spermatopositor.
Gnathosoma. Setae: a, m – anterior, middle seta of gena; h – hypostomal seta of mentum; v, l, d, cm, acm, ul, sul, vt, lt, sup, inf – palp setae; ω – palp tarsal solenidion; cha, chb – cheliceral setae. Other structures: br – rutellar brush; Ch – chelicera; ch.fr – cheliceral frame; ru – rutellum; sp – spicule of chelicera; Tg – Trägårdh's organ.
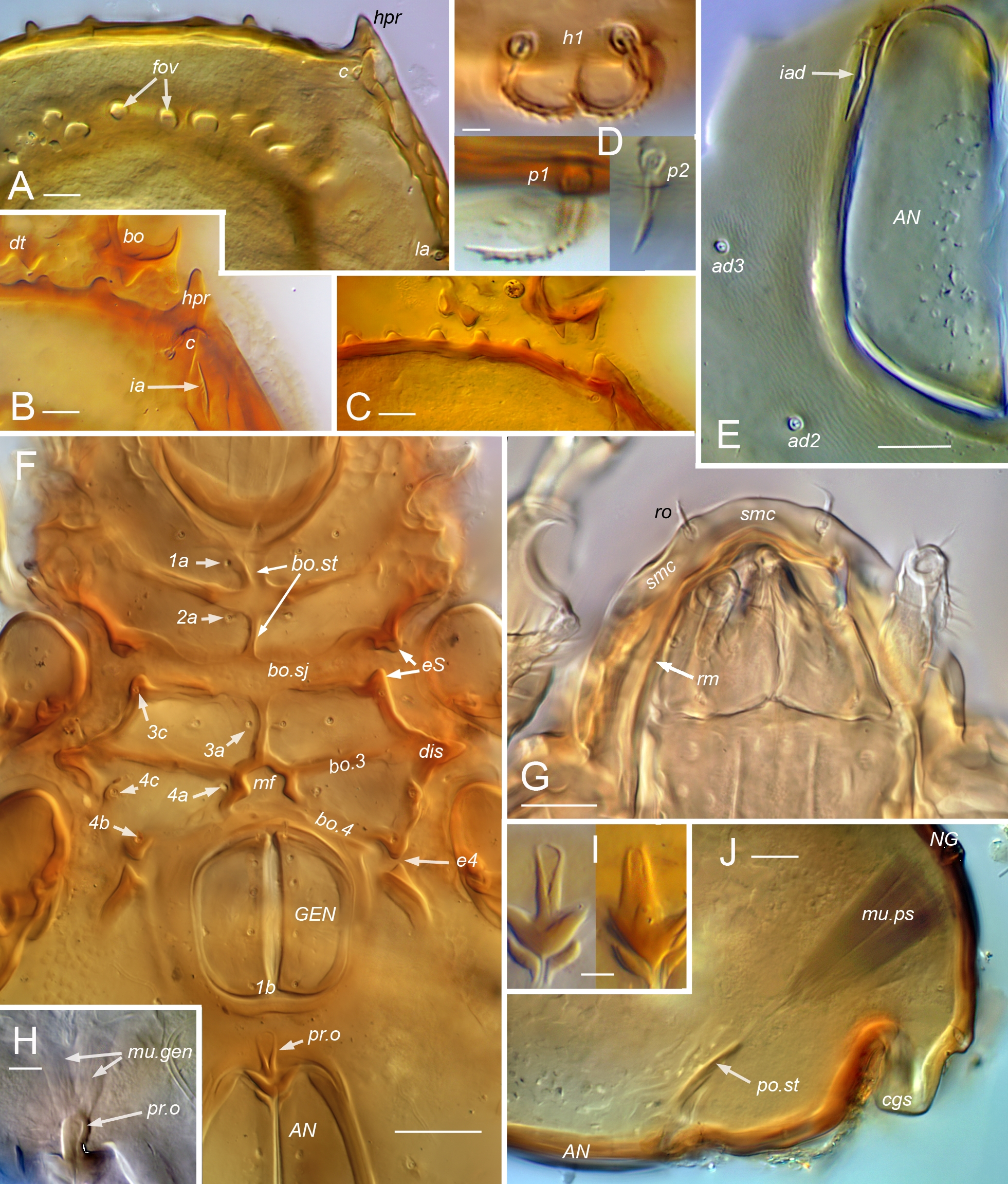
Legs. Setiform organs: σ, φ, ω – solenidia of genu, tibia and tarsus, respectively (with numeric subscript if relevant); e – famulus of tarsus I; d, l, v – dorsal, lateral, ventral setae of whorl, respectively; ev, bv – basal trochanteral setae; ft, tc, it, p, u, a, s, pv, pl – tarsal setae. Other structures: p.a – porose area; prt – protectum; ret – retrotectum; sac – saccule. Parentheses around leg setal notations denote the two members of a pseudosymmetrical pair on a given leg segment, rather than a true bilateral pair (unless otherwise indicated); when denoted separately, prime and second (', '') distinguish the seta on the anterior and posterior face, respectively.
In locality data, counties within USA states and Canadian provinces are indicated by Co.
Redescription of Caleremaeus
Berlese (1910) provided no diagnosis when Caleremaeus was proposed, but simply referred to the type species, C. monilipes. In early synopses (e.g. Sellnick 1928, Willmann 1931), genus-level characters were not distinguished. Nor did Grandjean (1965b) distinguish generic traits when he gave an extended diagnosis for the newly proposed Caleremaeidae, based only on C. monilipes. Generic characters seem not to have been isolated until Bulanova-Zachvatkina (1975) listed a single generic trait: the unique notogastral topography. Norton (1978) proposed including Veloppia as a second genus of Caleremaeidae and considered Grandjean's (1965b) diagnosis of this family as a diagnosis of Caleremaeus. Subsequently Subías and Arillo (2001) and Weigmann (2006) distinguished Caleremaeidae and Caleremaeus with brief diagnoses. Below, we use the latter three works and our own observations to propose a new diagnosis and expanded description of Caleremaeus. Parts relating to adults are based on all species known to us, both described and undescribed; those relating to nymphs are based on only C. monilipes, C. retractus (sensu stricto; additionally the `retractus' group from New York) and C. arboricolus; those relating to the larva are from C. arboricolus and the `retractus' group from New York, as well as the larval exuvial scalp of C. retractus, and literature data on C. monilipes. For C. monilipes, published information on adults is from references given above (Introduction), while that on juveniles is mainly from Michael (1882), Grandjean (1965b) and Seniczak and Seniczak (2019; but see R12).
Caleremaeus Berlese, 1910
Type species: Damaeus monilipes Michael, 1882 (p. 16). The original combination often has been given in the literature as Notaspis monilipes, but this was a later recombination by Michael (1888).
Etymology — Berlese (1910) did not indicate the etymology of Caleremaeus, but the stem eremaios is Greek (meaning solitary) and is the basis for the older genus name Eremaeus Koch, 1835. The prefix `cal', if also based on Greek, would be from kalos, meaning beautiful. While less likely, Berlese might have mixed languages: if `cal' were Latin-based it could relate to the habitat of the type species C. monilipes, which Michael (1882) collected from rotten wood (Latin: cala, piece of wood).
Diagnosis — Brachypylina with small to medium-sized adults (length 306–475 µm), overall shape elongate-pyriform in dorsoventral view. Integument with enveloping cerotegument, usually with dense, dome- to mushroom-shaped excrescences; sclerotized procuticle partly foveate to foveolate. Prodorsum with or without paired, ridge-like lamella and tutorium; with or without prodorsal enantiophysis. Rostrum with strongly developed submarginal crest. Bothridial seta with basal stalk and flattened, expanded head. Dorso- and pleurophragmata absent. Notogaster without porose organs; anterior margin nearly straight, with small dentate tubercles or knots; with distinct humeral process opposing tubercle(s) on posterior wall of bothridium to form humeral enantiophysis; with strong topography consisting of relatively flat lateral region and two strong bulges (transverse anterior bulge and longitudinal posterior bulge) separated by foveate transverse sulcus; with 10 pairs of setae, marginal to submarginal. Pedotectum I present, II absent; propodolateral apophysis absent; discidial ridge usually present, distinct discidium present or absent; circumpedal carina absent; lateral, parastigmatic and aggenital enantiophyses present; coxisternum with distinct medial fossa between setae 4a. Subcapitular rutellum atelobasic. Legs relatively short, tibiae I, II unusually large, with narrow basal stalk and swollen distal bulb, I with dorsodistal process; pretarsi monodactylous; seta d absent from genua I–III and all tibiae; iteral setal pair present on tarsi I–II, only it'' on III, none on IV. Nymphs plicate, eupheredermous, gastronotum with papilliform attachment cornicle; setal pair h1 adjacent on extension of pygidial sclerite; paraprocts atrichous in larva, proto- and deutonymph.
Adult
Facies, cuticle — Small to medium-sized (length 306–475 µm) Brachypylina, with overall elongate-pyriform shape in dorsoventral view (Figs 1, 8, 13). Integument with enveloping cerotegument conspicuously covering all exposed surfaces, mostly with dome- to mushroom-shaped excrescences of various sizes and densities (Figs 2C–D, 10B–C); prodorsal and notogastral setae usually with some type of isotropic coating or basal cerotegument nodule (Figs. 9J–K, 16A–D). Sclerotized procuticle medium-brown when mature, becoming paler, yellowish with alcohol preservation; partly foveate to foveolate. Usually with little or no adhering debris; exuvial scalps of juveniles not retained on notogaster.
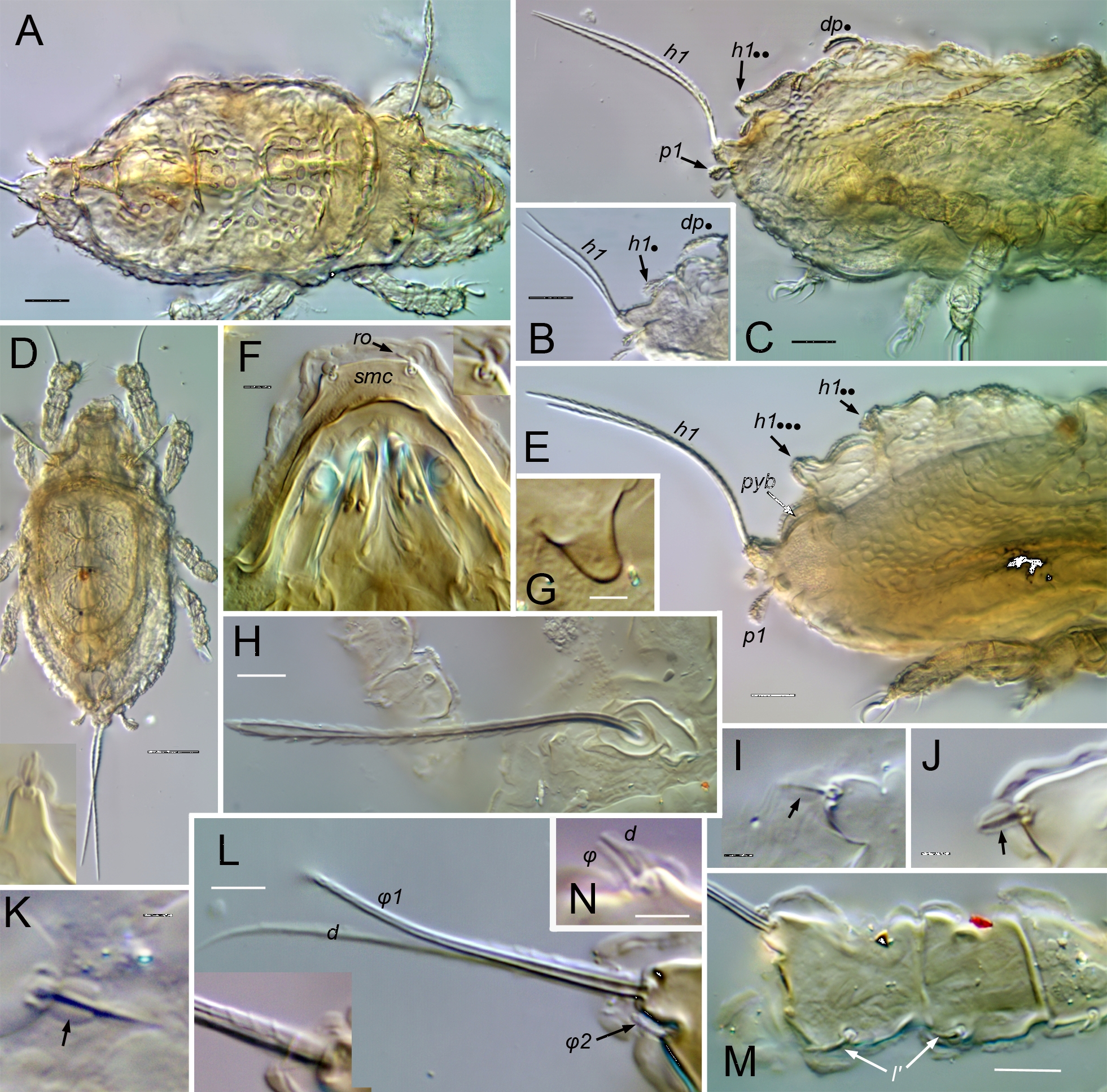
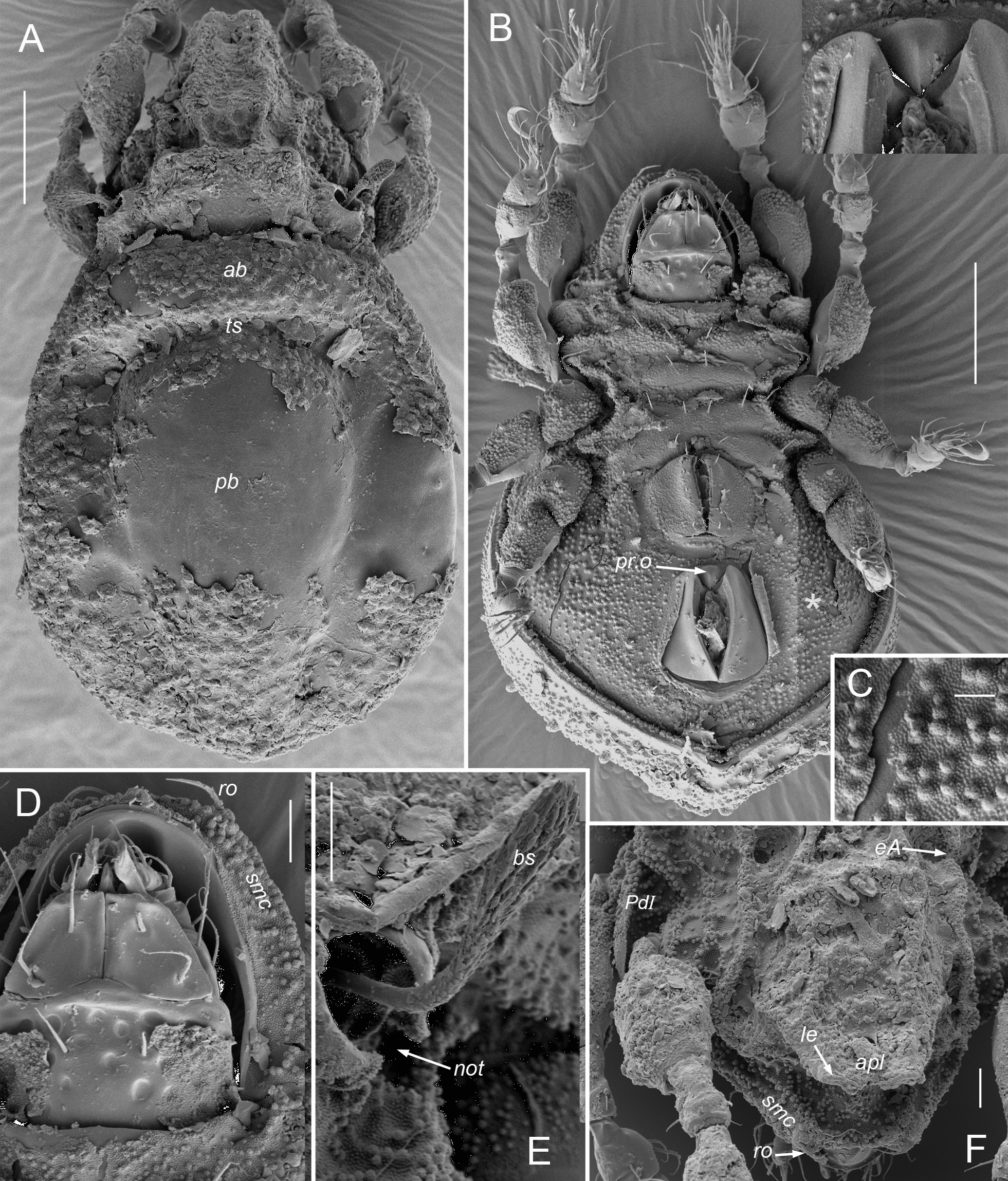
Prodorsum (Figs 1, 2, 8, 9, 13–15, 18) — With or without ridge-like lamella and tutorium (R1); with or without prodorsal enantiophysis (eA); with one (usually) to three pairs of small dorsosejugal tubercles (dt) anterior to sejugal groove, approximately aligned with seta in. Rostrum entire, broadly rounded to subtriangular in dorsal view. Rostral bulge (rb) conspicuous (e.g., Figs 1B, 2I, M, 9H) or not (Fig. 15E–F); ventral face of bulge and rostrophragma with (Figs 2K–M; 15F–H) or without embossed pattern (R2). Rostrum with shelf-like submarginal crest (Figs 2L, 3G, 15E, 18A; smc) extending from near acetabulum I to insertion of seta ro or beyond, forming most of lateral contour in dorsoventral view; without genal tooth or incision. Without dorsophragma or pleurophragma, gnathosomal muscles inserting directly on prodorsal cuticle (mu.gn; Fig. 9H); dorsal cheliceral retractor muscle attachments marked by paired longitudinal row of internal sigillae in oval to quadrilateral region between bothridia, usually with posterolateral corners of region marked by tubercle pair dt (Fig. 1A) or most medial tubercle if several present (Fig. 9E). Bothridium strongly projecting above surface, with well-sclerotized wall, broad anterolateral notch (Figs 9D, 14E; not), and one or more blunt teeth or tubercles on posterior wall; with two chambers—wide outer chamber smooth-walled in distal half, with several well-spaced circular ridges in proximal half; narrow inner chamber with dense fine striations, those of first part circular but abruptly changing to longitudinal in deeper, innermost part (Fig. 2F); without tracheal organ. Bothridial seta directed dorsolaterally, with basal stalk and flattened, expanded head, each occupying about half setal length; stalk smooth, head densely squamose with subtriangular, distally blunt scales, or with more vague ridges and elevations (Figs 2E, 9D–F, 14E). Seta in relatively small, at most about half mutual distance of pair; seta le inserted far distally, near or beyond level of ro, on cusp or other form of projection. Vestige of second bothridial seta (Figs 9I, 15E; exv) present (R3).
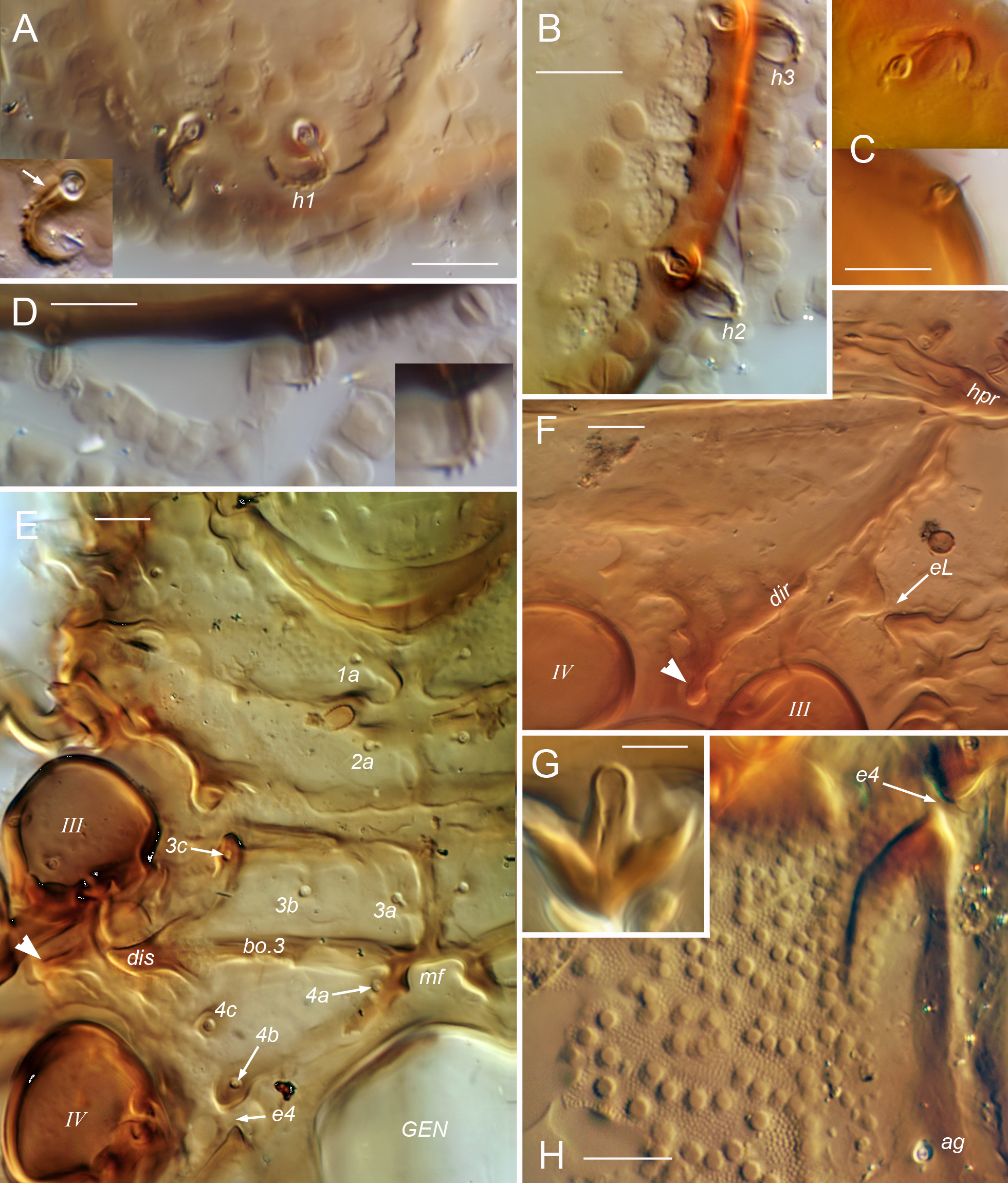
Notogaster — Without octotaxic system of porose organs. Fully fused to prodorsum across distinct dorsosejugal groove (Fig. 2M); circumgastric scissure broad posteriorly, but gradually narrowing anteriorly to efface in humeral region. Length about 1.2–1.3 times width, varying slightly with hysterosomal distension: slightly angular posteriorly when contracted; nearly truncate anteriorly with several to dozen knots or tubercles along margin (Fig. 3A–C). Humeral process (hpr) conspicuous, triangular in dorsal view, opposing bothridial tubercle(s) to form humeral enantiophysis (eH) across sejugal groove; not extended posteriorly as longitudinal carina (`crista'). Without marginal tectum. With strong topographic relief (Figs 2A–B, M; 14A), comprising relatively flat marginal zone and two tandem medial bulges separated by distinct transverse sulcus; anterior bulge (ab) transverse, between notogastral margin and sulcus, with setae of pair c on either side; larger posterior bulge (pb) longitudinal, tapering to nearly reach posterior margin. Bulges smooth (but see comments about C. gleso below, under C. arboricolus n. sp.), transverse sulcus with irregular row or narrow band of distinct foveae. Notogastral setae small to medium-sized; 10 pairs, six (c, la, lm, lp, h3, h2) forming single file in submarginal zone (Figs 1A–B, 8A, 13A); one pair (h1) inserting close together at posterior end of central bulge; three pairs (p1, p2, p3) inserting along posterior margin. With typical five pairs of lyrifissures; ia lateral to seta c, im near seta lm, obliquely oriented; ip lateral to central bulge, between setae p1 and h2 (Fig. 9M); ih and ips anterior to seta p3, positioned in tandem along posterolateral margin at approximate level of setae lp, h3, respectively (Fig. 1B). Opisthonotal gland opening (gla) inconspicuous, posterior to im.
Coxisternum and lateral podosoma (Figs 1B, 8B, 13B) — Coxisternum with well-defined transverse grooves associated with internally-marked epimeral borders (Fig. 14B). Muscle sigilla of coxisternum strongly developed, leaving most borders well defined in transmitted light (Fig. 3F): border bo.4 chevron-shaped, i.e. oblique on each side of midline; sternal border (bo.st) wide in epimere I, narrow in II and III. Apodemes 1–3 of typical size and form (Fig. 10E); without apodeme 4. Epimere IV with distinctly impressed, unpaired medial fossa (Fig. 3F; mf). Ventral and lateral podosoma with multiple tubercles: sejugal groove spanned by lateral enantiophysis (eL) dorsal to leg insertions and parastigmatic enantiophysis (eS) below insertions (R4); aggenital enantiophysis (e4) present, spanning groove along bo.4. Epimeres III, IV with (Fig. 3F) or without (Fig. 8B) well-defined ridges marking lateral depressions coapted to leg trochanters. Epimeral setation 3-1-3-3; seta 3c inserted on posterior tubercle of eS, 4b on anterior tubercle of e4 (R5). Pedotectum I small, with normal, scale-like form (R12); pedotectum II absent. With or without discrete discidium (dis), usually with oblique discidial ridge (dir) between acetabula III, IV running toward sejugal region (Figs 3F, 9I, 16E-F). Circumpedal carina absent. Tracheal system normal: trachea 1 and sejugal trachea bifurcated, trachea 3 simple.
Anogenital region (Figs 1B, 8B, 13B) — Curvature of ventral plate interrupted posteriorly by vague, rounded process, usually accommodated by vague notch in notogastral margin. Genital aperture subrectangular, well separated from anal aperture; latter wider posteriorly, pyriform to subpentagonal. Genital aperture of male proportionally smaller than that of female (relative to anal aperture: length 0.7, width 0.8 in male, 0.9, 1.0 in female). With 4–6 pairs of genital setae in single file near medial margin, distance between setae increasing posteriorly; single aggenital seta near posterolateral corner of each genital plate; two pairs of anal setae, near medial margin of plate; three pairs of adanal setae, ad1, ad2 posterior to anal plate, ad3 at about its mid-length. Preanal organ (pr.o; R6) with basal plate subtriangular, exposed between closed anal plates (Figs 8B, 14B); internalized apophysis (Fig. 3F, H, I) short, tubular to slightly expanding or tapering distally (i.e. internally). Lyrifissure ian absent; iad close to anterolateral corner of anal plate, parallel to margin (Fig. 3E). Posteromedial corner of each anal plate with long, narrow strut (Fig. 3J, po.st) extending internally to serve as attachment for postanal suspensor muscle (mu.ps). Ovipositor (Fig. 10F–H) of normal form and moderate size, length similar to body height when fully extended; three distal lobes long, narrow, occupying about half length beyond fold; with usual nine pairs of setae: three pairs of acute to acuminate coronal setae (ca. 10–12 µm), and four setae on each lobe; distal setae (ψ1, τ1) attenuate (ca. 20–30 µm), more proximal setae (ψ2 , τ2-4)\down acuminate (ca. 10–15 µm). Spermatopositor (Fig. 10D; spr) short, with typical form for Brachypylina; with seven pairs of acuminate to attenuate setae (ca. 6–10 µm) having typical distribution including pairs ψ1, ψ2 being closely adjacent posteriorly; mid-level seta (probably τ1) with alveolus distinctly larger than others (Fig. 10D, insert). Genital papillae with normal form, homogeneous.
Gnathosoma — Subcapitulum (Fig. 1C) diarthric, without axillary saccule at base of palp; rutellum (ru) atelobasic, with broad ventral lobe, two dorsal teeth and rutellar brush (br); setation normal, with single pair of hypostomal (h), two pairs of genal setae (a, m) and two pairs of inconspicuous adoral setae on narrow lateral lips; postpalpal seta baculiform, slightly curved. Palp (Fig. 1D) five-segmented with elongated cylindrical tarsus, about same length as genu and tibia combined; chaetome (trochanter to tarsus) 0-2-1-3-9(1), with tarsal setae relatively long, except acm, su and pair (ul) short, thick, eupathidial; solenidion ω independent, baculiform, nearly prone (R12). Chelicera (Figs 5E, 17D) without specialization: chelate dentate, with chela occupying about quarter total length; with few or no spicules (sp) on main body; with usual dorsal (cha) and abaxial (chb) setae and tapering Trägårdh's organ (Tr) on adaxial face.
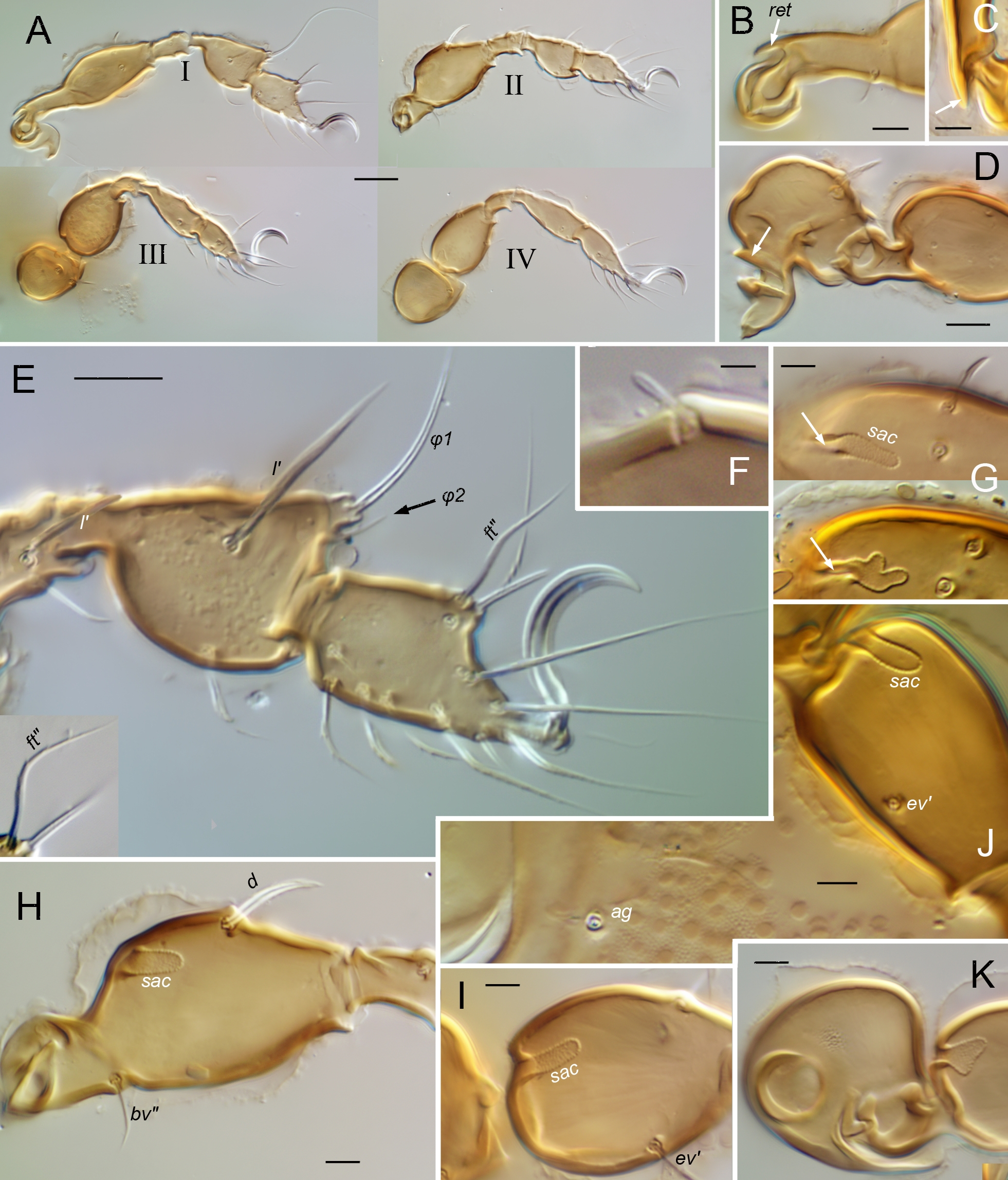
Legs (Figs 4-5, 11, 17) — Relatively short, about 0.4–0.5 times body length; legs II, III slightly shorter than I, IV. Articulations simple, without tecta except for partial retrotectum at base of femora I, II (Fig. 11B–C; ret) and trochanters III, IV, and partial ventrodistal protectum on trochanters III, IV (Fig. 5B–C; prt). Trochanters III, IV short, broad, somewhat asymmetrically mushroom shaped, short stalk hidden in lateral view; with porose area on adaxial face. Femora without ventral carinae, bulbous middle region gradually tapering distally, variously narrowed proximally to form short stalk; bulb with porose area or saccule. Genua with proximal half slightly narrowed, all similar in length. Tibiae I, II conspicuously bulbous distally, abruptly narrowed to short proximal stalk attached dorsally; tibia I distinctly deeper than tarsus I in lateral view, with broad dorsodistal apophysis bearing solenidia; tibia II with depth similar to tarsus II or only slightly greater; tibiae III, IV gradually tapered proximally. Tarsus I abruptly tapered distally at midlength, IV tapered gradually, II, III intermediate. Tibiae, tarsi without porose organ. Pretarsi monodactylous. Leg chaetome consistent among species (R7, R12), with salient features as follows. Solenidial counts (legs I–IV, genu, tibia, tarsus): 1-1-1-0, 2-1-1-1, 2-1-0-0; genual solenidia and tibial solenidion φ2 short, baculiform, φ1 flagellate (`tactile'), tarsal solenidia tapering but blunt (`ceratiform'). Setal counts (legs I–IV, trochanter to tarsus, famulus included,): 1-1-2-1, 4-4-3-2, 3-2-1-2, 4-4-3-3, 20-16-14-11; homologies in Table 1. Seta l″ of femora I and II very low on adaxial face. Seta d absent from genua I–III and from all tibiae. Tarsus I with proral (p) and subunguinal, s, setae eupathidial; fastigial setae differently shaped: ft′ nearly straight or slightly and gradually curved; ft″ smaller, bent at oblique angle or distally subflagellate (Figs 4A, 11E, 17C). Iteral pair present on tarsi I–II, only it″ present on III, no iteral seta on IV; proximal accessory setae present on tarsi I (l″, v′) and II (l″); seta a′ absent from tarsus IV. Famulus, e, isodiametric, baculiform.
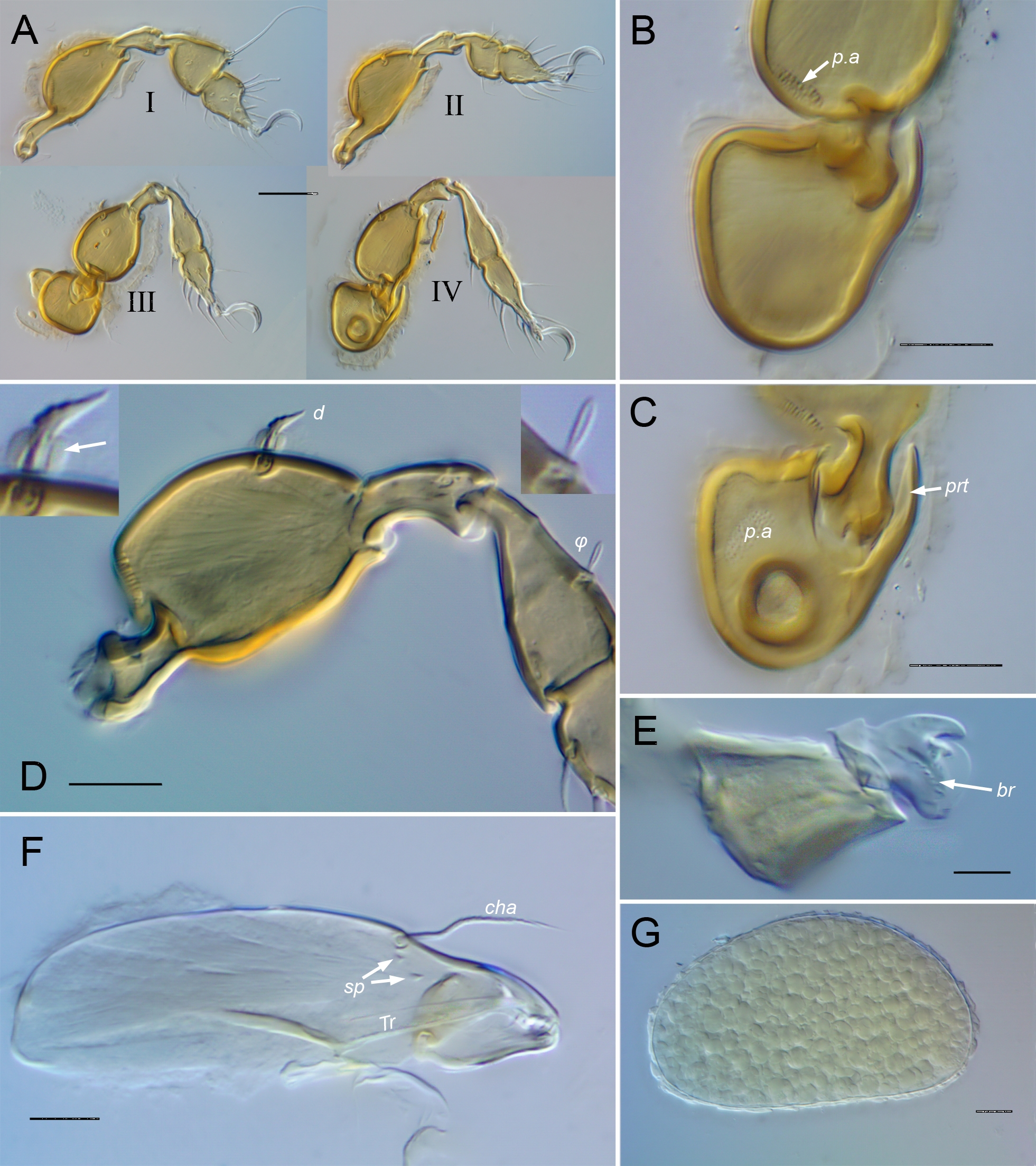
Juveniles
Figures 6–7, 12
Facies, cuticle — Preserved specimens colorless to light tan. Unsclerotized regions of gastronotic cuticle plicate, except smooth underneath exuvial scalps of nymphs; plicae generally vertical laterally, vaguely circumferential around opisthonotal gland opening and paraprocts. Hysterosomal line of dehiscence not evident. Cerotegument distinct, enveloping, with short excrescences of various sizes generally similar to those of adults or merged into irregular masses.
Prodorsum — With several ridges or folds, longitudinal laterally and transverse medially; one of latter bearing setal pair le. Rostrum usually truncate anteriorly (Fig. 7F). With normal setation, all but bothridial seta (bs) short to medium length. Bothridium and bs fully developed in all instars; bothridium projecting, cylindrical to slightly funnel-shaped, thin-walled, without tracheal organ; bs similar to that of adult, or narrower and proportionally longer.
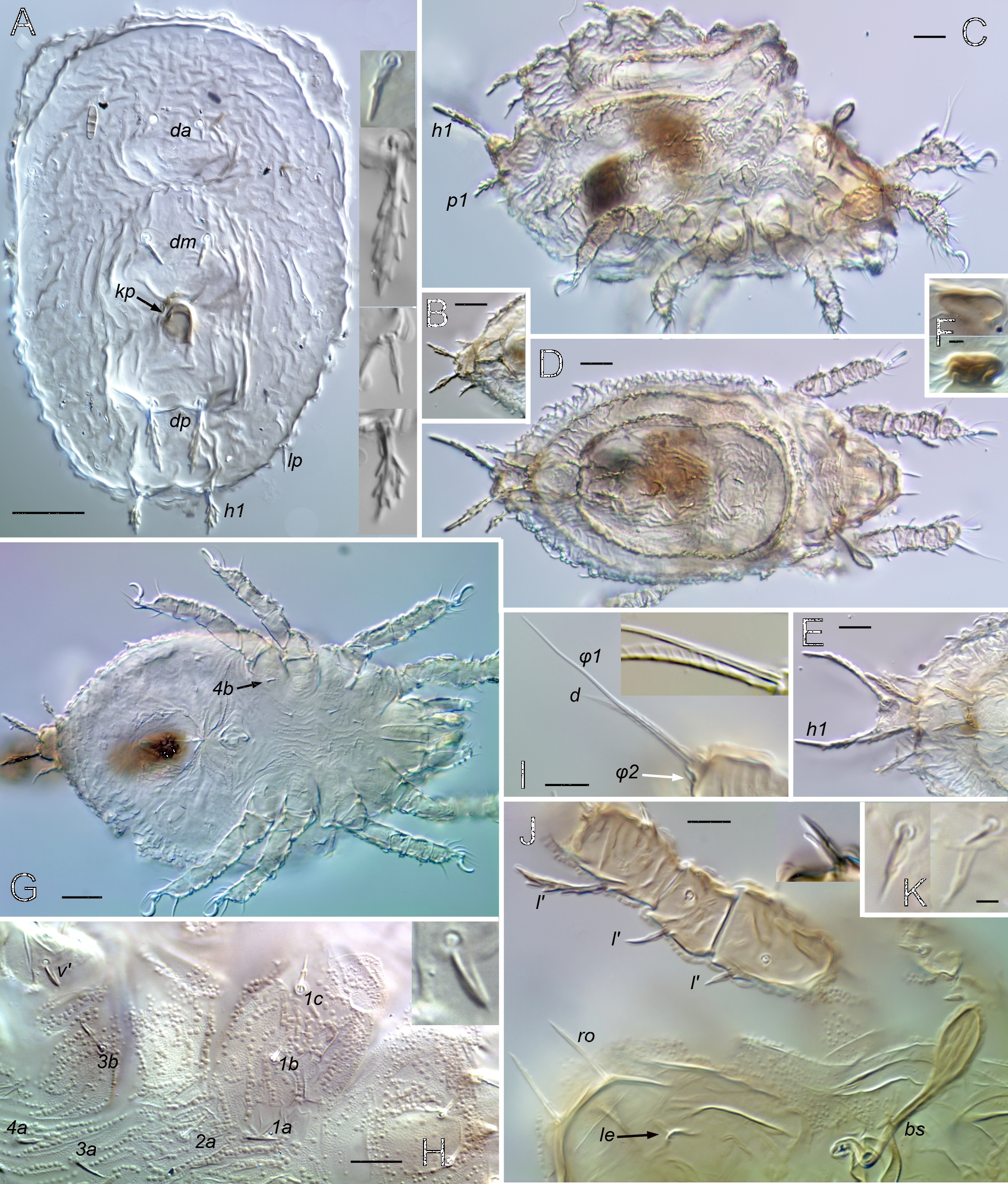
Gastronotum — Larva dorsally with strong topography comprising elevated anterior rim, three distinct tandem medial bulges and low, slightly bifid pygidial bulge (Figs 6A–B; 12A); with normal 12 pairs of setae: setae of pairs da, dm, dp adjacent on respective medial bulges, pair h1 on pygidial bulge. Gastronotum of nymphs with margins not covered by scalps, forming somewhat flattened rim, protruding most distinctly in anterior region (Figs 6B, 12C); without medial bulges; pygidial sclerite present, with bulge (Figs 6B, 7E; pyb) anteriorly and separate posterior lobe made terminally bifid by adjacent tubercles bearing setae h1. Nymphs with typical eupherederm gastronotic setation of 12 pairs, having lost dorsocentral setae (da, dm, dp) while gaining pseudanal setae p1, p2, p3. Pair h1 conspicuously enlarged in nymphs (R8). Exuvial scalps of previous instars tightly attached (R9) by means of small, papilliform, lightly sclerotized, centrally located cornicle (Fig. 7G; k); protonymphal cornicle inserting in coapted pocket (Figs 6A, 12A, F; kp) of larval scalp, under front slope of medial bulge bearing seta dp; cornicle of other nymphs nesting into previous respective cornicle (Fig. 12F). Scalps strongly reticulate or not, with setation typical of eupherederms: scalp of larva with c1, c2, da, dm, dp, la, lm, lp, h1; of proto- and deutonymph with c1, c2, la, lm, lp, h1-3, p1.
Coxisternum — Without brachytracheae or other representation of apodemato-acetabular tracheal system. With lightly sclerotized demi-epimeres, separated by broad band of soft cuticle having distinct to vague longitudinal plicae and rows of cerotegument granules (Fig. 12G). Claparède's organ of larva typical, emerging through slit-like opening, with seta 1c formed as protective scale; 1c normal in nymphs. Epimeral setation (larva to tritonymph; no change in adult): 3-1-2, 3-1-2-1, 3-1-2-2, 3-1-3-3; setae 1a, 2a, 3a, 4a all on soft medial cuticle, others on epimeral sclerite; seta 4a delayed to deutonymph, in normal manner (R5).
Anogenital region — Genital setation varies with species: protonymph with one pair, deutonymph with two or three, tritonymph with four or five. Aggenital seta first formed in deutonymph. Paraprocts atrichous in larva, proto- and deutonymph: setal rows p, ad, an first formed in proto-, deuto- and tritonymph, respectively. Ontogeny of cupules normal, cupule added with respective setal row; ian formed in tritonymph but half size of others (about diameter of setal alveolus), lost in adult.
Gnathosoma — Similar to that of adult, except for weaker sclerotization of subcapitulum making labiogenal articulation indistinct. Palp femoral seta inf first formed in protonymph.
Legs — With size proportions as in adult but most segments somewhat more tubular; tibia I with strong, cylindrical dorsodistal apophysis. Ontogeny of leg chaetome given in Table 1, with salient features as follows. Seta d present on all genua and tibiae, coupled in same alveolus with respective solenidion on all but genu IV; except on tibia I, seta and solenidion very short, equal in length or d very slightly longer (Figs 7N, 12J, 18D), solenidion difficult to see in some orientations. Seta d and φ1 of tibia I both long, inserting on dorsodistal apophysis; φ1 flagellate, d 2/3 to 3/4 as long, with barbs or cilia having clear, velum-like coating of various distinctness (Figs 7L, 12I). Protonymphal leg IV setation 0-0-0-0-7, with typical tarsal complement of (p), (u), (pv), ft″. On tarsus I, setae (p) appear to be eupathidial from larva (uncertain in early instars), but s formed as normal seta, becoming eupathidial only in adult; s located proximal to pair (a) in nymphs, but displaced distal to them when eupathidial, as usual. Ontogeny of iteral setae complex: pair forms in tritonymph on tarsus I and in adult on II, with solitary it″ tritonymphal on III (R7, R12).


Caleremaeus retractus (Banks, 1947)
Carabodoides retracta Banks, 1947; p. 123. [Nomen nudum in Pearse 1946, p. 148]
Caleremaeus retractus (Banks, 1947); Marshall et al. 1987, p. 225
Etymology — Banks (1947) presented no explanation of the species epithet, and there are no hints in his description. Marshall et al. (1987) treated `retracta' as an adjective and emended the name accordingly.
Type locality — The type specimens (below) derived from a study of soil animals of the Duke Forest (Durham Co., North Carolina) by Pearse (1946). The Duke Forest is somewhat fragmented and, since four different locations and habitats were studied, the exact origin of the types is unknown.
Type material — Banks (1947) reported two specimens in the type series. The holotype (original designation as `type') is a slide-mounted specimen in the arachnid collection of the MCZ. The label bears the following data in Banks' handwriting: `Duke Forest N. Car.; \#475; Pearse; Carabodoides retracta Bks; 3018 type.' The mite is broken by crushing but has an estimated total length of about 320 µm. The second specimen is a paratype in the mite collection of the USNM. It is a gravid female, also broken, with an estimated original length of 340 µm and with the following label data in Banks' handwriting: `Duke Forest, N.C. 238, Pearse, Jan. Carabodoides retracta Bks. Paratype.' The measurements contrast significantly with the `.55 to .6 mm' reported by Banks (1947).
Other material examined — Approximately 40 topotypic adults (24 females, 10 males, several undetermined) with the following data: North Carolina, Durham Co., Duke Forest (35° 58.9' N, 78° 56.6' W), 4-V-1979, L.J. Metz, col., from loblolly pine (Pinus taeda Linn.) and hardwood forest litter. Also available for study were 30 nymphs and more than 300 adults from a nearby location that we consider near-topotypes: North Carolina, Durham Co., G.W. Hill Demonstration Forest (36°12.1' N, 78°52.9' W), 1973 (various dates), L.J. Metz col., from loblolly pine forest litter and upper soil.
Diagnosis — Caleremaeus species with adults having total length 306–340 µm. Prodorsum with well-developed distal cusps. Each cusp longer than wide, pair usually close together and basally-connected; tip pointed, oblique beyond insertion of seta le. Lamella well defined proximally, with variable development more anteriorly; tutorium well developed, enantiophysis eA present. Usually with single small pair of dorsosejugal tubercles. Notogastral setae small (most 11–14 µm), inconspicuously barbed beyond basal cerotegument nodule. Epimeral groove 2 usually without bordering tubercles or knots; ventrosejugal groove with only enantiophysis eS. Female with five pairs of genital setae, male with 4–6. Leg femora each with porose area. Nymphs with bothridial seta elongate, nearly as long as prodorsum, squamose in distal half but without distinct head; gastronotic seta h1 with length similar to that of bs, narrow, barbed throughout, pair closely parallel and distinctly bowed; exuvial scalps appearing reticulated in transmitted light.
Adult
Figures 1–5



Dimensions — Total length of 20 measured topotypes 306–340 µm (mean 324); maximum width 172–203 µm (mean 189). Female (n = 10) length 323–340 µm (mean 333), maximum width 191–203 µm (mean 198); male (n = 10) length 306–328 µm (mean 313), width 172–191 µm (mean 179).
Integument, setae — Cerotegument excrescences mostly in form of near-spherical nodule with short, thick stalk (like unopened `button' mushroom). Nodules relatively uniform in size according to location: largest on mid-notogaster, 4–6 µm diameter (Fig. 2A, C, D), often well-spaced; slightly smaller (\textasciitilde3 µm) anteriorly and laterally on notogaster, densely packed, usually touching; smallest (\textasciitilde1–2 µm) on prodorsum, venter and legs; basal cerotegument layer on venter microgranular between nodules. Most dorsal setae (except bs) short, inconspicuous, acuminate; basal third hyaline, smooth, penetrating distinct, usually shaded, cerotegument nodule; distal region pigmented, with minute barbs on outer curvature (e.g. Figs 2G, 3D). Most ventral setae simple, nearly straight, without pigment or distinct cerotegument nodule.
Prodorsum — Surface usually foveolate, with well-spaced circular depressions mostly 5–8 µm diameter (Fig. 1A), foveate in some (Fig. 2G); tip of rostrum and subtriangular posteromedial region (with muscle sigilla) mostly smooth. With basally-connected pair of strongly projecting cusps (Fig. 2G; cu), longer than wide, each bearing lamellar seta (le) near tip; lateral margin of cusp distal to le insertion sharply oblique, forming pointed tip directed anteromedially at variable, often asymmetrical angle; paired tips often pincer-like, separated by only 2–8 µm. Cusp pair usually appear connected basally, with U-shaped inner margin, separated by less than their length, rarely with wider separation (Fig. 2H). Submarginal crest (smc) extending beyond insertion of rostral seta (ro), completely around front of rostrum (Figs 1B, 2J, I, 3G); cuticle scrobiculate immediately below crest. Rostral bulge (Fig. 2I, K, L) conspicuous, with embossed pattern on ventral surface. Lamella well defined posteriorly (level of acetabulum I); with variable development more anteriorly, either extending onto base of cusps (Fig. 2H) or (more commonly) effacing proximal to cusps (Figs 1A, 2G). Tutorium effacing anteriorly, well developed posteriorly to form anterior part of prodorsal enantiophysis eA (Fig. 1B); posterior tubercle of eA well-defined, conical, nearly touching tutorium. Short longitudinal ridge present laterally, between seta ex and acetabula I, II (Fig. 1B). With single pair of small, rounded to subrectangular dorsosejugal tubercles, located at corners of smooth sigillar region (Fig. 1A; dt); tubercle sometimes doubled or bilobed (Fig. 3B–C). Bothridial seta (bs; \textasciitilde55–60 µm) projecting dorsolaterally, slightly curved just outside bothridium, remainder straight; squamose head comprising half its length, lightly pigmented, usually with acute tip; stalk smooth, unpigmented (Figs 1A, 2E). Posterior wall of bothridium with strong, conical, tooth-like tubercle directed at humeral process of notogaster, and second more medial tubercle, usually smaller (Figs 1A, 3B–C). Seta le (\textasciitilde15 µm; Fig. 2G) strongly curved medially, tips of pair overlapping or not according to form of cusps; ro (\textasciitilde15 µm) curved anteroventrally, inserting on submarginal crest at level slightly posterior to le; seta in (15–20 µm; Fig. 3G) slightly curved or nearly straight, mutual distance 4–5 times length; seta ex (\textasciitilde15 µm) inserted between bothridium and short lateral ridge. Setal vestige exv ventral or posteroventral to ex, nearly touching its alveolus.



Notogaster — Length about 1.2 times width; usually evenly rounded posteriorly. Foveae mostly limited to transverse sulcus and to anterolateral region between setae c and la (Fig. 3A). Humeral process (hpr) medium-sized (8–10 µm long), tip usually not reaching bothridial tubercle across sejugal groove. Anterior margin (between hpr) irregular, with series of 4–12 small tubercles (up to 4 µm long), weak knots, or slight bulges (Fig. 3A-C). Most setae with form of prodorsal setae noted above, 11–14 µm; h1 with same form, but usually slightly larger (12–18 µm), medially curled, mutual distance of pair about equal to length; p2–p3 smaller (6–8 µm) and differently shaped, smooth, without pigment or basal cerotegument nodule (Fig. 3D). Lyrifissures ia, im, ip relatively large (10–14 µm; Fig. 3B), ips, ih smaller (\textasciitilde6 µm).
Venter and lateral podosoma — Foveate in acetabular region and lateral parts of coxisternum (Fig. 3F), and usually in single transverse row behind smooth mentotectum; coxisternum without foveae centrally; with irregular muscle sigilla on inner face of cuticle. Laterosejugal enantiophysis (eL) with strong conical to rounded tubercles. Epimeral groove 2 usually smooth. Enantiophysis eS variable in size, tubercles overlapping or not reaching each other; without other tubercles or knots along ventrosejugal groove. With strong, sculpted discidial ridge (dir) posterior to acetabulum III and separate conical discidium (dis) below it, near lateral end of epimeral border 3 (Fig. 1B). Lateral coaptive ridges well-formed, connecting posterior tubercle of eS, discidium, and (usually) anterior tubercle of e4 (Fig. 3F). Epimeral setae acuminate to attenuate (\textasciitilde12–15 µm). Aggenital enantiophysis (e4) strongly developed. Medial fossa of epimere IV often with small pair of projections on margin (Fig. 3F). Anogenital region relatively smooth, but with inconspicuous fine striation in adanal region (Fig. 3E). Apophysis of preanal organ slightly tapered to slightly expanded (Fig. 3F, H, I). Females consistently with five pairs of genital setae; males variable, 4–6 (of 18 male plates examined, nine with 4 setae, eight with 5, one with 6; asymmetrical in five of nine individuals). Anogenital setae acuminate; aggenitals and genitals \textasciitilde6–7 µm, anals \textasciitilde5–6 µm; adanals \textasciitilde6–9 µm (ad1 longest). Lyrifissure iad \textasciitilde12 µm (Fig. 3E).


Gnathosoma — Subcapitulum smooth or with few scattered foveolae; hypostomal (h, \textasciitilde14 µm) and genal (a, m, \textasciitilde15–18 µm) setae attenuate. Chelicera \textasciitilde80 µm long, with 0–2 small spicules; setae cha (\textasciitilde22 µm), chb (\textasciitilde15 µm) attenuate, barbed.
Legs (Figs 4–5) — Femora I, II similar in form: with abrupt transition from proximal stalk to bulb, junction at nearly right-angle; femur I \textasciitilde2.5, II \textasciitilde2.0 times longer than high in lateral view, stalk occupying \textasciitilde0.4 femoral length; stalk of femur II slightly broader than that of I. All femora with porose area, mostly on adaxial face of bulb. Tibia I with bulb markedly swollen, only \textasciitilde1.2 times longer than high. Tarsus I abruptly tapered in distal half, but without distinct projecting mid-dorsal bulge. Tarsus II without noticeable proximal stalk, depth similar to that of tibia in lateral view. Seta d of femora short, flame-shaped, similar in structure to dorsal body setae (pigmented, barbed, with conspicuous cerotegument nodule at base; Fig. 5D). Seta l' of genu and tibia I not conspicuously enlarged.





Juveniles
Figures 6–7



(Larva known only from exuvial scalp)
Length (without setae) and maximum width of protonymph 230 x 98 µm (n = 1); deutonymph 230–289 x 107–142 µm (n = 9); tritonymph 284–353 x 147–181 µm (n = 7). Bothridial seta (Figs 6A, 7H) straight, elongated, nearly as long as prodorsum, gradually thickening distally but without distinct head, slightly lanceolate with angular tip; distinctly squamose in distal half. Setae in, ex, le minute, hardly extending beyond basal cerotegument nodule; ro about twice as long but inconspicuous, curved ventrad. Seta le inserted on weak tubercle, slightly longer than wide; ro inserted on truncate rostral projection appearing like anterior part of submarginal crest of adult (Figs 6B, 7F). Gastronotic region of larva (based on exuvial scalp; Figs 6A, 7B–C) with setae c1, c2, la, lm, lp minute (\textasciitilde3–4 µm), smooth, acicular to nearly baculiform, hardly emerging from basal cerotegument nodule; dorsocentral setae closely paired (mutual distance 10–13 µm), da (\textasciitilde8–9 µm) and dm (\textasciitilde11–12 µm) slightly arched, with strong barbs on outer curvature, dp (\textasciitilde24–26 µm) weakly clavate, strongly squamose; h1 similar to dp but shorter (\textasciitilde15–17 µm). Setal pair h1 closely parallel, greatly elongated, \textasciitilde130–150 µm in tritonymph (length relative to body length \textasciitilde0.3 in proto-, 0.4–0.5 in deuto-, tritonymph); uniformly narrow except slightly tapered distally, with small but conspicuous barbs throughout. Nymphal seta p1 subclavate seen face-on but slightly flattened and cupped, strongly squamose on upper curvature (\textasciitilde20 µm in tritonymph); other gastronotic setae minute, simple, (4–8 µm in tritonymph), hardly emerging from basal cerotegument nodule (Fig. 7I–K). Ventral setae simple, acuminate, without cerotegument nodule; in tritonymph, epimeral setae 6–9 µm, genital and aggenital setae \textasciitilde4 µm, ad setae 6–7 µm, an 4–5 µm. Genital seta ontogeny variable: deutonymph with two or three pairs, tritonymph with four or five, valves sometimes with asymmetrical complement. Lateral setae of legs generally short, inconspicuous; l′ of tibia I hardly reaching distally to end of segment (Fig. 7M); seta d of tibia I with cilia and velum-like coating indistinct (Fig. 7L). Exuvial scalps conspicuously reticulated due to fovea-like excavations on underside (Fig. 6A–B, 7A–C, E); seta h1 consistently broken from nymphal exuviae.


Comparisons
Adults of Caleremaeus retractus are similar to those of C. monilipes (cf. Weigmann 2006, Miko and Travé 1996) and C. divisus in having a distinct lamella and tutorium and lamellar seta inserted on a strong cusp, traits that are absent from C. arboricolus and C. nasutus. According to the crude illustration of C. divisus, the cusp stops well short of the rostral margin, while reaching or surpassing it in C. monilipes and C. retractus; also, the anterior notogastral tubercles of C. divisus were drawn as far larger than those of other species, equal in size to the humeral process (Mihelčič 1952).
Adults of Caleremaeus retractus are distinguishable from those of C. monilipes (Cm) by: (1) having a less sculptured prodorsum, including weakly-developed lamella (stronger in Cm); (2) having nearly erect setae in, the pair separated by 4–5 times setal length (in slightly larger, curved mediad and separated by less than three times setal length in Cm); (3) lacking additional distinct knots or small tubercles along epimeral groove 2 and along the anterior edge of the sejugal groove between tubercle pair Sa (present in Cm); (4) having a modest humeral process that rarely reaches anteriorly to overlap bothridial tubercles (larger humeral process, overlapping with bothridial tubercle in Cm); (5) being smaller, with adult total length 306–340 µm (373–475 µm in Cm). Nymphs are distinguishable by: (1) the narrow, elongate form and closely parallel orientation of setae h1 in nymphs (distinctly clavate and divergent in Cm; Fig. 18C); (2) having generally smaller, less conspicuous leg setae (generally larger in Cm; cf. setae l' in Figs 7M, 18D); having smooth, small, inconspicuous seta ro (larger, conspicuous, with several strong barbs, projecting distinctly forward beyond rostral margin in Cm; Fig. 18D, see also Michael 1882).
Possible species group
As noted in the Introduction, morphometric and genetic evidence suggests that European records of Caleremaeus monilipes represent a complex of species (Krisper et al. 2017) that ultimately may be considered the `monilipes' species-group. The same may be true of the most similar North American species, C. retractus.
At an early stage of this study, we identified specimens of C. retractus from many locations in eastern North America, including the states of Alabama, Arkansas, Florida, Georgia, Louisiana, Illinois, Indiana, Mississippi, New Hampshire, New York, Vermont, Virginia and West Virginia, as well as the Canadian province of Quebec. Adults of these specimens are presently indistinguishable from those at the type location in North Carolina, except perhaps for a propensity of New York adults to have one or two small knots across epimeral groove 2 from seta 2a (Fig. 18E). But juveniles suggest that more than one species is involved. In addition to the near-topotypical material from Durham Co., North Carolina, we have juveniles from Florida and from New York (Onondaga Co.). The Florida nymphs are identical to the near-topotypes, but New York juveniles are easily distinguished by their shorter, straighter pygidial setae (Fig. 18G; h1), larger seta l' on tibia and genu I, and tibia I seta d with more distinct barbs and velum-like coating. As with near-topotypes, setae h1 of the New York population are adjacent, parallel and consistently broken from exuviae, unlike those of C. monilipes and C. arboricolus in which they are divergent and retained on exuviae.
The differences could represent geographic variation in these setae, but without further knowledge of juveniles from other locations, and especially without genetic data, we have no basis for judgement. At present, we prefer to assign specimens with the adult traits of C. retractus to a `retractus' species group unless juveniles are known and correspond with the near-topotypes.
Caleremaeus arboricolus n. sp.
ZOOBANK: CF4D144D-15F3-467B-94DF-67104DB0C404 ![]()
Etymology — The Latin species epithet `arboricolus' is an adjective referring to the microhabitat (tree bark) of this species.
Material examined — Holotype adult and 35 adult paratypes from: USA, New York, Onondaga Co.; Syracuse, Oakwood Cemetery (43° 01.9' N, 76° 07.9' W); 10.ix.2008; R.A. Norton and D.A. Saunders, col., from mixed lichens on boles of living trees (washed and sieved). Ten adult paratypes from same location, but 24.vi.2019. One larva and 16 nymphs (non-type) from same two collections. Holotype and three paratypes deposited in USNM; five paratypes in CNC; remainder in RNC. Other material (all adults; in CNC and RNC) as follows. USA: Alabama: Baldwin Co., Bon Secours National Wildlife Refuge, Jeff Friend Trail (30º 14.6' N, 87º 47.21' W), 6.iii.1994, V. Behan-Pelletier, col., 9 from lichens on horizontal tree trunk. Maine: Penobscot Co., Orono, University of Maine Experimental Forest, x.1987, C. Stubbs col., 19 from lichens on red maple (Acer rubrum Linn.) trunk. New Jersey: Middlesex Co., New Brunswick, 1978, J. Walker col., 1 (no other data). New York: Essex Co., Newcomb, Huntington Wildlife Forest, H. Root, col., vii-viii-2002, 11 from lichens on sugar maple (Acer saccharum Marshall) [as Caleremaeus sp. 1 in Root et al. 2007]; Onondaga Co., LaFayette Experiment Station (SUNY-ESF), 19.vii.1973, J.R. Philips col., 1 from regurgitated Great Horned Owl pellet in hardwood forest. Tennessee: Anderson Co., Great Smoky Mountain National Park, Indian Gap, 5200' (elev.), 18.vi.1957, W.R.M. Mason col. Virginia: Page Co., Shenandoah National Park, Stony Mountain Trail, Skyland area, 4000' elev., 14.viii.1986, E.E. Lindquist col., 1 from lichen mats on tree trunks and rocks. Canada: Ontario: Leeds-Grenville Co., Near Otter Lake (34º 34.87' N, 76º19.77' W), 23.vii.2003, J. Chen, V. Behan-Pelletier, J. Johnson col., 15 from cedar twigs (4m from ground), 5 from juniper twigs (<1m from ground).
Diagnosis — Caleremaeus species with adults having total length 311–353 µm. Prodorsum without distinct distal cusps; lamellar setae inserted on transverse ridge, each on slightly projecting tubercle; lamella represented by only indistinct apparent vestige; tutorium weakly developed; enantiophysis eA absent; oblique row of 2–3 knots in place of typical dorsosejugal tubercle. Notogastral setae conspicuous, erect, mostly thick and squamose, some with coating but without basal cerotegument nodule. Epimeral groove 2 usually without bordering tubercles or knots; ventrosejugal groove with only small, somewhat irregular and variable enantiophysis eS. Females and males consistently with five pairs of genital setae. Leg femora each with saccule. Nymphs with bothridial seta having squamose, flattened head, similar to that of adult; gastronotic seta h1 thick, straight, squamose, pair distinctly diverging; exuvial scalps not reticulated in transmitted light.
Adult
Figures 8–11
Dimensions — Total length (n = 20) 311–353 µm (mean 332); maximum width 174–216 µm (mean 195). Female (n = 10) length 328–353 µm (mean 342), maximum width 174–216 µm (mean 204); male (n = 10) length 311–333 µm (mean 322), maximum width 181–191 µm (mean 185).
Integument, setae — Cerotegument excrescences ranging from dome-like to near-spherical nodules on short, thick stalk (Fig. 10B). Excrescences mostly 4–5 µm diameter on notogaster, closely spaced (mostly less than their diameter apart); dark, dense band of smaller excrescences (2 µm) around posterior and lateral notogastral margin; prodorsum and ventral plate with inconspicuous, small (1–2 µm) domes interspersed with dense, minute granules (Fig. 10C). Setae of various forms, but without conspicuous basal cerotegument nodule; some with pigmented coating (possibly cerotegument; e.g. Fig. 9J–K).
Prodorsum — Central region foveate, with closely spaced circular depressions, mostly 6–8 µm diameter; foveation distinct between strong anterior transverse ridge and weakly-defined transverse ridge at level of acetabulum I (Fig. 9A). Lamella apparently vestigial, represented only by short, thin longitudinal carina. Without projecting cusp; setal pair le inserted on strong transverse ridge, each on distinct lateral tubercle (Fig. 9B–C). Interbothridial region with 2–3 knots (rarely one) in oblique transverse row on either side of central sigilla (Fig. 9E), sometimes with weakly-defined ridge parallel to row, between it and seta in (Fig. 8A). Submarginal crest ending anteriorly at seta ro; area between crest and rostral margin smooth, not scrobiculate (Fig. 9G). Rostral bulge moderately developed, without embossed pattern on ventral face (Fig. 9H). Tutorium weakly developed, gradually effacing both anteriorly and posteriorly; prodorsal enantiophysis absent. Lateral face highly sculptured (Fig. 9G): with several small tubercles or knots between bothridium and acetabulum I; with scalloped concentric carina or series of several short oblique ridges immediately anterior to parietal wall of acetabulum I. Variably foveate between pedotectum I and acetabulum II. With short transverse ridge above pedotectum I. Bothridium with anterior wall weakly foveate; posterior wall usually with one strong tooth (sometimes two) and 1–2 additional weak teeth or knots (Fig. 9D–E). Bothridial seta (50–60 µm; Fig. 9D–F) flattened, with clavate outline seen face-on, distally rounded or slightly acute; head strongly pigmented, vaguely squamose, with low, narrow, blunt scales or mounds in linear, slightly radiating pattern; stalk smooth, not noticeably pigmented. Setae in and le short, distinctly barbed, pigmented and with isotropic coating; in (\textasciitilde12–15 µm), acuminate, le (15–25 µm) isodiametric to slightly clavate (Fig. 9B–C). Seta ro (20–27 µm) unpigmented, uncoated, with weak, inconspicuous barbs. Seta ex simple, minute (4–5 µm); vestige exv posteroventral to ex, separated by at least one alveolar width (Fig. 9I).
Notogaster — With 6–12 knots on anterior margin, mostly 5–7 µm diameter, often weakly defined (Figs 8A, 9A); humeral process relatively small, not projecting beyond anterior margin. About 12–15 strongly defined foveae (5–8 µm diameter) in transverse sulcus; otherwise without fovea or foveolae. Posterior bulge rather evenly tapered posteriorly; each lateral margin of bulge with nearly linear series of 8–15 knots or short ridges (Figs 8A, 10A). Setae of several types: lp, h3, h2 (25–30 µm) lightly pigmented, flattened, subclavate seen face-on, somewhat cupped and curved, with numerous conspicuous barbs on outer face and pigmented coating of variable thickness (Fig. 9J–K); h1 similar but strongly curved mediad, mutual distance of pair equal to setal length or less; la, lm similar to lp but narrower, shorter (20–22 µm); seta c (12–15 µm) not broadened or only slightly so, and with little or no coating; p-row not broadened and without coating, p1 (\textasciitilde25 µm) usually with strong, sharp point and few but strong barbs, p2, p3 very short (8–9 µm), spiniform, smooth or with 1–2 small barbs (Fig. 9L).



Venter and lateral podosoma — Epimere I foveate laterally; other epimeres without depressed features other than epimeral grooves and medial fossa on epimere IV, though strongly marked internal muscle sigilla often give reticulate impression in transmitted light (Fig. 8B). Development of coxisternal knots and tubercles somewhat variable: usually with several minute knots anterolateral to seta 2a and 2–3 others opposing them on epimere I across shallow epimeral groove 2; usually with small tubercle bearing seta 3c opposing 1–2 others across sejugal groove (comprising enantiophysis eS); knot or small tubercle sometimes present closely anterolateral to seta 4c; epimeral groove 4 with relatively small aggenital enantiophysis (e4) but without other tubercles or knots. Lateral region of epimeres III, IV without distinct coaptive ridge associated with leg trochanters. Without discrete discidium, but with distinct discidial ridge reaching anterodorsally to sejugal groove (Fig. 9; dir), sometimes appearing like discidium in optical projection (Fig. 8B). Ventral setae simple, acicular, each inserting on small, low but distinct basal tubercle. Coxisternal setae 15–20 µm. Both genders consistently with five pairs of short (7–9 µm) genital setae; anal setae similar (10–12 µm). Adanal setae slightly longer: ad1 (18–20 µm) posteriorly positioned, slightly shorter than mutual distance of pair; ad2 similar, at posterolateral corner of plate; ad3 shorter (10–12 µm), inserted about its length from plate margin, at level slightly posterior to middle of plate. Lyrifissure iad 6–7 µm long. Apophysis of preanal organ tubular. Ventral plate outline slightly bulging posteriorly between setal pair ad1.



Gnathosoma — Subcapitulum without foveae, hypostomal (h, \textasciitilde15 µm) and genal (a, m, 15–17, 20–25 µm respectively) setae attenuate. Chelicera 75–80 µm long; without spicules; setae cha (\textasciitilde20 µm), chb (\textasciitilde15 µm) attenuate, barbed.



Legs — Femur I elongated, 4–4.5 times longer than maximum height in lateral view, with gradual transition between stalk and bulb, at distinctly oblique angle (Fig. 11A). Femur II (Fig. 11H) differently shaped: length only slightly more than twice height, with very short stalk, distinctly broader than that of femur I. All femora lacking surficial porose area but with short, broad saccule opening through short slit on proximal wall of bulb; saccule shape variable – simple, sausage-shaped or with irregular width (Fig. 11G–J). Trochanters III, IV with normal adaxial porose area. Genua and tibiae often with irregular dorsal contour due to several transverse ridges; genu and tibia I with enlarged seta l', that of genu surpassing distal end of segment, that of tibia equal or greater than maximum tibia height (Fig. 11E). Tarsus I with slight projection mid-dorsally, bearing solenidia, famulus and seta ft''. Femoral seta d normal, acute, straight to slightly curved, without basal cerotegument nodule (Fig. 11H).


Juveniles
Figure 12
Length (without setae) and maximum width of larva (La) 196 x 92 µm (n = 1); protonymph 220 x 99 µm (n = 1); deutonymph 252–284 x 123–137 µm (n = 4); tritonymph (Tn) 294–319 x 157–167 µm (n = 2). Setae without conspicuous basal cerotegument nodule, but many with clear, basal isotropic sleeve, most conspicuous in small setae (e.g. Fig 12A, lp). Bothridial seta similar to that of adult: with distinct flattened head, clavate seen face-on, vaguely squamose (Fig. 12J). Seta ro conspicuous, projecting forward (\textasciitilde17 µm in La, 25 µm in Tn), longer than mutual distance of pair, acicular, weakly barbed, with inconspicuous coating near base; setae le, in and ex minute (\textasciitilde5 µm in La, \textasciitilde7 µm in Tn), baculiform to acute, thickened by nearly complete isotropic coating, le of nymphs inserted on small tubercles. Larva with setae c2, c3 and dorsolateral series small, smooth, baculiform to acute, not or hardly emerging from clear sleeve (Fig 12A; lp). Middle row pigmented, with length and roughness increasing posteriorly, basal coating less conspicuous or apparently absent (Fig. 12A): c1 (\textasciitilde5 µm), da (\textasciitilde12 µm) and dm (\textasciitilde15 µm) blunt, with one to several coarse barbs; dp (\textasciitilde25 µm) coarsely squamose with acute tip, slightly longer than mutual distance of pair; seta h1 (\textasciitilde20 µm) directed straight posteriorly or slightly diverging, shorter than mutual distance, broadest and coarsely squamose in middle. Setae h2 (attenuate, \textasciitilde15 µm) and h3 (acicular, \textasciitilde5 µm) inserted close to paraprocts. Nymphs with gastronotal setae in c- and l-series small (8–12 µm in Tn), simple, baculiform to slightly tapered, extending little beyond coating. Nymphs with h1 large (\textasciitilde80 µm in Tn), distinctly diverging, pigmented, slightly broadened and squamose in middle, distally blunt, setae and supporting posterior lobe upturned in deuto- and tritonymph but not protonymph (Fig. 12C); h2,\down h3 (25–30 µm in Tn) pigmented, coarsely barbed to squamose. Seta p1 (30–40 µm in Tn) broadened and squamose in middle, distally blunt; p2 (\textasciitilde15 µm in Tn) simple, acicular to acuminate. Ventral setae simple, acuminate to attenuate, with coating in basal half; in Tn epimeral setae 10–15 µm, genital and aggenital setae 8–10 µm, ad setae 10–12 µm, an 4–5 µm. Genital seta ontogeny consistently 1-2-4-5 (protonymph to adult). Ventral cuticle with microgranulate cerotegument, small excrescences often arranged in short lines (Fig. 12H). Ventral setae and those of basal leg segments short, simple, with full isotropic coating, sometimes displaced during preparation (Fig. 12H, K). Lateral setae on legs well developed, conspicuous; l' of tibia I enlarged, coarsely barbed to squamose, extending distally well beyond end of segment (Fig. 12J); seta d of tibia I with distinct barbs and velum-like coating (Fig. 12I). Exuvial scalps not reticulated, ornamented only with surface plication (Fig. 12A); seta h1 consistently retained on all exuviae.


Comparisons
Adults of C. arboricolus are distinguishable from those of all described extant Caleremaeus species in: having a prodorsum lacking enantiophysis eA and having the dorsosejugal tubercle (dt) represented instead by a linear series of usually three knots; having a notogaster with a series of knots outlining the posterior bulge and setae that are conspicuous, squamose (except p2, p3); and saccules on leg femora instead of porose areas.
The most similar described species is C. gleso, which is known only from Baltic amber. Based on Sellnick's (1931) description and sketchy dorsal illustration of an adult, C. gleso shares with C. arboricolus the absence of distinct prodorsal cusps extending forward from a transverse ridge and notogastral setae that are clavate, at least in part. C. gleso differs from C. arboricolus in having: a prodorsum with larger, more clavate lamellar setae; a more sculptured central prodorsum (perhaps with more defined lamellae); and a depression on either side of the transverse sulcus. The notogaster was described as having foveate anterior and posterior bulges, but the sclerotized cuticle of these structures is smooth in all known extant species. Considering the difficulties of observing small amber inclusions, Sellnick may have mistaken the round cerotegument excrescences for `pits', but this can be confirmed only if the species is rediscovered.
Caleremaeus nasutus n. sp.
ZOOBANK: 38F837A3-7D6C-4D2C-B339-D4183DA29EBB ![]()
Etymology — The Latin species epithet `nasutus' is an adjective referring to the large nose-like anterior lobe of the prodorsum.
Material examined — Holotype and 13 paratype adults from: USA, Alabama, Randolph Co., State Route 48, ca. 1 km east of Woodland (33° 22.8' N, 85° 23.4' W);, 3.xii.1980, R.A. Norton col., from litter layer in young shortleaf pine (Pinus echinata Mill.) and mixed-oak (Quercus spp.) forest. Other material: Another 75 paratype adults are from Alabama, Lee Co., near Auburn, 7.xi.1975 (collector unknown), from litter in loblolly pine (Pinus taeda Linn.) forest. Holotype and 10 paratypes deposited in USNM; 15 paratypes in CNC, remainder in RNC.
Diagnosis — Caleremaeus species with adults having total length 284–348 µm. Prodorsum without cusp or distinct lamella; lamellar setae inserted on large, hollow, tongue-shaped anterior lobe; tutorium weakly developed, enantiophysis eA present; usually with single small pair of dorsosejugal tubercles. Notogastral setae small (mostly \textasciitilde10–15 µm), most barbed and curled beyond basal cerotegument nodule. Epimeral groove 2 without bordering tubercles or knots; ventrosejugal groove with only simple enantiophysis eS. Genital setation variable; females usually with five, males with 4–6 setae on each plate. Leg femora each with porose area. Juveniles unknown.
Adult
Figures 13–17



Dimensions — Total length of 20 paratypes 284–348 µm (mean 326); maximum width 162–196 µm (mean 180). Female (n = 10) length 336–348 µm (mean 342), maximum width 186–196 µm (mean 191); male (n = 10) length 284–323 µm (mean 309), width 162–181 µm (mean 169).
Integument, setae — Cerotegument excrescences mostly in form of dome- to mushroom-shaped or near-spherical excrescences, but partly irregular in form (Figs 14, 16A, B, H); largest on notogaster (mostly 3–5 µm diameter), smallest (1–2 µm) on venter where microgranules cover and fill intervening space (Fig. 14C–D). Dorsal setae (except bs) short, inconspicuous, acute to acuminate; basal third hyaline, birefringent, smooth, straight, surrounded by distinct and usually shaded cerotegument nodule; distal region isotropic, pigmented, curved to curled, with minute barbs on outer curvature (Fig. 16A, B, D), sometimes pulled away during preparations (Fig. 16C). Ventral setae (except adanal series) simple, nearly straight, without pigment or distinct basal cerotegument nodule.


Prodorsum — Surface foveate (depressions mostly 7–9 µm diameter) except posteromedial region (above sigilla for cheliceral retractor muscles) smooth. Without lamella or cusp; setal pair le inserting on large, hollow, tongue-shaped anterior lobe (Figs 14F, 15E; apl) that conspicuously overhangs rostrum; lobe distally rounded in dorsal view (Figs 13A, 15A) or medially indented (Figs 14A, 15B). Tutorium weakly developed, but enantiophysis eA present (Fig. 15A, E); lamella absent or represented by vaguely defined short longitudinal ridge medial to eA (Figs 14A, 15A, C). Rostral bulge inconspicuous under large prodorsal lobe; embossed pattern present (Fig. 15F–H). Submarginal crest complete anteriorly, between setal pair ro (Figs 13B, 14F); cuticle scrobiculate between crest and rostral margin (Fig. 15E). Short longitudinal ridge present laterally, above acetabula I–II. With single pair of small dorsosejugal tubercles. Bothridial seta (60–65 µm) with head strongly squamose (Figs 13A, 14E); bothridial wall foveate anteriorly (Fig. 15E), posteriorly with one strong tooth-like tubercle and with (Fig. 15C) or without second smaller adjacent (more medial) tubercle. Seta le short (13–18 µm), acuminate, slightly curved, pigmented and with minute barbs beyond basal nodule, inserted near anterior contour of prodorsal lobe with mutual distance at least three times length; ro similar in shape, with similar mutual distance; in similar but slightly shorter (11–13 µm); seta ex minute (\textasciitilde2 µm), vestige exv ventral or posteroventral to ex, nearly touching (Fig. 15E).



Notogaster — Anterior margin varying from irregular, with vaguely defined small tubercles and knots, to having half-dozen distinct, tooth-like tubercles; humeral process (\textasciitilde10 µm) usually overlapping main tubercle of bothridium. Transverse sulcus with about 10–12 foveae of different size (5–8 µm diameter); foveae also present anterolaterally, between hpr and seta la (Fig. 13A). Without series of knots along margin of posterior bulge. Setae distinctly bent or curled upon leaving basal cerotegument nodule; p2, p3 (\textasciitilde6 µm) unpigmented, fine, simple, acuminate; all others pigmented, with small coarse barbs and acuminate tip: c (8–10 µm), la-lp, h2, h3 (11–14 µm; Fig. 16B–D), h1 (14–17 µm; Fig. 16A).
Venter and lateral podosoma — Foveate in acetabular region and lateral parts of coxisternum, and sometimes in single transverse row behind smooth mentotectum. Coxisternum without tubercles or knots along epimeral groove 2; enantiophyses eS of modest size, each tubercle simple in form; e4 strongly developed (Fig. 16H); medial fossa with small pair of projections on inner margin (Fig. 16E). Discidium well-developed, usually appearing connected to posterior tubercle of eS by coaptive ridge, but rarely to anterior tubercle of e4. Enantiophysis eL and discidial ridge well defined (Fig. 16F); latter with distinct projection at ventral end, appearing like second discidium in ventral view (Figs 13B, 16E). Epimeral setae acuminate to attenuate (11–15 µm). Genital setation variable, 4–6 setae on each plate: of 10 females examined, seven symmetrical with 5/5 setae, three asymmetrical with 5/6; of 11 males examined, four symmetrical with 4/4, five with 5/5 and one each asymmetrical with 4/5, 4/6. Anogenital setae acicular; aggenital, genital and anal seate (5–8 µm) simple, adanal setae (10–13 µm) with basal nodule. Lyrifissure iad 8–10 µm. Apophysis of preanal organ tubular (Fig. 16G). Outline of ventral plate slightly bulging posteriorly, between setal pair ad1.


Gnathosoma — Subcapitulum foveolate, hypostomal (h, \textasciitilde14 µm) and genal (a, m, 12–13 µm) setae attenuate. Chelicera (Fig. 17D) \textasciitilde75-80 µm long; without spicules; setae cha (\textasciitilde23 µm), chb (\textasciitilde15 µm) attenuate, barbed.
Legs — Femur I \textasciitilde2.7 times longer than maximum height in lateral view, with sharp, nearly right-angle transition between stalk and bulb (Fig. 17A). Femur II (Fig. 17B) similar, but slightly shorter (\textasciitilde2.5); stalk occupying about third length of femur I, about quarter that of femur II. All femora with porose area, mostly on adaxial face of bulb. Tibia I \textasciitilde1.3 times longer than high in lateral view. Tarsus I abruptly tapering in distal half, but without distinct projecting mid-dorsal bulge (Fig. 17C). Tarsus II without noticeable proximal stalk, depth similar to that of tibia in lateral view. Seta d of femora short, flame-shaped (Fig. 17B), similar in structure to dorsal body setae (pigmented, barbed, with conspicuous cerotegument nodule at base). Seta l' of genu and tibia I not conspicuously enlarged.






Comparisons
Adults of C. nasutus are unique among described Caleremaeus species in having a prodorsum with a large, tongue-shaped anterior lobe, bearing the lamellar setae. Otherwise, they share several features with C. monilipes and C. retractus, including the presence of enantiophysis eA, the presence of an embossed pattern on the ventral face of the rostral lobe and rostrophragma, and ridges laterally on epimeres III-IV that outline depressions coapted to the respective trochanters. The form of dorsal setae (with basal cerotegument nodule and pigmented, barbed distal portion) is shared with C. retractus, but not C. monilipes.
Notes on biology
Reproduction — Gravid females of Caleremaeus species in this study carried a maximum of two eggs (one in each oviduct) but a single egg was most common. Eggs are slightly flattened unilaterally, about 1.8 times longer than broad (Fig. 5G). Females were more abundant than males, but all species seem bisexual; males accounted for about one-third to one-half of adults in our samples. This is consistent with data for C. monilipes presented by Seniczak and Seniczak (2019) but Grandjean (1941) reported a slight male bias (1: 0.8) for this species.
Food — Overall, species of Caleremaeus appear to be opportunistic feeders on fungi and decaying plant organic matter, as is typical of oribatid mites (Schneider et al. 2004). Based on very limited and unquantified information, adults and juveniles of both C. retractus (Fig. 19A–E) and C. arboricolus (Fig. 19F–H) ingest diverse fungal material—both hyphae and spores—and it dominated most boli and pellets we observed. Most other material was not identified but appeared to comprise fragments of plant-based organic matter. Boli and pellets in adults of C. nasutus (Fig. 19I–L) were generally like those of other species.
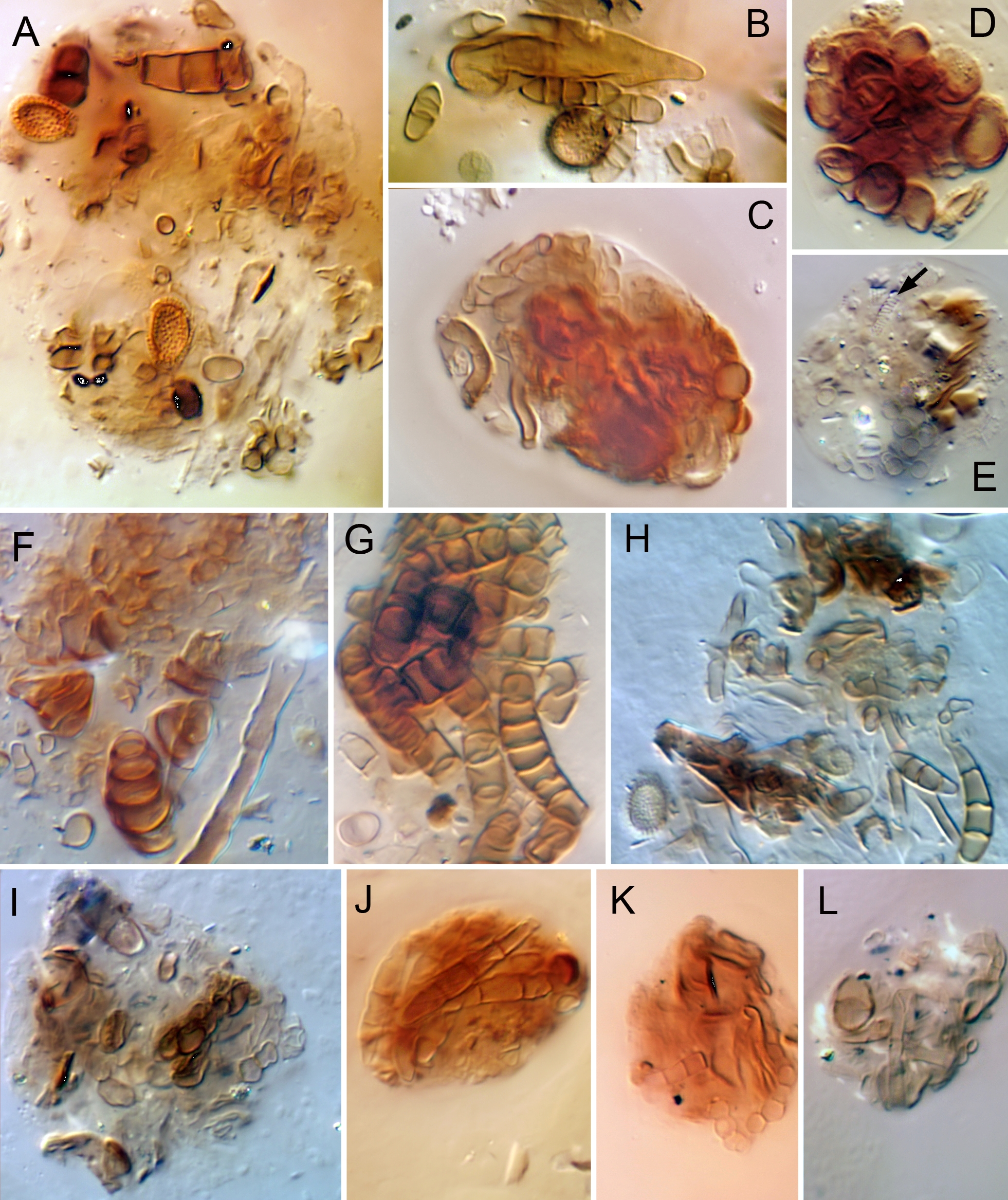


Despite its wide distribution, little has been written on the feeding biology of C. monilipes. Skubała and Maslak 2010 regarded C. monilipes as a xylophage, but neither of the two cited references (Luxton 1972; Schatz 1983) made such a claim. Fischer et al. (2010) found C. monilipes adults from tree trunks to have stable-isotope signatures more in line with those of soil-dwelling species, rather than lichen feeders; they speculated that they eat algae or other resources available on bark.
Habitat associations — Collectively, Caleremaeus species clearly show some affinity for above-ground microhabitats, primarily in forests. Caleremaeus nasutus and C. retractus (sensu stricto) have not been found outside soil microhabitats, but this may be due to a lack of appropriate sampling where these mites occur. Based on material in the CNC and RNC, members of the `retractus' group have been collected from diverse non-soil substrates, including arboreal and saxicolous lichens and mosses, bark of both coniferous and deciduous trees (including cankered bark of chestnut trees), rotting wood of logs and tree-holes and decaying woody fungal sporocarps, in addition to soil-litter. Caleremaeus arboricolus is consistently associated with arboreal microhabitats, including bark, twigs and lichens; the single non-arboreal collection was from a regurgitated owl-pellet lying on the soil surface. The similar C. gleso, known only as a Baltic amber fossil, was almost certainly arboreal since trees were the source of original resin-flows. While little is known of C. divisus, it has been reported only from arboricolus moss.
There are scattered reports of Caleremaeus monilipes being collected in low to moderate density from forest soil-litter (e.g. Moraza and Peña 2005), but the original collection was from decaying wood (Michael 1882) and woody substrates seem to be the primary microhabitat, particularly logs in early stages of decomposition (Siira-Pietikainen et al. 2008; Skubała and Maslak 2010 and included references; Seniczak and Seniczak 2019; Seniczak et al. 2019) or rot-holes in standing trees (Skubała and Gurgul 2011; Taylor and Ranius 2014). Other works suggest less fidelity to dead woody substrates. Travé (1963) considered it a predominantly saxicolous species that could also be found on trees. Subías (1977) considered it a muscicole, found with similar frequency on rocks and low on tree trunks; Fischer et al. (2010) also noted the lower-trunk affiliation. By contrast, Arroyo et al. (2013) found moderate numbers in oak-canopy mosses, and Seniczak and Seniczak (2019) reported an abundance on oak-trunk moss above 1.5 m, but absence from lower regions. Moss substrates also were indicated by Schweizer (1957) and Bonnet et al. (1975). Odd reports (at least some clearly related to transported substrates) include discoveries in nests of mice, birds and ants (Lebedeva and Poltavskaya 2013; Krawczyk et al. 2015; Elo et al. 2018). It remains to be determined how much the microhabitat diversity of C. monilipes reflects possible cryptic speciation (see Introduction).
Family-group classification
Composition
Caleremaeidae Grandjean, 1965b, was monogeneric when proposed, but as many as six other genera have been added since, according to author; these include Veloppia and five genera usually grouped as Anderemaeidae (Anderemaeus, Cristeremaeus, Epieremulus, Luxtoneremaeus, Yugaseremaeus). We believe that none of these additions (or others that have been informally suggested in unpublished internet documents: i.e. Megeremaeus, Caucaseremaeus; Subías 2019) can be supported in a phylogenetic context, and that the family should remain monogeneric.
The removal of Veloppia will be discussed in detail separately (Norton et al., in preparation). Woas (2002) doubted its inclusion in Caleremaeidae and perceived a closer relationship to Hungarobelbidae. With increased knowledge, we view the various adult similarities proposed by Norton (1978) as more widespread and possibly symplesiomorphic, and juvenile traits clearly show that Veloppia is not among the plicate eupheredermous groups.
The idea that the southern-hemisphere genus Anderemaeus and some other members of Anderemaeidae are confamilial with Caleremaeus recently was discussed and rejected by Norton and Ermilov (2019). In summary, inclusion of Anderemaeus in Caleremaeidae was first formalized by Woas (2002), but it had roots in earlier studies (Franklin and Woas 1992, Subías and Arillo 2001). By transferring the type-genus, Woas (2002) effectively subsumed Anderemaeidae within Caleremaeidae sensu lato. Subías (2004) followed this proposal but expanded it by including all genera of Anderemaeidae. Woas' proposal was based on three shared traits, paraphrased here: the presence of a lamella-tutorium complex; the presence of the aggenital enantiophysis (e4, U); and a tubular preanal organ. Norton and Ermilov (2019) considered these to be symplesiomorphies of the relevant taxa, and instead showed that Anderemaeus—and therefore its family, Anderemaeidae—are part of a clade of Brachypylina that is more highly derived than Caleremaeus. Based largely on the morphology of juveniles, they transferred Anderemaeidae to Gustavioidea.
Superfamily classification
Grandjean (1965b) proposed Caleremaeidae as part of a reorganization of what he called the `groupe E restant', i.e. remaining eupheredermous genera that, due to insufficient knowledge, were not treated in his iconic classification of oribatid mites (Grandjean 1954a). He included six of the families in the newly proposed Eremuloidea (= Ameroidea; R10) but left Caleremaeidae, Eremaeidae and Tenuialidae without higher assignment. Since then, Caleremaeidae most often has been included in Oppioidea (e.g., Balogh 1972; Bulanova-Zachvatkina 1975; Marshall et al. 1987; Fujikawa 1991; Balogh and Balogh 1992), but as concepts of that superfamily became more restricted, Caleremaeidae was omitted (e.g. Behan-Pelletier 1991).
Subías (2004) deconstructed the traditional Oppioidea and included his broad sense of Caleremaeidae (see above) in a newly recognized Eremelloidea, but none of the other included families is known to be eupheredermous. This classification has been little used by other authors. Informally, in unpublished but frequently-cited online annual updates, Subías (2016 and following) abandoned Eremelloidea and grouped Caleremaeidae sensu lato with Eremaeoidea. This had been suggested earlier—but not formalized—by Franklin and Woas (1992), though Subías' concept of the latter superfamily seems much broader than theirs. Weigmann (2006) noted that previous classifications of Caleremaeidae were problematic in a phylogenetic context and recognized the monofamilial Caleremaeoidea, though he did not indicate whether his sense of Caleremaeidae was broad or narrow.
Previously (Norton and Behan-Pelletier 2009; Schatz et al. 2011), we followed the tentative suggestions of Woas (2002) and included Caleremaeidae in an expanded concept of Ameroidea. It was the most recent of several expansions of the superfamily that began with Grandjean's (1966) addition of Staurobatidae. Curiously, he did not also include Basilobelbidae, despite their having the key traits of aggenital neotrichy and highly reduced proral setae on tarsi II-IV (see Grandjean 1959b, as Hammation), but this family was added by Balogh (1972) along with Heterobelbidae. Subsequently, Miko and Travé (1996) added Hungarobelbidae and Woas (2002) added Rhynchoribatidae, Spinozetidae and Oxyameridae. We relied upon the similarities of Caleremaeus and Hungarobelba noted by Travé (1961) and Miko and Travé (1996) to support adding Caleremaeidae to Ameroidea, but the resulting group of 14 families little resembles Grandjean's (1965b) original concept and even includes some families whose juveniles are not known to be eupheredermous. After our detailed studies of Caleremaeus herein, and reconsideration of traits—plus reference to two limited molecular studies—we no longer feel that this transfer to Ameroidea was reasonable; there are three general reasons.
First, the several purported similarities of Caleremaeus and Hungarobelba are not exclusive enough to be convincing synapomorphies or are not clearly homologous. Paraphrasing from Travé (1961) and Miko and Travé (1996), these are as follows. (1) The two genera share the laterosejugal enantiophysis (eL): however, this is found in several other eupheredermous taxa (R4). (2) They share the prodorsal enantiophysis (eA) and associated groove, though eA can be absent (presumed lost) from species in each genus: but eA is even more widespread than eL (see R4 and summary in Norton and Ermilov 2019). (3) They share an anteriorly truncate notogaster, with some type of humeral projection: again, this is not exclusive, being shared also by numerous other eupheredermous taxa—i.e. Veloppia, Megeremaeidae, Cepheidae—as well as some disparate apheredermous families such as Anderemaeidae, Autognetidae and Quadroppiidae, as well as Spinozetidae (with juveniles unknown). (4) They purportedly share a similar dimorphic type of genital papilla, with the posterior two pairs being more elongated, clavate, and slightly distant from the anterior pair: but these papillae are not perfectly rounded, and their shape can appear slightly different depending on orientation—we found no difference in shape or spacing among the papillae of any Caleremaeus species (see also Behan-Pelletier 1991). (5) They share a ridge near the rostral margin that bears seta ro: these are differently formed structures—that of Caleremaeus is a uniform shelf-like structure that may extend around the front of the rostrum while that of Hungarobelba is a much less conspicuous carina that effaces anteriorly; it is not unusual in Brachypylina for a carina (lateral ridge) to be directed anteriorly from acetabulum I, though typically it lies close to or even merges with the rostral margin (e.g. Grandjean 1960, 1962; Behan-Pelletier 1990) rather than reaching seta ro. (6) They supposedly share a long famulus on tarsus I: but this is vague, and a matter of degree—that of Hungarobelba is indeed unusually long and tapered but so is that of Basilobelbidae (Grandjean 1959b), and a long, tapered famulus is common in Damaeidae; the famulus of Caleremaeus is more modest in length and baculiform.
Second, the most distinctive trait of Ameroidea—at least in its original context (Grandjean 1965b, 1966)—that might be considered apomorphic among eupheredermous taxa is aggenital neotrichy, and several of the component families also have a more general ventral neotrichy. Caleremaeus exhibits no neotrichy on the body or legs. Other unusual traits of Ameroidea are not universal in the group. Most, but not all families exhibit size regression of proral setae on legs II–IV, where they are small, spiniform and often inconspicuous. In Caleremaeus these setae are plesiomorphic: normally formed and conspicuous. Caleremaeus also has a rather plesiomorphic form of preanal organ, in which the internalized apodematal extension is a simple, hollow, tubular or caecum-like structure. This does not occur in Ameroidea, but no clearly derived state for the group has been characterized. In its expanded context of 14 families, Ameroidea seems unjustifiable by synapomorphies; it has been defined (Norton and Behan-Pelletier 2009) by a collection of some widespread plesiomorphies and others that are present or absent according to family.
Third, the only existing molecular phylogeny study that includes Caleremaeus and members of Ameroidea contradicts the inclusion of Caleremaeidae in Ameroidea. With a focus on relationships among members of Zetorchestoidea, Lienhard et al. (2013) examined tree topology for seven eupheredermous families. Their consensus tree, based on a combined data set of mitochondrial (COI) and nuclear (EF-1α) genes, showed Caleremaeus as the sister-group of Niphocepheus (Niphocepheidae); in turn, this clade was sister-taxon to the families Ctenobelbidae and Damaeolidae, which are unquestioned members of Ameroidea. Numerous eupheredermous families were not represented, and not all branches had high statistical support, but the strong linkage of Caleremaeus to Niphocepheidae, rather than the ameroid families, seems significant. Like Caleremaeus (but unlike Ameroidea), juveniles of Niphocepheidae—and other members of Zetorchestoidea (sensu Schatz et al. 2011), as well as Neoliodoidea and Plateremaeoidea—have plicate cuticle, a trait that we consider plesiomorphic in Brachypylina.
The only other molecular work that included Caleremaeus was based on the 18S ribosomal RNA gene. In a study encompassing representatives of many Brachypylina families, but none in Ameroidea, Schaefer and Caruso (2019; supplemental online material) presented two detailed trees. A maximum likelihood tree included Caleremaeus within a clade that contained four species in the family Damaeidae (in the monofamilial Damaeoidea), while in a Bayesian inference tree Caleremaeus formed a trichotomy with two different clades of those Damaeidae species. Since no Damaeidae were included in the Lienhard et al. (2013) work, direct comparison is not possible.
Faced with a seemingly mosaic distribution of morphological traits among the main eupheredermous taxa (Miko and Travé 1996; Woas 2002), at present we can offer no strong argument for including Caleremaeidae in any of the larger superfamilies. For example, the characteristic topography of the Caleremaeus notogaster is approximated in certain Plateremaeoidea—Pedrocortesellidae (e.g. Hunt 1996, his Fig. 34A)—but no other similarities seem important and differences are many. The prodorsum of at least some Caleremaeus species seems most similar to that of Megeremaeidae (Eremaeoidea) in having a lamella-tutorium complex, eA, eL, eH and an alveolar vestige of the second exobothridial seta, but most hysterosomal and leg traits differ. The scalp-attachment cornicle of Caleremaeus nymphs is seen also in Damaeoidea, but nymphs of the latter are not plicate, and adults differ in many ways.
Therefore, we adopt Weigmann's (2006) monofamilial Caleremaeoidea, but view Caleremaeidae in its strictest sense, i.e. including only Caleremaeus. Its diagnosis, as well as that of the family, would be identical to that of the genus, given above. Such a redundant classification has no phylogenetic content, but in this instance it seems preferable to retaining Caleremaeidae in an obviously polyphyletic superfamily, such as Ameroidea in the sense used by Woas (2002), Subías (2004), Norton and Behan-Pelletier (2009) and Schatz et al. (2011). Caleremaeoidea joins Neoliodoidea, Plateremaeoidea and Zetorchestoidea in a paraphyletic cluster of superfamilies near the base of Brachypylina phylogeny, characterized by eupheredermous nymphs with plicate cuticle.
Remarks on morphology and classification
1. Role and evolution of lamella and tutorium — It seems likely that the lamella and tutorium appeared early in the evolution of Brachypylina, in a ridge- or rib-like form such as in Megeremaeidae (Aoki and Fujikawa 1971; Behan-Pelletier 1990; see also Woas 2002, Norton and Ermilov 2019). As known for 150 years (Michael and George 1879; Berlese 1896), the two structures play a role in defense by providing a place for coaptation of leg I, the distal parts of which typically lie between them when legs are adducted following disturbance. In more derived oribatid mites they often are highly developed as blade-like projections that overhang the adducted leg from above and below, respectively (Grandjean 1952; Fernandez et al. 2013).
Regression also has occurred, with transitions starting from both blade - and ridge-like forms. In some families or genera of the derived, poronotic superfamilies Ceratozetoidea and Oripodoidea, large blade-like lamellae and tutoria have regressed to narrow carina or ridges, or have disappeared. Similarly, in Caleremaeus monilipes and C. divisus the ridge-like lamella-tutorium complex seems sufficiently developed to provide a coaptive space (see Seniczak and Seniczak 2019, their Fig. 4), while in North American species this complex ranges from partially regressed (C. retractus, C. nasutus) to being almost entirely lost (C. arboricolus). Whether the defensive leg adduction occurs in any Caleremaeus species is unknown, as behavior has not been reported.
2. Rostral bulge — This bulge is a centrally located convexity in the solid limb of the rostral tectum that appears to accommodate distal elements of the gnathosoma—probably the cheliceral tips—when the mite is in a defensive posture with chelicerae retracted and the subcapitulum elevated. It is a common feature in brachypyline oribatid mites and often it seems `excavated' on the inner face to make the bulge very thin-walled, though this is not true of Caleremaeus species. Also, the pattern of excavation may result in an apparent central tooth projecting into the bulge (Norton and Ermilov 2017). Caleremaeus has no such tooth, but a frontal view of some species may give such an impression, since a unique, raised central rib is seen in optical cross-section (Fig. 2L). The rib is the central element of an embossed `scorpion-like' pattern on the lower surface of the bulge (Figs 2K; 15F–H; 18A) that is present in all examined species except C. arboricolus. The central rib is crossed by a symmetrical series of about six raised transverse striae directed perpendicularly from it. The transverse striae are concentrated at the base of the rostral bulge, but the `tail' of the scorpion appears to continue onto the freely-projecting rostrophragma, to which the soft cheliceral frame is attached (Fig. 15H; rph, ch.fr).
3. Exobothridial seta vestige — Close to the exobothridial seta in the adult of all examined Caleremaeus species is a circular pale spot that in transmitted light looks much like the alveolus of seta ex, except no seta emerges from it. We consider it an alveolar vestige (exv) of the second exobothridial seta, a seta that has been lost from all members of Brachypylina. Among Brachypylina, we know of a similar vestige only in adults of Eremaeidae and Megeremaeidae (Behan-Pelletier 1993; as em). Though interesting, we interpret this similarity as a symplesiomorphy and therefore not evidence of a phylogenetic relationship among these families. A similar vestige is widespread in the outgroup Nothrina, many members of which also have a single exobothridial seta (e.g. Norton et al. 1996), and even in some Enarthronota (Grandjean 1963).
4. Enantiophyses — Adults of Caleremaeus species are rich in enantiophyses, tubercles that oppose each other across a cuticular groove. The grooves and tubercles seem to function in holding and anchoring an air film that has contact with the stigmata of the apodematal-acetabular tracheal system (Chen et al. 2004). It is obvious how such localized plastrons are important for intertidal taxa (e.g. Pfingstl and Krisper 2014), but even for fully terrestrial species they could be important whenever the immediate environment is inundated with water for extended periods of time.
Grandjean (1954b) proposed the term `enantiophysis' in reference to Damaeidae and later (1960) refined his ideas and nomenclature, but the structures are widespread, particularly in early- to middle-derivative eupheredermous families. Grandjean (1960, 1966) doubted that enantiophysis homology among taxa could be established on a general scale, but he did note the widespread taxonomic distribution of two, both of which occur in Caleremaeus (Figs 1, 13), These span the important sejugal groove, which encircles the body and in which one of the three pairs of stigmata open. The parastigmatic enantiophysis (eS or S) spans the groove just below the sejugal stigma, and the humeral enantiophysis (eH or H) spans it behind the bothridium, from which a tubercle on its posterior wall opposes a humeral projection from the notogaster. Since then, it has become apparent that three other enantiophyses are consistently placed and readily identified.
The laterosejugal enantiophysis (eL or L) also spans the sejugal groove, but mid-laterally, above the acetabula (Norton 1978). In the literature it has been reported for relatively few taxa—Caleremaeidae, Hungarobelbidae, Gymnodampia (Ameridae), Veloppia—but it also exists in Megeremaeidae (e.g. Behan-Pelletier 1990, her Fig. 32). Moreover, many descriptions of oribatid mites omit details of the lateral podosoma, so this is certainly an incomplete list. An almost certain independent evolution occurred in some Selenoribatidae, intertidal mites that rely on plastron respiration (Pfingstl 2013, 2015). In this family the anterior tubercle bears the opening of the coxal gland (z), which is not true of other taxa and reinforces the idea that this is an independent appearance of eL. Grandjean (1968) considered this a parastigmatic enantiophysis but it is distinctly dorsal to the acetabula, whereas in its usual sense eS is below them.
Two others are associated with different grooves. The prodorsal (eA, or A) enantiophysis spans a dorsolateral groove on the prodorsum between the levels of acetabula I and II. If a tutorium exists, its posterior end forms the anterior tubercle of eA, with a separate tubercle immediately posterior to the groove. In the absence of a tutorium there can be a separate small tubercle on the anterior side of the groove. This enantiophysis is constant, or nearly so, in some eupheredermous families (Megeremaeidae, Pheroliodidae), but has variable presence in Caleremaeidae, Hungarobelbidae and Veloppia. It may be absent from most members of a family, but present in one or more genera, as in Damaeidae (Tokukobelba, some Kunstidamaeus), Ameridae (Gymnodampia) and the apheredermous family Anderemaeidae.
The aggenital enantiophysis (e4, U, G, co.ag) spans epimeral groove 4, with the anterior tubercle often bearing seta 4b (see R5). It is widespread but scattered among the non-poronotic Brachypylina, as summarized by Norton and Ermilov (2019). Among proven eupheredermous taxa it is present in all species of Caleremaeidae and Veloppia, some Cepheidae (Eupterotegaeus) and some Eutegaeidae (Neoeutegaeus); among `presumed' eupheredermous families—i.e. with unknown juveniles but classified in Cepheoidea and Polypterozetoidea—it seems to be universal in Microtegeidae, Cerocepheidae and Nodocepheidae. Among apheredermous taxa, it is typical of Anderemaeidae and is found in one genus each of Otocepheidae (Fissicepheus; Aoki 1967) and Nosybeidae (Topalia; Colloff 2019). It is absent from Megeremaeidae and Hungarobelbidae, and is questionable in a few Damaeidae (a tubercle bearing seta 4b may be hypertrophied, but no posterior tubercle is present (e.g. Tokukobelba; Lamos 2016).
5. Epimeral setation — Herein (Figs 3F, 8B, 10D, 13B), we follow the modification of Grandjean's (1934) chaetotaxy for epimeral setae proposed by Norton and Franklin (2018; their Remark \#15). The fundamental difference is that Grandjean focused on the order of setal appearance on each epimere, while the modification focuses on positional correspondence and therefore probably reflects metameric homology. This modification especially affects the notations for 4a and 4b, setae on an epimere that is first formed in the protonymph. Like the many leg IV setae that are delayed to the deutonymph, we feel seta 4a (= 4b in the widely used Grandjean chaetotaxy) is also delayed to the deutonymph; when it appears, it is well aligned with larval setae 1a, 2a and 3a on the soft sternal cuticle (Fig. 12H). By contrast, protonymphal seta 4b (= 4a in the Grandjean chaetotaxy) is inserted on the paired epimeral sclerite (Fig. 12G; out of view in Fig. 12H), as are larval setae 1b and 3b.
6. Preanal organ — This sclerotized structure serves for the origin of paired adductor muscles that act on the genital valves (Grandjean 1969). It appears to have evolved from a simple unpaired plate on the anterior wall of the anal vestibule, as in many Nothrina, to become a largely internalized apodeme or apophysis in most Brachypylina. When the anal valves are tightly closed, the base of the organ either may be entirely hidden in the vestibule or it may be partly exposed; this depends on the size of the base and especially whether or not the anterior tectum of the two anal valves fully meet. In Caleremaeus species the organ is much like that of Damaeidae (Grandjean 1969, his Fig. 5A; LF), though the shape of the internal apophysis differs. The exposed base of the organ is relatively large, subtriangular in ventral view (Figs 8B, 13B, 14B; pr.o); the muscles originate on the internalized tubular apophysis and insert directly on the genital plate (Figs 3F, H, I, 16G). Weigmann (2006; his Fig. 121b) illustrated the anal plates of C. monilipes as completely covering the preanal organ, but this appears to be an error: in all our specimens of C. monilipes the base of the organ is exposed when anal plates are closed.
7. Leg setation — Variation. The complement of setae and solenidia on legs of both adult and juvenile instars is remarkably consistent in Caleremaeus, such that Table 1 expresses the ontogeny in all examined species to the extent that data exist (see R12). No interspecific difference was noted among adults, and a single example relates to ontogeny: seta l' of tibia III is tritonymphal in C. monilipes, but deutonymphal in the other studied species (Table 1). Noted intraspecific variation includes only the following: (1) in the near-topotypical population of C. retractus, genu I seta v' was absent from two of nine tritonymphal legs examined; (2) in the topotypical population of C. arboricolus, tibia IV seta l' was absent from one of eight tritonymphal legs examined; (3) in the same population, tarsal seta l'' was absent from one of 10 adult legs I examined and (4) tarsal seta pl' was absent from one (different) adult leg. The last example presumably is anomalous, since pl' is a fundamental, eustasic tarsal seta that forms in the larva. The previous three relate to delays in the formation of amphistasic setae (setae variable among species or populations regarding the instar of first appearance). In examples 1 and 2 the setae presumably would have appeared in the adult, since these setae were present on all adults examined; in example 3, the delay in an adult-forming seta represented a loss by retardation in the concepts of F. Grandjean (see review in Norton 1977).
Trochanter III. Caleremaeus species are unusual in having both setae of trochanter III—v' and l'—formed in the deutonymph. The many oribatid mites for which setal development is known exhibit a variety of ontogenies on trochanter III, but usually these setae are formed in successive instars. We know of only four other species with the Caleremaeus pattern. Of these, only Niphocepheus nivalis (Schweizer, 1922) is a member of Brachypylina (Travé 1959); the other three are in the nothrine superfamily Malaconothroidea and include Mainothrus badius (Berlese, 1905), Tyrphonothrus maior (Berlese, 1910; as Trimalaconothrus novus and denoted v'1 , v'2) and Allonothrus giganticus Haq, 1978 (data respectively from Seniczak et al. 1998; Knülle 1957 and R.A.N. unpublished).
Lateral setae. On femora and tibiae I and II of Caleremaeus species, the lateral pseudosymmetrical pair have positions that seem far from being `paired' (Fig. 4A, B). The adaxial member (l') is high on the inner face, whereas the abaxial member (l'') is low on the outer face. On tibiae I and II l'' can easily be mistaken for a ventral seta prior to the tritonymph, when v'' first appears below it. On tibiae III and IV (Fig. 4C), which lack l'', it is seta l' that is abaxial and very low on the outer face, but in these instances v' is already present by the time l' forms, so the potential confusion is less. In essence, the setal verticil of these segments seems somewhat rotated on the segment, turned clockwise on legs I and II and counterclockwise on III and IV, but the shifts are most obvious in the lateral setae. Wauthy and Fain (1991) referred to such asymmetries—resulting from one member of a pair rising from its ancestral position and the opposite member falling—as `basculations'. Those noted here are abaxial (=antiaxial) basculations ('' on I/II and ' on III/IV); they are not uncommon in oribatid mites, but they seem unusually strong in Caleremaeus.
Iteral setae. The iteral pair (it) of tarsal setae are amphistasic, always post-larval, and always appear in the same position between the proral and tectal pairs, which are in contrast eustasic larval setae. But the specifics of this development among oribatid mites are surprisingly diverse, with more than a dozen ontogenetic patterns known (Grandjean (1961a, 1964a; see also Norton and Franklin 2018). The presence and instar of appearance of iteral setae vary among taxa, among the four tarsi, and rarely even between members of the pseudosymmetrical pair. The iteral ontogeny of Caleremaeus is interesting for two reasons. First, the pattern is complex, differing on each leg. Grandjean (1964a) noted this for C. monilipes by a formula that indicated first appearance of iteral setae on tarsi I-IV: (n3—Ad—[0, Ad]—0). In other words, the iteral pair appears in the tritonymph on tarsus I, in the adult on tarsus II, and fails to form on tarsus IV; tarsus III is asymmetrical in this regard, with it' failing to form but it'' appearing in the adult. The second interesting point is that this complex formula, which is unique among oribatid mites, appears to be a generic trait (though only the adult setation is known in C. nasutus and we have no setation data for C. divisus; also, see R12).
8. Nomenclature of posterior gastronotic setae h1 vs p1 — In numerous families of oribatid mites, particularly eupheredermous taxa, the hysterosoma of nymphs terminates in a posterior process, typically with an indistinct pygidial (or caudal) sclerite, on which two pairs of setae insert. One pair usually is closer together than the other and slightly more dorsal, though in some taxa they are at equal height; most often the medial, higher pair is significantly larger. When applying his opisthonotal chaetotaxy to Porobelba spinosa (Damaeidae) nymphs, Grandjean (1954c) was uncertain which of these pairs represented h1 and which was p1 This is understandable, given that the pygidial region represents the dorsal part of terminal hysterosomal segments and therefore undergoes the least amount of spreading as segments are added to the caudal bend during anamorphosis (Grandjean 1939). Slightly later, for the similar pygidium of Polypterozetes (Polypterozetidae), Grandjean (1959a) clearly labeled the larger, more dorso-medial pair as h1.
By contrast, regarding Caleremaeus monilipes, Grandjean (1965b; p. 719) specifically and clearly considered the larger, more medial setae on the pygidial sclerite (therein called the `croupion') to be pair p1; we infer therefore that he considered the smaller, more separated, slightly lower pair to be h1. We found no explanation in his writings, but this seeming reversal of his hypothesis on homology is consistent with his labeling of setae in studies of at least three other eupheredermous taxa —Mongaillardia (Ameroidea; Grandjean 1961b), Pheroliodes (Plateremaeidae; Grandjean 1964b) and Fosseremus (Damaeolidae; Grandjean 1965a)—though the medial pair are not enlarged in these groups.
Without noting this apparent contradiction, most subsequent authors appear to have followed the earlier Polypterozetes-model in considering the adjacent and usually larger middle pair to belong to segment H (with the notation h1). Some examples relate to Neoliodoidea (Seniczak et al. 2018b, Platyliodes), Plateremaeoidea (Ermilov et al. 2010, Pedrocortesella, Aleurodamaeus), Damaeoidea (Miko and Mourek 2008, Kunstidamaeus), Gustavioidea (Seniczak et al. 2018a, Hafenrefferia) and C. monilipes (Seniczak and Seniczak 2019). We follow this view herein. The slightly more dorsal position of the middle pair in the typical formation argues for their belonging to segment H instead of PS, which is not part of the opisthonotum until the protonymph. This opinion is not universal among recent authors: Weigmann 2002 considered the large medial setae to be p1 in nymphs of Eremobelba (Ameroidea).
9. Attachment of scalps — In many oribatid mites whose nymphs carry exuvial scalps there is no obvious attachment mechanism, but some taxa have evolved a structural specialization for this purpose. For example, in the poronotic family Oribatellidae opisthonotal seta dp is either structurally modified or it inserts in a sheath-like callosity under the previous exuvium (Grandjean 1953), and in some Tegoribatidae (Tegoribates) a small bulge around this same seta forms a convexity in exuvia that nest together (Behan-Pelletier 2017).
The best-known mechanism is that of the Damaeidae, in which a distinct projecting cornicle on the opisthonotum inserts into the cornicle of the previous exuvium, forming a nested attachment structure. The cornicle may be short and simple or elongate and variously curved or twisted, according to species. In his review of this structure in Damaeidae, Ermilov (2012) indicated that it was unique to this family, but in fact there are two other examples. One is Caleremaeus, first noted by Grandjean (1965b). As described above (see also Seniczak and Seniczak 2019) its cornicle is a simple papilla, not very different from the simplest damaeid cornicle, e.g., that of some Damaeus species (Grandjean 1960). The other is Basilobelbidae, in which nymphal exuvia attach through a nesting `stylus' that is not essentially different from the damaeid cornicle; the truly unique aspect of scalp attachment in this family is the strange mechanism by which the tritonymphal scalp is anteriorly fixed to the adult notogaster (Grandjean 1959b). In the related family Heterobelbidae, which has yet another unique mechanism for attachment of the tritonymphal exuvium to the notogaster, there is no cornicle or other obvious mechanism connecting the scalps themselves (Beck 1962; Okayama 1980).
Caleremaeidae, Damaeidae and Basilobelbidae share a similar apomorphic attachment mechanism, but it seems unlikely that they form a single clade within the eupheredermous taxa. Nor is there a taxon with an obvious precursor to the cornicle-mechanism, though Eremobelbidae gives a hint as to how it might have evolved. Weigmann (2002) described a small stalk on the opisthonotum of Eremobelba juveniles, which apparently adheres to the previous exuvium. If true, one could envision the stalk becoming hollow, like a cornicle, but it seems more parsimonious to evolve a cornicle from a simple bulge (cf. Tegoribates, above). Seniczak and Seniczak (2019) considered the cornicles of Caleremaeus and Damaeidae to be convergences, and at present we have no strong contradictory evidence.
10. Ameroidea: nomenclature and content — Applying the principle of coordinate categories, Marshall et al. 1987 recognized that Eremuloidea was not the oldest appropriate name for the superfamily that Grandjean (1965b) proposed to include Amerobelbidae, Ctenobelbidae, Eremulidae, Damaeolidae, Eremobelbidae and Ameridae (adding Staurobatidae in 1966). They considered Amerobelboidea Grandjean, 1954 to be the oldest available name for this group and considered Eremuloidea a junior synonym. But they cited the wrong date for Amerobelbidae, which was a nomen nudum when it first appeared in the literature (Grandjean 1954a) as noted by Schatz et al. (2011); it was formally published by Grandjean (1961b). But they also cited an incorrect author and date—Grandjean 1965b—for the family-group name Ameridae. Ameridae was proposed by Bulanova-Zachvatkina (1957) and, since this is the oldest name in the group, Ameroidea is the proper name for Grandjean's Eremuloidea. The first correct use of Ameroidea seems to have been by Pérez-Iñigo (1997).
11. Corrections to Norton and Behan-Pelletier (2009) — In 2009, Caleremaeidae was included in Ameroidea, as noted above. But a more complete knowledge of the family, with its plicate juveniles, raises doubts about this grouping. Stated traits of Ameroidea (p. 457) that are not found in Caleremaeidae include: (1) absence of a tutorium (it is present in most species); (2) absence of a discidium (it is present in some species); (3) genital setation 6 pairs (4–6 can be present); (4) preanal organ without caecum (the internalized process is hollow, essentially like a caecum); and (5) proral setae of legs short and spiniform (they are normally formed setae). The diagnostic couplet (p. 483, couplet 88) distinguishing Caleremaeidae from Anderemaeidae also is wrong or misleading (cf. Norton and Ermilov 2019). The first character should be deleted: as noted above, Caleremaeidae species have ring-like ridges within the bothridium. The character `circular depressions between setae c and la' refers to the foveae of the transverse sulcus; more important is the absence in Anderemaeidae of the unique notogastral topography (bulges and sulcus) found in Caleremaeidae. Enantiophysis A (= eA) is said to be absent from Anderemaeidae and present in Caleremaeidae, but in fact it is typical of Anderemaeidae and present or absent in Caleremaeidae. The cheliceral character should be ignored: it does not apply to most taxa currently included in Anderemaeidae.
12. Discrepancies with Seniczak and Seniczak (2019) — This important paper, based on material from Norway, redescribed the adult of the type species of Caleremaeus, C. monilipes, and presented the first complete assessment of ontogeny. However, during our study of C. monilipes specimens from Germany, Spain and Sweden, we noted several discrepancies with their results. After reexamination of these issues, Anna Seniczak kindly provided explanations and in some cases corrections (personal communication with R.A.N., 2019).
Enantiophyses. Their Fig. 3 shows the absence of enantiophysis eL, which is present on all our specimens (and was illustrated but not labeled by Miko and Travé 1996; their Fig. 8C). The specimen illustrated for their Fig. 3 was a light brown (teneral) specimen on which eL was not conspicuous, but eL clearly is present in the darker, more mature adults among their material. Also, they used some unconventional notations for enantiophyses (see R4); most significant was the use of Va-Vp (usually reserved for the ventrosejugal enantiophysis) for the aggenital enantiophysis spanning epimeral groove 4, and E4a-E4p for small tubercles on either side of epimeral groove 3.
Discidium. They illustrated and labeled a discidium (their Fig. 2; dis), whereas Grandjean (1965b) had indicated the absence of a discidium or discidial ridge in this species. According to A. Seniczak, their darker specimens match the figures of Miko and Travé (1996; their Figs 8C, 13B), which show a low, irregular and vaguely defined elevation in the region between acetabula III and IV. Our specimens also match the latter figures: when viewed from below in transmitted light, the irregular elevation appears darker by projection, but the impression disappears in lateral view. As described above, the development of a discidium or discidial ridge is variable among species of Caleremaeus, and that of C. monilipes seems weakest of all.
Pedotectum I. All species of Caleremaeus have a typical, well-defined pedotectum I in the form of a `cupped' scale that posteroventrally envelops the base of leg I. This is well-shown in their Figs 2 and 4. However, their Fig. 1 is misleading in showing it as an isolated structure, distant from the leg, and their text incorrectly designates it as a propodolateral apophysis (P) instead of a pedotectum.
Leg setation. There are three discrepancies between their description of leg setation (their Fig. 13 and Table 2) and the results of our studies on C. monilipes. Upon reexamination of their material they found the following: only one iteral seta, it'', develops on tarsus III and only one antelateral seta, a', develops on tarsus IV; both setae v' and l' form on trochanter III in the deutonymph. Therefore, there is no conflict with our generic description.
Palp solenidion. In their Fig. 3C, the palp is illustrated as having solenidion ω entirely fused to seta acm, but in correspondence they expressed some uncertainty, not having directly distinguished the two components. In all our studied material of Caleremaeus, including C. monilipes, ω is independent from acm and lies prone on the tarsal cuticle, where it is inconspicuous. Grandjean (1965b) also noted this independent, prone form in C. monilipes (presumably a French population).
Acknowledgements
We are grateful to numerous individuals whose assistance enhanced our study. Barry Flahey and Barb Eamer, both retired from the Research Branch, Agriculture and Agri-Food Canada, inked the figures and prepared the SEMs, respectively. Dr. Günther Krisper (Karl-Franzens-Universität Graz, Austria) generously shared the results of unpublished studies on Caleremaeus monilipes that were conducted in his laboratory, including two unpublished posters presented at meetings: (1) `Caleremaeus monilipes Michael, 1882 (Oribatida, Caleremaeidae) – More than one species!' by Krisper, G., Neuhold, P. and Lienhard, A., presented at the 7th Symposium of the European Association of Acarologists in Vienna, Austria (July, 2012); (2) `Caleremaeus (Oribatida, Caleremaeidae): An underestimated taxon' by Lienhard, A. and Krisper, G., presented at the 8th Symposium of the European Association of Acarologists in Valencia, Spain (September 2016). Dr. Mark Judson (Muséum national d'Histoire naturelle, Paris, France) kindly advised us on nomenclatural issues. We are particularly grateful to Drs. Anna and Stanisław Seniczak (University of Bergen, Norway; Kazimierz Wielki University, Bydgoszcz, Poland) for their patience and careful responses to our questions concerning their recent study of C. monilipes. Much of our work depended on the generosity of collectors noted above, particularly the late Dr. Louis J. Metz, USDA Forest Service, Raleigh, North Carolina, who was inspirational in R.A.N.'s early career. We also are grateful for the opportunity to study type specimens of C. retractus at the USNM and MCZ collections.
References
Aoki J. 1967. A preliminary revision of the family Otocepheidae (Acari, Cryptostigmata). II. Subfamily Tetracondylinae. Bul. Nat. Mus. Nat. Sci., Tokyo, 10: 297–359.
Aoki J., Fujikawa T. 1971. Occurrence of a Japanese representative of the North American genus Megeremaeus (Acari, Megeremaeidae). Taxonomic notes on oribatid mites of Hokkaido. IV. Annot. Zool. Japon., 44: 109–112.
Arroyo J., Kenny J., Bolger T. 2013. Oribatid and gamasid mite assemblages occurring in aerial and floor habitats of native forestry at Killarney National Park. Irish Natur. J., 32: 121–131.
Baker E.W., Wharton G.W. 1952. An Introduction to Acarology. New York: The MacMillan Co. pp. 465.
Balogh J. 1961. Identification keys of world oribatid (Acari) families and genera. Acta Zool. Acad. Sci. Hung., 7: 243–344.
Balogh J. 1963. Identification keys of holarctic oribatid mites (Acari) families and genera. Acta Zool. Acad. Sci. Hung., 9: 1–60.
Balogh J. 1972. The oribatid genera of the world. Budapest: Akademiai Kiadó. pp. 188.
Balogh J., Balogh P. 1992. The oribatid mites genera of the world. vol. 1. Budapest: The Hungarian National Museum Press. pp. 263.
Banks N. 1947. On some Acarina from North Carolina. Psyche, Cambridge, 54: 110–141. doi:10.1155/1947/70181 ![]()
Beck L. 1962. Beiträge zur Kenntnis der neotropischen Oribatidenfauna. 2. Nothridae, Camisiidae, Heterobelbidae (Arach., Acari). Senck. biol., Frankfurt, 43: 385–407.
Behan-Pelletier V.M. 1990. Redefinition of Megeremaeus (Acari: Megeremaeidae) with description of new species and nymphs of M. montanus Higgins and Woolley. Can. Entomol., 122: 875–900. doi:10.4039/Ent122875-9 ![]()
Behan-Pelletier V.M. 1991. Observations on genital papillae of pycnonotic Brachypylina (Acari: Oribatida). Acarologia, 32: 71–78.
Behan-Pelletier V.M. 1993. Eremaeidae (Acari: Oribatida) of North America. Mem. Entomol. Soc. Canada, 168: 1–193. doi:10.4039/entm125168fv ![]()
Behan-Pelletier V.M. 2017. Tegoribatidae of North America, with proposal of Protectoribates gen. nov., and new species (Acari, Oribatida, Tegoribatidae). Zootaxa, 4337: 151–197. doi:10.11646/zootaxa.4337.2.1 ![]()
Berlese A. 1896. Acari, Myriapoda et Scorpiones hucusque in Italia reperta. Ordo Cryptostigmata II (Oribatidae). Portici, Padova. pp. 98.
Berlese A. 1910. Lista di nuove specie e nuove generi di Acari. Redia, 6: 242–271.
Bonnet L., Cassagnau P., Travé J. 1975. L'écologie des arthropodes muscicoles à la lumière de l'analyse des correspondantes: Collemboles et Oribates du Sidobre (Tarn, France). Oecologia, 21: 359–373. doi:10.1007/BF00345827 ![]()
Bulanova-Zachvatkina E.M. 1957. The club-legged armored mite family Damaeidae Berl. (Acariformes, Oribatei). Communication I. Zool. Zhurnal, 36: 1167–1186. [in Russian]
Bulanova-Zachvatkina E.M. 1975. Family Caleremaeidae. In: Gilyarov M. S., Krivolutsky D. A. (Eds). A key to the soil-inhabiting mites. Sarcoptiformes. Moscow: Nauka, pp. 193–194.
Chen J., Norton R.A., Behan-Pelletier V.M, Wang H.-f. 2004. Analysis of the genus Gymnodampia (Acari: Oribatida), with redescription of G. setata and description of two new species from North America. Can. Entomol., 136: 793–821. doi:10.4039/n04-017 ![]()
Colloff M.J. 2019. The oribatid mite genus Topalia in Australia (Oribatida: Nosybeidae) and the taxonomic status of related families and genera. Zootaxa, 4647: 290–321. doi:10.11646/zootaxa.4647.1.18 ![]()
Elo R.A., Penttinen R., Sorvari J. 2018. Distribution of oribatid mites is moisture-related within red wood ant Formica polyctena nest mounds. Appl. Soil Ecol., 124: 203–210. doi:10.1016/j.apsoil.2017.11.013 ![]()
Ermilov S.G. 2012. Morphology of cornicles of oribatid mites of the family Damaeidae (Acari, Oribatida). Zool. Zhurnal, 91: 529–536. [in Russian; English version: Entomol. Rev., 92: 576–582]
Ermilov S.G., Sidorchuk E.A., Rybalov L.B. 2010. Morphology of juvenile stages of Pedrocortesella africana Pletzen, 1963 and Aleurodamaeus africanus Mahunka, 1984 (Acari: Oribatida). Ann. Zool. (Warszawa), 60: 391–406. doi:10.3161/000345410X535389 ![]()
Ezhova E.E., Kostyashova Z.V. 1997. Specimens of fossil oribatids (Acarina, Oribatida) from Baltic amber in the museums of Kaliningrad (Russia). Bernstein-Symposium Bochum, 16.–17. Sept. 1996. Sonderheft Metalla. pp: 45–50.
Fernandez N., Theron P., Rollard C. 2013. The family Carabodidae (Acari: Oribatida) I. Description of a new genus, Bovicarabodes with three new species, and the redescription of Hardybodes mirabilis Balogh. Intern. J. Acarol., 39: 26–57. doi:10.1080/01647954.2012.741144 ![]()
Fischer B.M., Schatz H., Maraun M. 2010. Community structure, trophic position and reproductive mode of soil and bark-living oribatid mites in an alpine grassland ecosystem. Exper. Appl. Acarol., 52: 221–237. doi:10.1007/s10493-010-9366-8 ![]()
Franklin E., Woas S. 1992. Some oribatid mites of the family Oppiidae (Acari, Oribatida) from Amazonia. Andrias, Karlsruhe, 9: 5–56.
Fujikawa T. 1991. List of oribatid families and genera of the world. Edaphologia, 46: (i–vii) 1–132.
Grandjean F. 1934. Les poils des épimères chez les Oribates (Acariens). Bull. Mus. nat. Hist. natur., 2e série, 6: 504–512.
Grandjean F. 1939. Les segments post-larvaires de l'hystérosoma chez les Oribates (Acariens). Bull. Soc. zool. France, 64: 273–284.
Grandjean, F. 1941. Statistique sexuelle et parthénogenèse chez les Oribates (Acariens). C.R. Séanc. Ac. Sci., 212: 463–467.
Grandjean F. 1949. Observation et conservation des très petits arthropodes. Bull. Mus. nat. Hist. natur., 2e série, 21: 363–370.
Grandjean F. 1952. Au sujet de l'ectosquelette du podosoma chez les Oribates superieurs et de sa terminologie. Bull. Soc. zool. France, 77: 13–36.
Grandjean F. 1953. Observations sur les Oribates (27e série). Bull. Mus. nat. Hist. natur., 2e série, 25: 469–476.
Grandjean F. 1954a. Essai de classification des Oribates (Acariens). Bull. Soc. zool. France, 78: 421–446.
Grandjean F. 1954b. Observations sur les Oribates (29e série) Bull. Mus. nat. Hist. natur., 2e série, 26: 334–341.
Grandjean F. 1954c. Observations sur les Oribates (30e série). Bull. Mus. nat. Hist. natur., 2e série, 26: 482–490.
Grandjean F. 1959a. Polypterozetes cherubin Berl. 1916 (Oribate). Acarologia, 1: 147–180.
Grandjean F. 1959b. Hammation sollertius n.g., n.sp. (Acarien, Oribate). Mem. Mus. nat. Hist. natur. (n.s.), ser. A, Zool., 16: 173–198.
Grandjean F. 1960. Damaeus arvernensis n. sp. (Oribate). Acarologia, 2: 250–275.
Grandjean F. 1961a. Nouvelles observations sur les Oribates (1re série). Acarologia, 3: 206–231.
Grandjean F. 1961b. Les Amerobelbidae (Oribates) (1re partie). Acarologia, 3: 303–343.
Grandjean F. 1962. Le genre Tegeocranellus Berl. 1913 (Oribates). Acarologia, 4: 78–100.
Grandjean F. 1963 Sur deux espèces de Brachychthoniidae et leur développement (Oribates). Acarologia, 5: 122–151.
Grandjean F. 1964a. Nouvelles observations sur les Oribates (3e série). Acarologia, 6: 170–198.
Grandjean F. 1964b. Pheroliodes wehnckei (Willmann) (Oribate). Acarologia, 6: 353–386.
Grandjean F. 1965a. Fosseremus quadripertitus nom.nov. (Oribate). Acarologia, 7: 343–375.
Grandjean F. 1965b. Complément à mon travail de 1953 sur la classification des Oribates. Acarologia, 7: 713–734.
Grandjean F. 1966. Les Staurobatidae n.fam. (Oribates). Acarologia, 8: 696–727.
Grandjean F. 1968. Schusteria littorea n.g., n.sp. et les Selenoribatidae (Oribates). Acarologia, 10: 116–150.
Grandjean F. 1969. Observation sur les muscles de fermeture des volets anaux et génitaux et sur la structure progénitale chez les Oribates supérieures adultes. Acarologia, 11: 317–349.
Hammen L. van der. 1980. Glossary of acarological terminology. Volume I. General terminology. The Hague: Dr. W. Junk Publ. pp. 244.
Harris, R. 1979. A glossary of surface sculpturing. Occas. Pap. Entomol., 28: 1–31.
Hunt G.S. 1996. A review of the genus Pedrocortesella Hammer in Australia (Acarina: Cryptostigmata: Pedrocortesellidae). Rec. Austral. Mus., 48: 223–286. doi:10.3853/j.0067-1975.48.1996.430 ![]()
Knülle W. 1957. Morphologische und entwicklungsgeschichtliche Untersuchungen zum phylogenetischen System der Acari. Acariformes Zachv. I. Oribatei, Malaconothridae. Mitt. Zool. Mus., Berlin, 33: 97–213. doi:10.1002/mmnz.4830330103 ![]()
Krawczyk A.J., Augustiničová G., Gwiazdowicz D.J., Konwerski S., Kucharczyk H., Olejniczak I., Rutkowski T., Skubala P., Solarz K., Zdrojewska Z., Tryjanowski P. 2015. Nests of the harvest mouse (Micromys minutus) as habitat for invertebrates. Biologia, 70: 1637–1647. doi:10.1515/biolog-2015-0186 ![]()
Krisper G., Schatz H., Schuster R. 2017. Oribatida (Arachnida: Acari). Checklisten der Fauna Österreichs, Nr. 9, Biosystematics and Ecology Series, Band 33. Verlag der Österreichischen Akademie der Wissenschaften, Wien. pp: 25–90. doi:10.2307/j.ctvdjrr69.5 ![]()
Krivolutsky D.A., Druk A.Ja., Eitminaviciute I.S., Laskova L.M., Karppinen E. 1990. Fossil oribatid mites. Vilnius: Mokslas Publishers. pp. 109 pp. [in Russian]
Krivolutsky D.A., Lebrun P., Kunst M., et al. 1995. Oribatid mites. Morphology, development, phylogeny, ecology, methods of study and characteristics of the model species Nothrus palustris C.L.Koch, 1839. Moscow: Nauka Publishers. pp. 224 pp. [in Russian]
Kunst M. 1971. Key to the fauna of Czechoslovakia – Supercohort Oribatei. In: Daniel M., Cerny V. (Eds). Klic zvireny CSSR IV. Praha: Academia. pp. 531–580. [in Czech]
Labandeira C.C., Phillips T.L., Norton R.A. 1997. Oribatid mites and the decomposition of plant tissues in paleozoic coal swamp forests. Palaios, 12: 319–353. doi:10.2307/3515334 ![]()
Lamos R.A. 2016. Tokukobelba gen. nov. (Acari: Oribatida: Damaeidae). Carolinea, 74: 53–102.
Lebedeva N.V., Poltavskaya M.P. 2013. Oribatid mites (Acari, Oribatida) of plain area of the Southern European Russia. Zootaxa, 3709: 101–133. doi:10.11646/zootaxa.3709.2.1 ![]()
Lienhard A., Schäffer S., Krisper G., Sturmbauer C. 2013. Reverse evolution and cryptic diversity in putative sister families of the Oribatida (Acari). J. Zool. Syst. Evol. Res. 52 (2014): 86–93. doi:10.1111/jzs.12037 ![]()
Luxton M. 1972. Studies on the oribatid mites of a Danish beech wood soil. I. Nutritional biology. Pedobiologia, 12: 434–463.
Marshall V.G., Reeves R.M., Norton R.A. 1987. Catalogue of the Oribatida (Acari) of continental United States and Canada. Mem. Entomol. Soc. Canada, 139. pp. 418. doi:10.4039/entm119139fv ![]()
Michael A.D. 1882. Further notes on British Oribatidae. J. Roy. Micr. Soc., ser. 2, London, 2: 1–18.
Michael A.D. 1888. British Oribatidae. Vol. II. London: Ray Society. pp. 337–657.
Michael A.D., George, C.F. 1879. A contribution to the knowledge of the British Oribatidae. J. Roy. Micr. Soc., London, 2: 225–251. doi:10.1111/j.1365-2818.1879.tb01649.x ![]()
Mihelčič F. 1952. Beitrag zur Kenntnis der Oribatei und Collembolen der Humusböden. Arch. Zool. Ital., 37: 93–106.
Miko L., Mourek J. 2008. Taxonomy of European Damaeidae (Acari: Oribatida) I. Kunstidamaeus Miko, 2006, with comments on Damaeus sensu lato. Zootaxa, 1820: 1–26. doi:10.11646/zootaxa.1820.1.1 ![]()
Miko L., Travé J. 1996. Hungarobelbidae n. fam., with a description of Hungarobelba pyrenaica n. sp. (Acarina, Oribatida). Acarologia, 37: 133–155.
Moraza M.L., Peña M.A. 2005. Oribatid mites (Acari, Oribatida) in selected habitats of La Gomera (Canary Islands, Spain). Boln. Asoc. Esp. Ent., 29: 39–54.
Norton R.A. 1977. A review of F. Grandjean's system of leg-chaetotaxy in the Oribatei and its application to the Damaeidae. In: Dindal D.L. (Ed.): Biology of oribatid mites. Syracuse: State Univ. New York. pp. 33–62.
Norton R.A. 1978. Veloppia kananaskis n. sp., with notes on the familial affinities of Veloppia Hammer (Acari: Oribatei). Intern. J. Acarol., 4: 71–84. doi:10.1080/01647957808684027 ![]()
Norton R.A., Behan-Pelletier V.M., Wang H.-f. 1996. The aquatic oribatid mite genus Mucronothrus in Canada and the Western USA (Acari, Trhypochthoniidae). Can. J. Zool., 74: 926–949. doi:10.1139/z96-106 ![]()
Norton R.A., Behan-Pelletier V.M. 2009. Chapter 15, Oribatida. In: Krantz G.W., Walter D. E. (Eds). A manual of acarology. Lubbock, Texas: Texas Tech University Press. pp. 430–564.
Norton R.A., Ermilov S.G. 2014. Catalogue and historical overview of juvenile instars of oribatid mites (Acari: Oribatida). Zootaxa, 3833: 1–132. doi:10.11646/zootaxa.3833.1.1 ![]()
Norton R.A., Ermilov S.G. 2017. Identity of the oribatid mite Oribata curva and transfer to Trichogalumna (Acari, Oribatida, Galumnidae), with discussion of nomenclatural and biogeographic issues in the 'curva' species-group. Zootaxa, 4272: 551–564. doi:10.11646/zootaxa.4272.4.4 ![]()
Norton R.A., Ermilov S.G. 2019. Anderemaeus (Acari, Oribatida)-overview, three new species from South America and reassessment of Anderemaeidae supported by ontogeny. Zootaxa, 4647: 241–289. doi:10.11646/zootaxa.4647.1.17 ![]()
Norton R.A., Franklin E. 2018. Paraquanothrus n. gen. from freshwater rock pools in the USA, with new diagnoses of Aquanothrus, Aquanothrinae, and Ameronothridae (Acari, Oribatida). Acarologia, 58: 557–627.
Okayama T. 1980. Taxonomic studies on the Japanese oribatid mites wearing nymphal exuviae. I. Heterobelba stellifera, sp. n. Acta Arachnol., 29: 83–89. doi:10.2476/asjaa.29.83 ![]()
Pearse A.S. 1946. Observations of the microfauna of the Duke Forest. Ecol. Monogr., 16: 127–250. doi:10.2307/1943104 ![]()
Pérez-Iñigo C. 1997. Acari. Oribatei. Gymnonota I. In: Ramos A. et al. (Eds): Fauna Iberica, vol. 9. Madrid: Museo de Ciencias Naturales, 373 pp.
Pfingstl T. 2013. Habitat use, feeding and reproductive traits of rocky-shore intertidal mites from Bermuda (Oribatida: Fortuyniidae and Selenoribatidae). Acarologia, 53: 369–382. doi:10.1051/acarologia/20132101 ![]()
Pfingstl T. 2015. Morphological diversity in Selenoribates (Acari, Oribatida): new species from coasts of the Red Sea and the Indo-Pacific. Intern. J. Acarol., 41: 356–370. doi:10.1080/01647954.2015.1035321 ![]()
Pfingstl T., Krisper G. 2014. Plastron respiration in marine intertidal oribatid mites (Acari, Fortuyniidae and Selenoribatidae). Zoomorphology, 133: 3359–3378. doi:10.1007/s00435-014-0228-5 ![]()
Radford C.D. 1950. Systematic check list of mite genera and type species. Intern. Union Biol. Sci., 1: 1–232.
Schaefer, I., Caruso, T. 2019. Oribatid mites show that soil food web complexity and close aboveground-belowground linkages emerged in the early Paleozoic. Commun. Biol. (2019) 2: 387. doi:10.1038/s42003-019-0628-7 ![]()
Schatz H. 1983. U.-Ordn.: Oribatei, Hornmilben. Catalogus Faunae Austriae, Wien, Teil IXi. pp. 118.
Schatz H., Schuster R. 2009. Acari – Oribatida, Hornmilben. In: Rabitsch W., Essl F. (Eds): Endemiten in Österreich. Kostbarkeiten in Österreichs Tier- und Pflanzenwelt. Wien: Naturwissenschaftlicher Verein für Kärnten, Klagenfurt und Umweltbundesamt GmbH, Klagenfurt. pp. 464–475.
Schatz H., Behan-Pelletier V.M., OConnor B.M., Norton R.A. 2011. Suborder Oribatida van der Hammen, 1968. In: Zhang Z.-Q. (Ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148: 141–148. doi:10.11646/zootaxa.3148.1.26 ![]()
Schneider K., Renker K., Scheu S., Maraun M. 2004. Feeding biology of oribatid mites: a minireview. In: Weigmann G., Alberti G., Wohltmann A., Ragusa S. (Eds): Acarine Biodiversity in the Natural and Human Sphere. Proceedings of the V Symposium of the European Association of Acarologists (Berlin 2004). Phytophaga (Palermo), 14: 247–256.
Schweizer J. 1957. Die Landmilben des Schweizerischen Nationalparkes. 4. Teil: Ihr Lebensraum, ihre Vergesellschaftung unter sich und ihre Lebensweise. Erg. wiss. Unters. Schweiz. Nat. Park, N.F., Liestal, 6: 11–107.
Sellnick M. 1928. Formenkreis: Hornmilben, Oribatei. In: Brohmer P., Ehrmann P., Ulmer G. (Eds): Die Tierwelt Mitteleuropas, vol. 3 (9). Leipzig: Quelle und Meyer. pp: 1–42.
Sellnick M. 1931. Milben im Bernstein. In: Andree K. (Ed.): Bernsteinforschungen, 2: 148–180.
Seniczak A., Seniczak S. 2019. Morphological ontogeny of Caleremaeus monilipes (Acari: Oribatida: Caleremaeidae), with comments on Caleremaeus Berlese. Syst. Appl. Acarol. 24: 1995–2009. doi:10.11158/saa.24.11.3 ![]()
Seniczak A., Bolger T., Roth S., Seniczak S., Djursvoll P., Jordal, B.H. 2019. Diverse mite communities (Acari: Oribatida, Mesostigmata) from a broadleaf forest in western Norway. Ann. Zool. Fennici 56: 121–136. doi:10.5735/086.056.0111 ![]()
Seniczak A., Seniczak S., Graczyk R., Pacek S. 2018a. Morphological ontogeny of Hafenrefferia gilvipes (Acari: Oribatida: Tenuialidae), with comments on Hafenrefferia Oudemans. Syst. Appl. Acarol. 23: 1265–1277. doi:10.11158/saa.23.7.5 ![]()
Seniczak S., Norton R.A., Wang H.-f. 1998. The morphology of juvenile stages of moss mites of the family Trhypochthoniidae (Acari: Oribatida), and the taxonomic status of some genera and species. Zool. Anz., 237: 85–95.
Seniczak S., Seniczak A., Kaczmarek S. 2018b. Morphological ontogeny of Platyliodes scaliger (Acari, Oribatida, Neoliodidae), with comments on Platyliodes Berlese. Syst. Appl. Acarol., 23: 25–41. doi:10.11158/saa.23.1.3 ![]()
Siira-Pietikäinen A., Penttinen R., Huhta V. 2008. Oribatid mites (Acari: Oribatida) in boreal forest floor and decaying wood. Pedobiologia, 52: 111–118. doi:10.1016/j.pedobi.2008.05.001 ![]()
Skubała P., Gurgul B. 2011. Importance of tree hollows for biodiversity of mites (Acari) in the forest reserve "Śrubita" (Carpathian Mountains, south Poland). Biological Letters, 48: 97–106. doi:10.2478/v10120-011-0010-z ![]()
Skubała P., Maslak M. 2010. Succession of oribatid fauna (Acari, Oribatida) in fallen spruce trees: Deadwood promotes species and functional diversity. In: Sabelis M.W., Bruin J. (Eds): Trends in Acarology, Proceedings of the XII International Congress of Acarology, Amsterdam (2006). Dordrecht: Springer-Science + Business Media B.V. pp. 123–128. doi:10.1007/978-90-481-9837-5_19 ![]()
Subías L.S. 1977. Taxonomía y ecología de los oribátidos saxícolas y arborícolas de la Sierra del Guadarrama (Acarida, Oribatida). Trabajos de la Cátedra de Artrópodos, Facultad de Biología, Universidad Complutense de Madrid, 24. pp. 379.
Subías L.S. 2004. Listado sistemático, sinonímico y biogeográfico de los Ácaros Oribátidos (Acariformes, Oribatida) del mundo (1758–2002). Graellsia, 60: 3–305. doi:10.3989/graellsia.2004.v60.iExtra.218 ![]()
Subías L.S. 2016. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles). Unpublished online version accessed March 2016, 593 pp.
Subías L.S. 2019. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles). Unpublished online version accessed March 2019, 536 pp. http://bba.bioucm.es/cont/docs/RO
_1.pdf ![]()
Subías L.S., Arillo A. 2001. Acari, Oribatei, Gymnonota II. Oppioidea. In: Ramos A. et al. (Eds): Fauna Iberica, vol. 15. Madrid: Museo de Ciencias Naturales. pp. 289.
Taylor A.R., Ranius T. 2014. Tree hollows harbour a specialised oribatid mite fauna. J. Insect Conserv., 18:39–55. doi:10.1007/s10841-014-9613-0 ![]()
Travé J. 1959. Sur le genre Niphocepheus Balogh 1943, les Niphocepheidae, Famille nouvelle (Acariens, Oribates). Acarologia, 1: 475–498.
Travé J. 1961. Sur deux interessantes espèces d'Oribates (Acariens). Vie et Milieu, 11: 683–686.
Travé J. 1963. Écologie et biologie des Oribates (Acariens) saxicoles et arboricoles. Vie et Milieu, Supplément, 14: 1–267.
Travé J., André H.M., Taberly G., Bernini F. 1996. Les Acariens Oribates. Wavre, Belgium: AGAR & Société internationale des Acarologues de Langue française. pp. 110.
Travé J., Vachon M. 1975. François Grandjean 1882–1975 (Notice biographique et bibliographique). Acarologia, 17: 1–19.
Vazquez M.M., Prieto Trueba D. 1999. Oribátida. In: Vazquez M.M. (Ed.): Fauna edáfica de las selvas tropicales de Quintana Roo. Chetumal: Universidad de Quintana Roo. pp, 73–90.
Vitzthum H. 1931. Acari = Milben. 9. Ordnung der Arachnida. In: Handbuch der Zoologie, vol. 3 (2). Berlin: De Gruyter. pp. 160.
Vitzthum H. 1943. Acarina. In: Bronn H.G. (Ed.). Klassen und Ordnungen des Tierreiches, 5. Band: Arthropoda, IV. Abteilung: Arachnoidea, 5. Buch: Acarina. Leipzig: Becker & Erler Kom.-Ges. pp. 1011.
Wauthy G., Fain A. 1991. Observations on the legs of Trimalaconothrus maniculatus Fain et Lambrechts, 1987 (Acari, Oribatida). Part 1. Larva, leg IV of nymphs and fundamental phanerotaxy. Acarologia, 32: 415–434.
Weigmann G. 2002. Morphologie, Biogeographie und Ökologie einer in Zentraleuropa neuen Hornmilbe: Eremobelba geographica Berlese, 1908 (Acari, Oribatida, Eremobelbidae). Abh. Ber. Naturkundemus., Görlitz, 74: 31–36.
Weigmann G. 2006. Hornmilben (Oribatida). Die Tierwelt Deutschlands. Teil 76. Keltern: Goecke & Evers. pp. 520.
Willmann C. 1931. Moosmilben oder Oribatiden (Cryptostigmata). In: Dahl F. (Ed.). Die Tierwelt Deutschlands, Band 22, vol. 5. Jena: Gustav Fischer. pp. 79–200.
Woas S. 2002. Acari: Oribatida. In: Adis J. (Ed.). Amazonian Arachnida and Myriapoda. Sofia, Moscow: Pensoft Publishers. pp. 21–291.



2020-03-02
Date accepted:
2020-05-01
Date published:
2020-05-11
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Norton, Roy A. and Behan-Pelletier, Valerie M.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



