Phytoseiidae of La Réunion Island (Acari: Mesostigmata): three new species and two males described, new synonymies, and new records
Kreiter, Serge1 ; Payet, Rose-My2 ; Douin, Martial3 ; Fontaine, Olivier4 ; Fillâtre, Jacques5 and Le Bellec, Fabrice6
1✉ Montpellier SupAgro, UMR CBGP INRAE/ IRD/ CIRAD/ SupAgro, 755 Avenue du Campus Agropolis (Baillarguet), CS 30016, 34988 Montferrier-sur-Lez cedex, France.
2CIRAD, Hortsys, Station de Bassin-Plat, 97410, Saint-Pierre, Réunion, France.
3Montpellier SupAgro, UMR CBGP INRAE/ IRD/ CIRAD/ SupAgro, 755 Avenue du Campus Agropolis (Baillarguet), CS 30016, 34988 Montferrier-sur-Lez cedex, France.
4SARL La Coccinelle, 6 Chemin Beaurivage, 97410 Saint-Pierre (La Réunion), France.
5Armeflhor, 1 Chemin de l'IRFA, 97410 Saint-Pierre, Réunion, France.
6HortSys, Université de Montpellier, CIRAD, TA B-103/C, Campus international de Baillarguet, 34398 Montpellier Cedex 5, France.
2020 - Volume: 60 Issue: 1 pages: 111-195
https://doi.org/10.24349/acarologia/20204361ZooBank LSID: E7376941-8C9E-44B1-82F5-00D4A010E079
Original research
Keywords
Abstract
Introduction
Several species in the family Phytoseiidae are important natural enemies of phytophagous mite and small insects in natural habitats, outdoor and protected crops all around the world (McMurtry and Croft 1997; McMurtry et al. 2013). However, despite the huge numbers of faunistic surveys carried out for more than 60 years, the fauna of some countries and particular ecosystems remain little explored. Consequently, it is important to survey phytoseiid faunas in poorly known areas in order to document the biodiversity of these areas, especially in biodiversity hotspots, as well as to discover new potential biological control agents (BCA). This is especially important given the context of new international and state regulations concerning import-export of BCA (Kreiter et al. 2020a, b).
The family Phytoseiidae is widespread all over the world and consists of 2,521 valid species dispatched in three sub-families and 94 genera (Demite et al. 2019).
Several terrestrial areas of the Indian Ocean constitute one of the world's hotspots of biodiversity. Myers (1988) defined the hotspot of biodiversity concept in order to identify the areas of biodiversity in most urgent need of conservation/protection. These world hotspots are characterized by high levels of endemism and have lost at least 70 % of their original natural vegetation (Myers et al. 2000). The characterization of the phytoseiid mite diversity in these areas is thus contributing to this general topic of conservation. Located in the Indian Ocean at 700–800 km from the Eastern coast of Madagascar, La Réunion is the main island of the Mascareignes Archipelago (with the two other main islands being Mauritius and Rodrigues).
Ueckermann and Loots (1985) published the first paper concerning Phytoseiidae of La Réunion Island 35 years ago. It concerns a description of a new species to Science, Phytoscutus reunionensis (Ueckermann and Loots) found on Prunus persica (L.) in La Plaine des Cafres in 1983 by Dr Serge Quilici (Ueckermann and Loots 1985). In a study of the Tetranychidae and their predators, Guttierrez and Etienne (1986) mentioned two species of Phytoseiidae for La Réunion, Euseius ovaloides (Blommers) and Phytoseiulus persimilis Athias-Henriot, the last probably escaped in the neighbouring areas of greenhouses after releases. Quilici et al. (1988) mentioned then two species on litchi (Litchi chinensis Sonn.) in Bassin-Martin CIRAD research station, Amblyseius largoensis (Muma) and E. ovaloides, bringing to four the number of known species. In a further study focussing on mites of various crops of the Island, Quilici et al. (1997) mentioned six additional species and Quilici et al. (2000) added 14 species recorded for the first time in La Réunion. Just after, Kreiter et al. (2002) described seven new species from the Island, bringing to eight the number of new species described and to 31 the total number of species recorded from this island. Only one was collected in another place after its description, Typhlodromus (Anthoseius) moraesi Kreiter and Ueckermann, very far from La Réunion, in Guadeloupe and then also in Martinique, both in the Caribbean (Kreiter et al. 2013, 2018c). Moraes et al. (2012) added another species for La Réunion, Neoseiulus recifensis Gondim Jr. and Moraes, originally described from Brasil. Surprisingly, this species was found in samples from a survey for selection of a BCA against Raoiella indica Hirst in Brazil, along with two other common species, A. largoensis and T. ( A.) moraesi. Finally, Kreiter et al. (2016a, b) mentioned the unexpected occurrence of Amblyseius swirskii Athias-Henriot in La Réunion, never collected and mentioned in previous studies, collected suddenly in high population in 2015 and 2016, bringing to 33 the number of the known species present in La Réunion Island.
We report in this 10th paper on La Réunion phytoseiids the results of additional surveys conducted from 2015 to 2018.
Material and methods
The survey took place in La Réunion Island in 2015, 2016, 2017 and 2018. Plant-inhabiting mites were collected from various cultivated and wild plants in various locations of La Réunion. Mites were directly collected on leaves with a fine brush or by using the leaf ''dipping-shaking-washing-filtering (dswf)'' method of Boller (1984) or by beating the plants (mainly shrubs or trees) and collecting the mites in a black plastic rectangular saucer 45 x 30 cm (Ref. STR 45, BHR, 71370 Saint-Germain-du-Plain, France). The method selected was dependent on the plant investigated: large leaves of shrubs and trees were sampled using the direct collection method or with dswf; small leaves of shrubs and trees with the dswf or by beating and herbaceous plants with dswf.
An experiment was conducted at the Bassin-Plat CIRAD Research Station, Saint-Pierre, in La Réunion Island (altitude above sea level = aasl: 153 m, 55°29'18'' E; 21°19'25'' S). The experimental site was a 0.3 ha citrus orchard (Citrus sinensis x C. reticulata cv. Tangor grafted on Citrange Carrizo) with 149 meters spaced trees planted in March 2012, after a 2-year spontaneous fallow. Tree rows were planted six meters apart. The wet season spans from November to April and the dry season from June to October. The local average annual precipitation for the period 2014 to 2016 was 1025 mm. From March 2012 to February 2014 (before the experiment started), weeds around the tree base and within 50-cm radii around them were controlled with the herbicide glyphosate (360 g.L-1 and 4 L.ha-1). Weeds in the 5-m-wide area between rows were controlled with a hammer mill.
The experiment began in March 2014. We used a complete bloc design with six replicates to test the effects of different weed management methods on the composition of the ground cover plant community. Four weed management treatments were compared: tillage (T), mowing (M), hammer mill (HM), and herbicide (H). These four treatments were distributed haphazardly within each replicate (four plots of 13 m x 5 m). Mowing and herbicide-spraying treatments were carried out in the same inter-rows so that they would not be disturbed by the tractors used for tillage or hammer mill. Hammer mill is the most commonly used engine for weed management in citrus orchards on the Réunion Island. Mowing was done with an adapted hedge-trimmer, which cuts up weeds at 10 cm above ground. A hammer mill [SML 155 SEPPI®, Caldaro (Bolzano), Italy] was used to crush weeds at the soil level. The herbicide treatment using glyphosate (360 g.L-1 at 4 L.ha-1) eliminated all weeds. A disk harrow (Grégoire and Besson®, Montigné-Montfaucon, France) was implemented once or twice in order to destroy the maximum of weeds. Weed management activities were activated when the ground cover was estimated by a farmer as being too high (70 to 80 cm height for at least one treatment). The timing for treatment was the same for all four treatments, and their timing was spaced out by a period of 93 + 27 days (mean ± SD) depending on the season.
Plots with different weed management (weed communities are variable in space and time and it is impossible to give a precise list of weed species for each date of sampling) were sampled in order to collect arthropods, including phytoseiid mites. Mites collected were then transferred with a brush into small plastic vials containing 70 % ethanol. Mites were then all mounted on slides using Hoyer's medium and identified using a phase or interferential contrast microscope (DMLB, Leica Microsystèmes SAS, Nanterre, France). Characters of specimens were measured using a graduate eyepiece (Leica, see above). We used Chant and McMurtry's (1994, 2007) concepts of the taxonomy of Phytoseiidae and the world catalogue database of Demite et al. (2019) for faunistical and biogeographical aspects. Only females were measured unless males were available. Immature will be measured and described in another paper.
In the (re)description of species, the setal nomenclature system adopted was that of Lindquist and Evans (1965) and Lindquist (1994), as adapted by Rowell et al. (1978) for the dorsum and by Chant and Yoshida-Shaul (1991) for the venter. The idiosomal setal pattern follows Chant and Yoshida-Shaul (1992). The notation for gland pores (solenostomes) or lyrifissures (poroids) is based on Athias-Henriot (1975).
Measurements of the main morphological characters were made as follows: dorsal shield length from the anterior to posterior shield margins along the midline; width between lateral margins at the level of setae s4; length of genital shield from the anterior margin of hyaline surface to the posterior margin of the shield; width of genital shield as the distance at the level of setae st5 and between posterior corners of the shield; ventrianal shield length as the distance between anterior and posterior margins; ventrianal shield width between insertions of ZV2 and at level of anus, of paranal setae between margins of the shield; cheliceral movable digit length was measured from basal articulation to tip of the digit; the fixed digit from the dorsal lyrifissure to the tip. Numbers of teeth on the fixed and movable cheliceral digits do not include the respective apical hook. Setae not referred to in the Results section should be considered as absent.
All measurements are given in micrometers (µm) and presented as the mean in bold followed by the range in parenthesis. New measurements for holotypes and paratypes of already described species are presented in tables in bold and underlined. Specimens of all species are deposited in the mite collections of Montpellier SupAgro conserved in UMR CBGP INRA/IRD/CIRAD/SupAgro. Specimens collected in fields in La Réunion within this survey were all identified. Very few single males collected alone within this study were not taken into account.
The following type or additional material have been borrowed and studied:
• The holotype of Neoseiulus houstoni (Schicha), from the reference collection of the Biosecurity Collections (NSW [= New South Wales] Department of Primary Industries), Orange NSW, Australia;
• The holotype, one paratype and additional material of Neoseiulus recifensis Gondim Jr and Moraes, housed in the mites reference collection of the Department Entomology and Acarology, Escuela Superior de Agricultura Luiz des Queiroz (ESALQ), University of Sao Paulo (USP), Piracicaba, Brasil.
We have also examined type specimens of Amblyseius longipilus Kreiter and Ueckermann (the holotype and 10 paratype females), eckermannseius nesiotus Ueckermann and Kreiter (one paratype female), Phytoseius haroldi Ueckermann and Kreiter (three paratype females), and Neoseiulus barreti Kreiter (one paratype female) of the mite collections of Montpellier SupAgro conserved in UMR CBGP, in order to complete descriptions and compared to specimens collected during this study.
The following abbreviations are used in this paper for morphological characters: dsl = dorsal shield length just under j1 to just below J5; dsw = dorsal shield width at the level of s4; Per. ext.: peritreme extension; gd = solenostome; Z4 ser., Z5 ser. = Z4, Z5 serrated (if Z4 and Z5 without ser. = not serrated); knob. = knobbed; gensl = genital shield length; gensw st5 = genital shield width at the level of setae st5; gensw post. corn. = genital shield width between posterior corners; lisl = largest inguinal sigilla (= ''metapodal plate'') length; lisw = largest inguinal sigilla (= ''metapodal plate'') width; sisl = smallest inguinal sigilla (= ''metapodal plate'') length; vsl = ventrianal shield length; gv3 = solenostome on ventrianal shield; gv3 dist. = distance between pre-anal solenostomes on the ventrianal shield; vsw ZV2 and vsw anus = ventrianal shield width at ZV2 level and at paranal setae level; asl = anal shield length; acw = anal shield width at the level of paranal setae; scl = total spermatheca length (calyx + neck or cervix + atrium); calyx l.: calyx length; scw = calyx largest width; FD = fixed digit; Fdl = fixed digit length; MD = movable digit; Mdl = movable digit length; Nb teeth Fd = number of teeth on the fixed digit; Nb teeth Md = number of teeth on the movable digit; Shaft = length of the shaft of spermatodactyl. We also used the following abbreviation: im. = immatures; BCA = Biological control agents; FCI = French Caribbean Islands; VCW: various countries in the world.
The following abbreviations are used in this paper for institutions: CBGP = Centre de Biologie pour la Gestion des Populations; CIRAD = Centre International de Recherche Agronomique pour le Développement; INRA = Institut National de la Recherche Agronomique; IRD = Institut de Recherche pour le Développement; MSA = Montpellier SupAgro, France; UMR = Unité Mixte de Recherche; UPR = Unité Propre de Recherche, Hortsys = Name of the CIRAD research unit o Agroecology functioning and performances of horticultural systems ; PVBMT = Plant Populations and Bio-aggressors in Tropical Ecosystems Joint Research Unit from University of La Réunion Island, CIRAD and INRA; NSW = New South Wales; ESALQ = Escola Superior de Agricultura Luiz de Queiroz; USP = Universidade de São Paulo.
Results and discussion
A total of 44 species were found from the beginning of 2015 to the end of 2018 in our surveys.
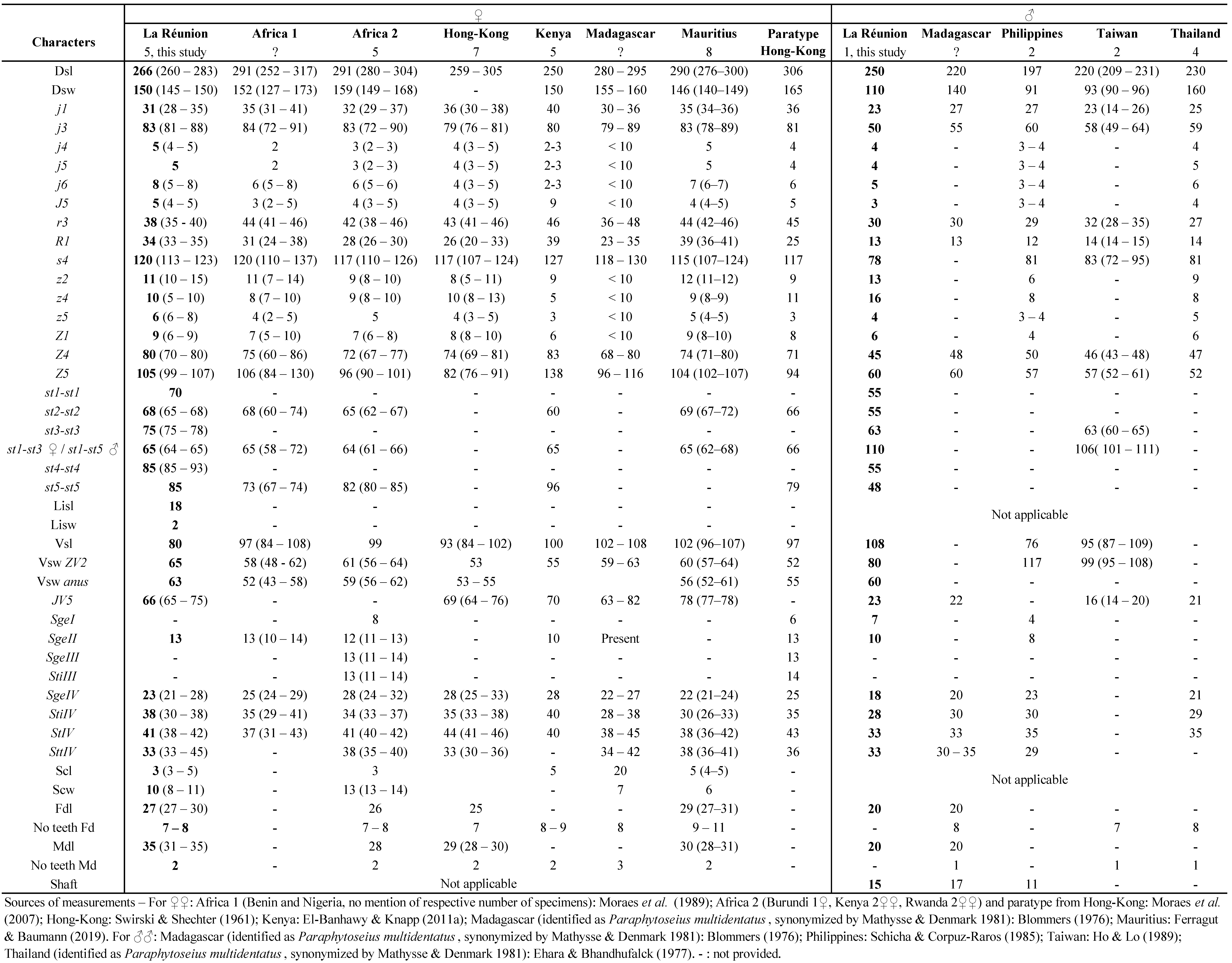
• Four species were already well-known in the literature, very common in La Réunion and already recorded; A. largoensis was commonly found in the near past in different localities (Moraes et al. 2012) and several hundred specimens of this species as well as of the next species (see below), A. swirskii, were collected; A. swirskii (see Kreiter et al. 2016a) of which ten females and five males were measured.; E. ovaloides (mentioned in Quilici et al. 1997, 2000), not measured in the present paper but this species has very characteristic features and is probably the most common species in La Réunion Island. Five hundred specimens were collected in the present study and everywhere in the Island; T. (Anth.) moraesi (see Kreiter et al. 2002), for which only eight females and one male collected in La Réunion were measured. This species is very characteristic and very common on weeds and low plants where several hundred specimens were collected during this study, mainly in experimental plots of Bassin-Plant CIRAD Research Station. Several specimens of this species was previously measured in the Caribbean islands (Kreiter et al. 2013, 2018c) and measurements of specimens of La Réunion agree well with measurements of these Caribbean specimens. These four species are very common and widespread in the island. Measurements of individuals of these four species are very largely overlap with those of original descriptions and of measurements published in other studies.
For the remaining 40 species, we give measurements and details in this paper:
• Nineteen have been already mentioned in previous papers (Quilici et al. 1997, 2000; Kreiter et al. 2002; Moraes et al. 2012) but they are rare and their occurrence have been given without any measurements which are still very interesting for further identifications: Neoseiulus arkeri Hughes, N. bayviewensis (Schicha), N. scapilatus (van der Merwe), N. teke (Pritchard and Baker), Paraphytoseius orientalis Narayanan, Kaur and Ghai, Phytoseiulus persimilis Athias-Henriot, Scapulaseius reptans (Blommers), Amblyseius herbicolus (Chant), A. tamatavensis (Blommers), Proprioseiopsis mexicanus (Garman), Typhlodromalus spinosus (Meyer and Rodrigues), Ueckermannseius nesiotus (Ueckermann and Kreiter), Euseius hima (Pritchard and Baker), Phytoseius amba Pritchard and Baker, P. crinitus Swirski and Shechter, P. haroldi Ueckermann and Kreiter, P. intermedius Evans and MacFarlane, Kuzinellus scytinus (Chazeau), Typhlodromus (Anthoseius) transvaalensis (Nesbitt).
• For one species, A. longipilus, 14 females have been measured in the original description of Kreiter et al. (2002) which is considered enough for a good estimate of the specific variability (Tixier 2012). Some character measures were however lacking in this original description and we provide here additional/complementary data on the species.
• For one species already known from the Island, N. recifensis, we were suspecting a synonymy with two other similar species of Neoseiulus (N. barreti and N. houstoni). These three species are compared thereafter and the male of one of this species is described for the first time.
• Seventeen species are new for the Island, namely: Neoseiulus baraki (Athias-Henriot), N. californicus (McGregor), N. houstoni, N. longispinosus (Evans), N. lula (Pritchard and Baker), N. paspalivorus (De Leon), Paraphytoseius horrifer (Pritchard and Baker), Typhlodromips culmulus (van der Merwe), Transeius soniae Zannou, Moraes and Oliveira, Amblyseius neoankaratrae (Ueckermann and Loots), Proprioseiopsis ovatus (Garman), Ueckermannseius parahavu Moraes, Zannou and Oliveira, Amblydromalus nakuruensis Moraes, Zannou and Oliveira, Phytoseius punicae Chinniah and Mohanasundaram, P. woodburyi De Leon, Platyseiella eliahui Ueckermann, Typhlodromus (Anthoseius) ndibu Pritchard and Baker. The male of A. neoankaratrae was previously unknown and is herein described.
• And finally, three species are new to Science, one belonging to the genus Amblyseius and two belonging to the genus Transeius. These three species are described in this paper.
All results concerning locations, measurements and some biological details for the 40 species are given thereafter.
Subfamily Amblyseiinae Muma
Amblyseiinae Muma, 1961: 273.
Tribe Neoseiulini Chant & McMurtry
Neoseiulini Chant & McMurtry 2003a: 6.
Genus Neoseiulus Hughes
Neoseiulus Hughes, 1948: 141.
Neoseiulus baraki (Athias-Henriot)
Amblyseius baraki Athias-Henriot 1966: 211.
Amblyseius (Amblyseius) baraki, Ehara & Bhandhufalck 1977: 54.
Amblyseius (Neoseiulus) baraki, Gupta 1986: 104.
Neoseiulus baraki, Moraes et al. 1986: 70; Chant & McMurtry 2003a: 27; Moraes et al. 2004a: 149; 2004b: 104; Zannou et al. 2006: 248; Chant & McMurtry 2007: 25.
Amblyseius dhooriai Gupta 1977: 30 (synonymy according to Gupta 1986).
This species belongs to the paspalivorus species group of the genus Neoseiulus as the female ventrianal shield is large, rectangular, rounded posteriorly, and the dorsal shield has marked ''shoulders'' at the level of setae r3 (Chant and McMurtry 2003a). Neoseiulus baraki is a Mediterranean and subtropical species often found on monocotyledonous plants, and mainly on Poaceae. It is also a predatory mite associated with the coconut mite Aceria guerreronis Keifer in many parts of the world (Moraes et al. 2004b; Lawson-Balagbo et al. 2008). It is known to disperse from herbaceous weeds to the coconut ''trees''. It has a flattened idiosoma with a small cross-sectional diameter (Moraes et al. 2004b), which enables it to reach the area underneath leaf bracts where the coconut mite feeds. Moreover, it shows a strong temporal relationship with the abundance of the coconut mite on palms (Fernando et al. 2003). Hence, N. baraki is considered a potential BCA against the coconut mite. However, in nature they are unable to maintain the coconut mite populations below the expected economic levels and so, additions of N. baraki to the environment to supplement natural populations for controlling the coconut mite has been considered. An essential pre-requisite to field augmentation is an effective mass rearing method. Use of coconut mites to mass rear N. baraki in the laboratory is expensive and time consuming. Eggs of Tetranychus urticae Koch, coconut pollen and maize pollen were found to be suitable alternative foods for rearing N. baraki but it can be more easily reared on Tyrophagus putrescentiae Shrank, which can be easily reared on cheap supports issued from agricultural products transformation.
This is the first mention of the occurrence of this species in La Réunion Island, the first mention in the Indian Ocean and the second mention from Sub-Saharan Africa sensu lato, in addition to Zannou et al. (2006).
Specimens examined: 16 ♀♀ + 8 ♂♂ in total, 12 ♀♀ + 5 ♂♂ measured. Saint-Pierre - Bassin-Plat CIRAD Research Station (altitude above sea level = aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 2 ♀♀ + 1 ♂ on Melinis repens (Willd.), 20/2/2017; 6 ♀♀ + 3 ♂♂ in CC (Cover crop), HM (Hammer Mill), and M (Mowing) and on Digitaria ramularis (Trin.), 20/3, 3 and 6/4 and 20/6/2017; 1 ♂ on Leucaena leucocephala (Lam.), 30/03/2017; 3 ♀♀ + 2 ♂ + 1 im. on Panicum maximum Jacq., and 5 ♀♀ + 1 ♂ + 6 im. on Bidens pilosa L., 20/2, 30/3 and 20/6/2017.
Remarks: The measurements of characters of adult females (Table 1) are similar to those published in the literature, especially with those from specimens of Tanzania, except for the number of teeth on fixed digits of chelicerae that is higher in specimens of Tanzania.



The measurements of adult males (Table 1) are also close to those published, and closest to those obtained from specimens from Tanzania, except for the number of teeth (same remark for females).
Neoseiulus barkeri Hughes
Neoseiulus barkeri Hughes 1948: 141; Chant & McMurtry 2003a: 35; Moraes et al. 1986:70; Moraes et al. 2004: 104.
Typhlodromus (Neoseiulus) barkeri, Nesbitt 1951: 35.
Typhlodromus (Typhlodromus) barkeri, Chant 1959: 63.
Typhlodromus (Amblyseius) barkeri, Hughes 1961: 222.
Typhlodromus barkeri, Hirschmann 1962: 9.
Amblyseius barkeri, Athias-Henriot 1961: 440; Moraes et al. 1989: 95.
Amblyseius (Amblyseius) barkeri, van der Merwe 1968: 112.
Neoseiulus bakeri, Ryu et al. 2001: 8; Chant & McMurtry 2003a: 33; Moraes et al. 2004a: 104; Chant & McMurtry 2007: 25.
Amblyseius masiaka Blommers & Chazeau 1974: 308 (Synonymy according to Ueckermann & Loots 1988).
Amblyseius mckenziei Schuster & Pritchard 1963: 268 (Synonymy according to Ragusa & Athias-Henriot 1983).
Amblyseius mycophilus Karg 1970: 290 (Synonymy according to Ragusa & Athias-Henriot 1983).
Amblyseius oahuensis Prasad 1965: 1518 (Synonymy according to Ragusa & Athias-Henriot 1983).
Amblyseius picketti Specht 1968: 681 (Synonymy according to Ragusa & Athias-Henriot 1983).
Amblyseius usitatus van der Merwe 1965: 71 (Synonymy according to Ueckermann & Loots 1988).
This species belongs to the barkeri species group of the genus Neoseiulus, as the spermathecal atrium is large and forked at junction with major duct. It belongs to the barkeri species subgroup as the calyx is not markedly constricted at junction with the atrium, the atrium is deeply forked at the junction with major duct without vacuolated area, and the major duct, atrium and calyx are of approximately the same width (Chant and McMurtry 2003a).
Neoseiulus barkeri has a worldwide distribution (Moraes et al. 2004a; Demite et al. 2019). Various studies have shown its ability to control Frankliniella occidentalis Pergande (Rodriguez-Reina et al. 1992), Thrips tabaci (Lindeman) (Broodsgaard and Hansen 1992) and T. urticae in cucumber (Fan and Petitt 1994b). Fan and Petitt (1994a) showed that augmentative releases of N. barkeri provided control of broad mite, Polyphagotarsonemus latus (Banks), on peppers. Neoseiulus barkeri constitutes a potential BCA for several crops especially in vegetables greenhouses.
This species has been mentioned by Quilici et al., (2000) in La Réunion, with locations listed but without morphological measurements presented. Measurements of specimens collected during this study are provided in the table 2.
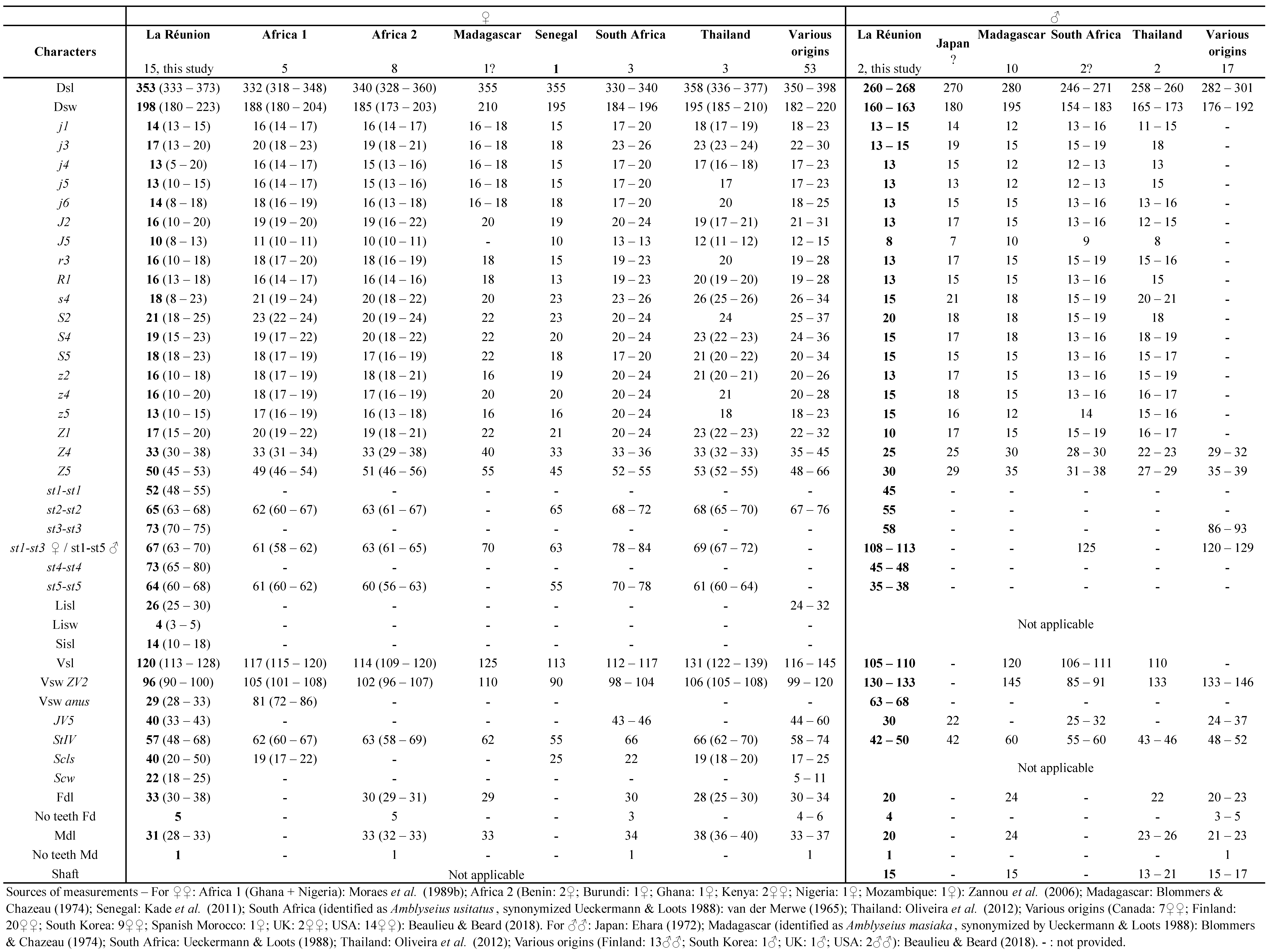


Specimens examined: 51 ♀♀ + 2 ♂♂ in total, 15 ♀♀ + 2 ♂♂ measured. St-Pierre - Ligne Paradis, La Coccinelle Inc. (aasl 164 m, Long 55°28'59'' E, Lat 21°18'55'' S), 5 ♀♀ in rearings of Proprioseiopsis mexicanus (Garman), 1/1/2017; Vincendo – Delaunay Jean-Max farm (aasl 110 m, Long 55°67'14'' E, Lat 21°38'' S), 45 ♀♀ + 2 ♂♂ on Capsicum annuum L., 11 and 18/1/2016 and 15/12/2016; Montvert-les-Hauts – EARL Le Mont Vert farm (aasl 582 m, Long 55°32'19'' E, Lat 21°19'42'' S), 1 ♀ on C. annuum, 19/9/2016.
Remarks: measurements of characters of females from La Réunion Island are only slightly different from female specimens from other countries, with less than 10 % differences (Table 2). All setae of specimens from South Africa (Ueckermann and Meyer 1988) are just slightly longer from those from other countries. Measurements of characters of males from La Réunion Island are also slightly different from male specimens from other countries, with < 10% differences (Table 2). In general, setae of specimens from La Réunion appears a bit smaller than those from other African countries but dorsal shied is larger.
Comparisons with N. barkeri measurements of female and male (Table 2) specimens of various origins in Beaulieu and Beard (2018) shows shorter dimensions of all characters of La Réunion specimens (all ranges of La Réunion specimens are in the lower parts of the ranges mentioned by these authors). These authors already mention in their paper the shorter dimensions of dorsal setae of African female and male specimens (lower part of observed ranges) compared to their own measurements (Beaulieu and Beard 2018).
Neoseiulus bayviewensis (Schicha)
Amblyseius bayviewensis Schicha 1977b: 394; Schicha 1987: 107.
Neoseiulus bayviewensis, Moraes et al. 1986: 72; Quilici et al. 1997: 285, 2000: 101; Beard 2001: 97; Kreiter et al. 2002: 348; Chant & McMurtry 2003: 21; Moraes et al. 2004: 107; Chant & McMurtry 2007: 25.
This species belongs to the cucumeris species group of Neoseiulus as dorsocentral setae are not as short relative to dorsolateral setae, and the ratio s4/j6 is 1.3 to 3.5. The spermatheca is without a stalk between calyx and atrium, the atrium is differentiated, joined directly to calyx. The species belong to the cucumeris species subgroup (Chant and McMurtry 2003a).
This species was only known from Australia for a long time (Demite et al. 2019). Quilici et al. (1997, 2000) collected this species on Hibiscus sp., associated with populations of the eriophyid Aceria hibisci (Nalepa), which is common in La Réunion. However, the biology of this predator remains unknown.
Collection data were provided in previous papers (Quilici et al. 1997, 2000) but without measurements of specimens. Measurements of specimens collected during this study are provided in table 3.



Specimens examined: 2 ♀♀ in total, both measured. Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 2 ♀♀ in the plot H (Herbicide), 4/4/2017 (see Material and methods).
Remarks: measurements of characters of La Réunion Island female specimens largely overlap with those of female specimens from Australia (Table 3). Measurements of La Réunion specimens are in general just a few percent's shorter, except for some setae or character dimensions which are quite shorter in La Réunion specimens, for example macrosetae of leg IV and size of ventral shields.
Neoseiulus californicus (McGregor)
Typhlodromus californicus McGregor 1954: 89.
Amblyseius californicus, Schuster & Pritchard 1963: 271.
Cydnodromus californicus, Athias-Henriot 1977: 62.
Amblyseius (Amblyseius) californicus, Ueckermann & Loots 1988: 150; Ehara et al. 1994: 126.
Amblyseius (Neoseiulus) californicus, Ehara & Amano 1998: 33.
Neoseiulus californicus, Moraes et al. 1986: 73; Chant & McMurtry 2003a: 21; Moraes et al. 2004a: 109; Chant & McMurtry 2007: 25; Guanilo et al. 2008a: 27, 2008b: 19.
Neoseiulus chilenensis Dosse 1958: 55 (synonymy according to McMurtry & Badii 1989).
Neoseiulus mungeri McGregor 1954: 92 (synonymy according to Schuster & Pritchard 1963).
Neoseiulus wearnei Schicha 1987: 103 (Synonymy according to Tixier et al. 2014).
Like the previous species, N. californicus belongs also to the cucumeris species group of Neoseiulus (Chant and McMurtry 2003a).
This widespread species (Moraes et al. 2004; Demite et al. 2019) is considered by McMurtry and Croft (1997) to be a specialized predator, Type 2. Nevertheless, it has characteristics of both specialist and generalist predatory mites (Castagnoli and Simoni 2003). It prefers to feed on spider mites (Gomez et al. 2009), but can also consume other mite species like tarsonemid mites [Phytonemus pallidus (Banks)] (Easterbrook et al. 2001), small insects such as thrips (Rodriguez-Reina et al. 1992) and even pollen when the primary prey is unavailable (Rhodes and Liburd 2006). It can migrate from grasses to fruit trees or grapevines and vice versa (Auger et al. 1999). It is a specialist predator of T. urticae on annual plants and woody species, and of Panonychus ulmi (Koch) and various Tetranychus spp. (and perhaps eriophyid mites) on trees and less frequently on grapevines (Auger et al. 1999). N. californicus is well-known as a BCA sold in many countries around the world for the management of spider mites in greenhouses but also in outdoor crops such as fruit crops in Europe. This is the first mention of that species for La Réunion Island.
Specimens examined: 18 ♀♀ + 2 ♂♂ in total, 14 ♀♀ + 2 ♂♂ measured. Ravine des Cabris – Ligne des Bambous, Lassay (aasl 221 m, Long 55°29'38'' E, Lat 21°17'17'' S), 1 ♀ on Amaranthus viridis L., 5/12/2016; Le Tampon – Grand Tampon (aasl 1100 m, Long 55°34'12" E, Lat 21°16'48" S), 6 ♀♀ + 1 ♂ on Cyperus rotundus L., 1 ♀ + 1 ♂ on Plantago lanceolata L. and 1 ♀ on Solanum mauritianum Scop., 18/1/2017; Montvert-les-Hauts – EARL Le Mont Vert farm (aasl 582 m, Long 55°32'19'' E, Lat 21°19'42'' S), 1 ♀ on Fragaria sp., 4/8/2015, 1 ♀ on C. annuum, 23/8/2016; Le 19e – Plaine des Caffres, JL Robert farm (aasl 1000 m, Long 55°32'9'' E, Lat 21°14'16'' S), 1 ♀ on Physalis peruviana L., and 2 ♀♀ on Emilia sonchifolia (L.) DC., 15/12/2015; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 4 ♀♀ in plots BM and CC, 4/4/2017.
Remarks: measurements of characters of the 14 female specimens (Table 4) fit well with those obtained for populations of other countries, such as those obtained from Tixier et al. (2008).



Values of measurements of La Réunion specimens are generally only slightly smaller (few percents variations). The same remark can be addressed for the adult male specimens (Table 4).
Comparisons with N. californicus measurements of a large number of female (Table 4) specimens of various origins in Beaulieu and Beard (2018) shows shorter dimensions of all characters of La Réunion specimens (all ranges of La Réunion specimens are in the lower parts of the ranges mentioned by these authors).
This is interesting to notice that it is not the case for males (Table 4), measurements of male specimens from La Réunion covering ranges or being in the middle of ranges mentioned by Beaulieu and Beard (2018) in their redescription.
Neoseiulus houstoni Schicha
Neoseiulus houstoni, Schicha 1987: 111; Chant & McMurtry 2003a: 23; Moraes et al. 2004a: 123.
Neoseiulus recifensis Gondim Jr. & Moraes 2001: 77; Chant & McMurtry 2003a: 23; Moraes et al. 2004a: 140, new synonymy.
Neoseiulus barreti Kreiter, in Furtado et al. 2005: 135, new synonymy.
These three species belong also to the cucumeris species group of Neoseiulus like previous species. However, whereas Chant and McMurtry (2003a) classified N. recifensis in the cucumeris species subgroup, N. houstoni was placed in the paraki species subgroup (also in the cucumeris species group), despite the two species having identical spermathecae (Chant and McMurtry 2003a). Neoseiulus barreti was described later than 2003, is not mentioned in Chant and McMurtry (2007) but the spermatheca is also identical to that of the two former species.
Neoseiulus houstoni was the first species collected and described in 1987 on Vigna unguiculata (L.) Walp. in Queensland, Australia (Schicha 1987).
Neoseiulus recifensis was discovered for the first time in Brazil in 2001 (Gondim Jr. and Moraes 2001), collected on Cocos nucifera L. in Recife and Itamaraca, Pernambuco, Brazil. It was collected then in 2007 and 2008 in Brazil in the states of Alagoas, Bahia, Cera, Paraiba and Rio Grande do Norte (Fiaboe et al. 2007; Lawson-Balagbo et al. 2008). Finally, it was collected in La Réunion Island in 2011 within a survey investigating potential predators of R. indica on coconut (Moares et al. 2012).
Two females of N. barreti were collected, only one time, in Brazil in 2004 on Solanum paniculatum L. in Itapaje, Ceara, Brazil and described later (Furtado et al. 2005).
Biology of the three species remain unknown. Males of the three species are unknown.
Specimens examined: 37 ♀♀ + 8 ♂♂ + 4 im. in total, 24 ♀♀ + 8 ♂♂ measured. Langevin – Jacqueline Waterfall (aasl 5 m, Long 55°64'40'' E, Lat 21°38'69'' S), 4 ♀♀ + 2 ♂♂ on Casuarina equisetifolia L., and 3 ♀♀ + 1 im. on Scaevola taccada Vahl, 19/7/2017; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 1 ♀ in plot H, 12/4/2016; 1 ♀ on B. pilosa, 1 ♀ on Euphorbia hypericifolia Lam., and 1 ♀ on P. maximum, 20/2/2017; 1 ♀ on Artocarpus heterophyllus Lam., 5 ♀♀ on C. equisetifolia, 1 ♀ on Acacia mearnsii De Wild., and 2 ♂ on Crotalaria retusa L., 27/2/2017; 4 ♀♀ + 2 im. on B. pilosa, 2 ♀♀ on L. leucocephala, 1 ♀ on Malvastrum coromandelianum (L.), and 8 ♀♀ + 4 ♂♂ + 1 im. on Parthenium hysterophorus L., 30/3/2017; 1 ♀ in plot CC, 3/04/2017 and 1 ♀ in plot CC, 6/04/2017; 1 ♀ on B. pilosa, 19/6/2017; 1 ♀ on A. viridis, 20/6/2017.
We have also examined the following type or additional material:
• The holotype of N. houstoni;
• The holotype, paratypes female and additional female material of N. recifensis;
• One paratype female of N. barreti.
Remarks: character measurements of the 24 females collected in La Réunion (Table 5) agree very well with those obtained from females of N. barreti or N. recifensis from Brazil and females of N. houstoni from Australia described previously (Gondim Jr. and Moraes 2001, Schicha 1987, Furtado et al. 2005). We consider so far that our specimens can be anyone of the three species and that examination of the specimens collected in this study can lead to anyone of the three species. Consequently, the morphometrics strongly suggest synonymy.
There are however some discrepancies between our measurements and observations and previous descriptions of the three species. In the three descriptions:
• Dorsal shield is reticulated in the description of N. barreti in the anterior lateral margins and starting to the back of s4 and on all the posterior part of the dorsal shield except the center;
• VA shield has straight slightly convex margins in Schicha 1987 and not in the two other species as a concave part exists in this margin just after ZV2 position;
• Z4 and Z5 are progressively tapered in drawings of N. barreti and Z5 seems longer (Furtado et al. 2005) vs for other species they are blunt/rounded apically (or more regularly parallel-sided);
• Macrosetae SgeIV, StiIV and StIV are mentioned as setaceous for N. recifensis (Moraes and Gondim Jr 2001) but seem slightly knobbed on illustrations of the species. SgeIV and StIV are slightly knobbed for N. barreti and N. houstoni and StiIV is not mentioned for N. houstoni;
• two teeth on the fixed digit and no tooth on the movable digit are mentioned for N. barreti and N. recifensis but three teeth for fixed digit and one recurved tooth for the movable digit are mentioned for N. houstoni;
• Spermathecae of N. barreti and N. recifensis are mentioned as trumpet-shaped but as bell-shaped for N. houstoni;
• Setae JV5 seem longer in N. barreti description and shorter in those of N. houstoni and N. recifensis;
• 6 poroids around genital/ventrianal shield are drawn for N. recifensis but only 4 for N. barreti and 0 for N. houstoni;
• Occurrence of JV3 is mentioned in the text of description of N. barreti but not illustrated (Furtado et al. 2005). These setae are not indicated for description of N. houstoni and N. recifensis.
• Chaetotactic formulae are not precised in the description of N. houstoni but mentioned only for genu II for N. barreti and for genua II and III for N. recifensis.
Our examination of the type material for N. barreti (one paratype ♀), N. houstoni (the single specimen found, the ♀ holotype) and N. recifensis (the ♀ holotype, one ♀ paratype and seven additional ♀) shows:
• dorsal shields of the three species present exactly the same reticulation as drawn in Furtado et al. (2005) for the description of N. barreti;
• VA shield present only a slight concavity after ZV2 position in N. houstoni;
• Z4 and Z5 are progressively tapered regularly parallel-sided in the three species and Z5 is of the same length (see table 8);
• Macrosetae SgeIV, StiIV and StIV are: rounded apically for SgeIV, pointed apically for StiIV and slightly knobbed for StIV for the three species;
• Three teeth on fixed digit (two strong anterior and one small tooth posterior to pilus dentilis) and one small recurved tooth in the anterior part of the movable digit for the three species, just as drawn by Schicha (1987);
• Spermathecae of N. barreti and N. recifensis are drawn exactly in the same way in original descriptions (Schicha 1987; Moraes and Gondim Jr. 2001; Furtado et al. 2005) and are identical in the three species after our examination (and ''bell-shaped'' seems more appropriate for the shape description);
• Setae JV5 are of similar length for the three species (see table 5);
• 6 poroids are present around genital/ventrianal shield for the three species;
• The mention of JV3 was an error in the text of the description of N. barreti. This seta is not present in the venter of that species and also not present in the two others;
• Chaetotactic formulae are identical for the three species: Genu II: 2-2/0 - 2/0-1 (seven setae);
Genu III: 1-2/1 - 2/0-1 (seven setae).
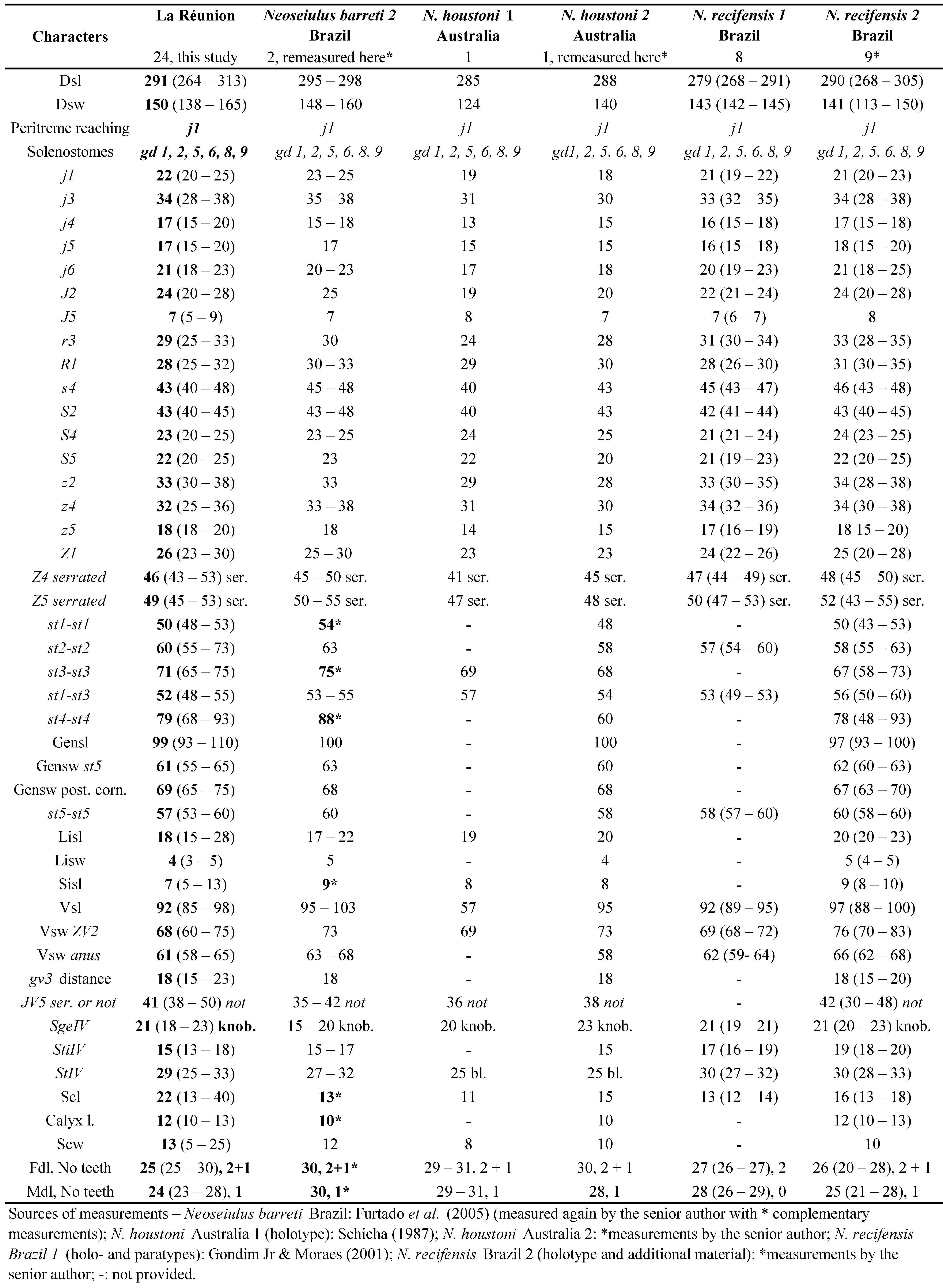


Considering all these information, we can conclude that the three species are synonyms. Consequently, our specimens are identified as the first species described among the three species, N. houstoni. The valid species name is thus Neoseiulus houstoni (Schicha). Previous specimens collected in La Réunion Island and identified as N. recifensis are renamed N. houstoni. Consequently, this is the first report of that species in La Réunion Island.
The male (for the three species) being unknown, it is herein described for the first time, based on La Réunion specimens.
Description of the adult male of Neoseiulus houstoni (Schicha)
n = 8 (Figs 1a-c)
Diagnosis — The following combination of characters indicated below in the description of the male of this species is quite similar to a lot of species of Neoseiulus belonging to the cucumeris and paraki species subgroups of the cucumeris species group. Not many characters allow to distinguish it from all males of other species if no females are collected in the same time: the peritreme reaching the level between j1 and j3, a limited reticulation compared to several species of these two subgroups, some dorsal setae length, especially s4, S2, Z4 and JV5 approximately of the same length (30 – 35) and longest setae after Z5, a spermatodactyl with a terminal part recurved as an open U (hook-like), no additional macrosetae on other legs than leg IV compared to several species of these two subgroups that have macrosetae on leg III, sometimes II, only three pairs of preanal setae instead of 4 pairs in males of several species of these subgroups, one pair of crateriform gv3 very close to setae JV2.
Dorsum — (Fig. 1a). Dorsal shield fused with the peritremal shield at the level of j1 position, with slight reticulations all around the edge and in the posterior part of the dorsal shield, 200 (183 – 213) long and 129 (115 – 140) wide, with six pairs of solenostomes (gd 1, 2, 5, 6, 8, 9). The dorsal shield bears 17 pairs of dorsal setae and 2 pairs of sub-lateral setae on the dorsal shield: j1 not visible, j3 28, j4 13, j5 15, j6 18, J2 15, J5 5, z2 25, z4 28, z5 18, Z1 15, Z4 35, Z5 40, s4 38, S2 33, S4 18, S5 15, r3 20, R1 20. All setae smooth except Z4 and Z5 serrated.
Peritreme — (Fig. 1a). Extending between j1 and j3. Peritremal shield fused with dorsal shield.
Venter — (Fig. 1b). Sternal shield smooth. Distances between st1 – st1 43, st2 – st2 50, st3 – st3 53, st1 – st5 98, st4 – st4 43, st5 – st5 33. Ventrianal shield with three pairs of pre-anal setae, JV1, JV2, and ZV2, and one pair crateriform gv3, mesad of the VAS, just a bit after the insertion line of setae JV2 but very close from these setae. Three pairs of poroids discernible on specimen ventrianal shields examined. Soft cuticle surrounding ventrianal shield with one pair of setae (JV5); ventrianal shield 83 long, 120 wide at anterior corners and 60 wide at level of paranal setae. JV5 smooth, 35 long. A pair of lyrifissures near JV5.
Chelicera — Fixed digit 20 long, no tooth discerned and movable digit 18 long with no tooth discerned because chelicerae are dorsoventrally oriented. Spermatodactyl with an open U-shaped foot, shaft (Fig. 1c) 13 long.
Legs — Legs IV with three macrosetae like in the female: SgeIV slightly knobbed 18, StiIV pointed 15, StIV very slightly knobbed 23. Chaetotactic formula of genu II and III similar to that of females.



Specimens examined — 8 ♂♂ collected, 8 ♂♂ measured. Langevin – Jacqueline Waterfall (aasl 5 m, Long 55°64'40'' E, Lat 21°38'69'' S), 2 ♂♂ on C. equisetifolia, 19/7/2017; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 2 ♂ on C. retusa, 27/2/2017; 4 ♂♂ + 1 im. on P. hysterophorus, 30/3/2017.
Type material — Eight paratype males in total. Three paratype males deposited in Montpellier SupAgro – INRA Acarology collection, Montpellier and five paratype males deposited in Bassin-Plat CIRAD Research station collection in La Réunion.
Remarks — This combination of characters of the male of this species are not unique and do not allow to distinguish it from all males of other species of Neoseiulus belonging to the cucumeris and paraki species sub-groups without collected females of the species. The male peritreme is shorter than the female peritreme. Other characters are very similar.
Neoseiulus longispinosus (Evans)
Typhlodromus longispinosus Evans 1952: 413; Evans 1953: 465; Womersley 1954: 177; Ehara 1958: 55.
Typhlodromus (Amblyseius) longispinosus, Chant 1959: 74.
Amblyseius longispinosus, Corpuz & Rimando 1966: 129; Schicha 1975: 103.
Neoseiulus longispinosus, Moraes et al. 1986: 85; 2000: 245; Chant & McMurtry 2003a: 37; Moraes et al. 2004a: 129; Chant and McMurtry 2007: 29.
This species belongs to the barkeri species group like N. barkeri (see above). It belongs to the womersleyi species subgroup as the calyx is markedly constricted at the junction with the atrium, the atrium is deeply forked at the junction with the major duct, and the major duct, atrium and calyx are not of the same width (Chant and McMurtry 2003a).
This species is distributed in many countries of the world, mainly in tropical areas (Moraes et al. 2000; Mailloux et al. 2010; Kreiter et al. 2013, 2018 a, c; Demite et al. 2019). It was found rarely in surveys made in Guadeloupe, Martinique and La Réunion except in studies on companion plants in citrus orchards (Mailloux et al. 2010; Kreiter et al. 2013, 2018c; Le Bellec et al., unpub. data). This species seems actually to be more common on weeds with populations of tetranychid mites. Neoseiulus longispinosus, a type II phytoseiid predatory mite, as is N. californicus (McMurtry et al. 2013), has received increasing attention in Asia for the control of different spider mites (of Eutetranychus, Oligonychus, and Tetranychus) since 2010 (Nusartlert et al. 2011). The feeding, development, predation, cannibalism, intra-guild predation and behaviour have thus been extensively studied by several authors (see for example Luong et al. 2017) for pest control purposes. Neoseiulus longispinosus is well-known as a BCA sell in several countries in the world for the management of spider mites. The recent results of Huyen et al.(2017) show that at least in controlled laboratory conditions, N. longispinosus is a potential biological control agent against the citrus red spider mite P. citri.
This is the first record of this species for La Réunion Island.
Specimens examined:37 ♀♀ + 1 ♂ + 4 im. in total, 18 ♀♀ + 1 ♂ measured. Saint-Paul – Savannah (aasl 61 m, Long 55°29'43'' E, Lat 21°20'41'' S), 8 ♀♀ in flowers of Phaseolus vulgaris L. 28/07/2015; Saint-Pierre – Eastern entrance of the city (aasl 61 m, Long 55°29'43'' E, Lat 21°20'41'' S), 1 ♀ on Ricinus communis L., 16/12/2015; Ravine des Cabris – Ligne des Bambous, Lassay (aasl 221 m, Long 55°29'38'' E, Lat 21°17'17'' S), 1 ♀ on Solanum torvum Swartz, 2/12/2016; 1 ♀ + 1 ♂ + 1 im. on Mirabilis jalapa L., 4/12/2016; Saint-Pierre – Bassin Martin, ARMEFLHOR Station (aasl 450 m, Long 55°31'9'' E, Lat 21°18'14'' S), 11 ♀♀ on Fragaria sp. + A. viridis, 18/1/2017; Saint-Gilles – Pépinières du Théâtre (aasl 70 m, Long 56°13'58'' E, Lat 21°2'50'' S), 1 ♀ on A. viridis, 14/2/2017; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 1 ♀ in plot H, 16/12/2016; 1 ♀ on B. pilosa, 2 ♀♀ + 1 im. on M. repens, 1 ♀ on Ipomoea obscura (L.), 1 ♀ on Teramnus labialis (L.f.), 12/2/2017; 1 ♀ + 2 im. on I. obscura, 1 ♀ on Digitaria radicosa (J. Presl), 30/3/2017; 6 ♀♀ in plots CC, M, HM and H, 3 and 6/04/2017.
Remarks: measurements of specimens of La Réunion females and males (Table 6) overlap with those obtained for populations of various countries. Measurements are slightly greater than those obtained on specimens of F.C.I. except for setae j4, J2, z5, StIV. On Comoros specimens, setae are longer except sternal shield length (st1-st3), inguinal sigilla (metapodal plates) and of macrosetae of basitarsus IV.



Neoseiulus lula (Pritchard & Baker)
Amblyseius (Amblyseius) lula Pritchard & Baker, 1962: 239.
Neoseiulus lula, Schicha 1981b: 212; Moraes et al. 1986: 87; Chant & McMurtry 2003a: 27; Moraes et al. 2004a: 130; Chant & McMurtry 2007: 29.
Amblyseius (Amblyseius) insignitus van der Merwe, 1968: 138 (synonymy according to Ueckermann & Loots, 1988).
Like N. baraki (see above), N. lula belongs to the paspalivorus species group (Chant and McMurtry 2003a).
The biology of this species remains unknown.
It is distributed in several countries and islands of sub-Saharan Africa but also in Cuba (Moraes et al. 2004a).
This is the first record of this species for La Réunion Island.
Specimens examined: a single ♀, measured. Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 1 ♀ on Chrysopogon zizanioides (L.), 2/3/2017.
Remarks: the measurements of the single female of Neoseiulus lula (Table 7) fit well measurements of specimens of the original description from Central Africa (Schicha 1981b) and those of specimens from Africa (Zannou et al. 2006), except for j6 and S2 which are smaller (\textgreater20 %) in the single specimen of La Réunion. Specimens from Africa (Zannou et al. 2006) have longer Z4 but a reduced ventrianal shield. Specimens from South Africa and Madagascar (van der Merwe 1968; Ueckermann and Loots 1988) have in general greater dimensions than those obtained for specimens from La Réunion Island.



Neoseiulus paspalivorus De Leon
Typhlodromus paspalivorus De Leon, 1957: 143.
Amblyseius paspalivorus, Schicha 1981b: 210.
Neoseiulus paspalivorus, Muma & Denmark 1970: 110; Moraes et al. 1986: 92; Chant & McMurtry 2003a: 27; Moraes et al. 2004b: 137.
Like N. baraki and N. lula, N. paspalivorus belongs to the paspalivorus species group (see above) (Chant and McMurtry 2003a).
N. paspalivorus was found only on coconut and on fruits, in association with A. guerreronis (Moraes et al. 2004b). This species is a promising candidate for the biological control of the coconut eriophyid (Lawson-Balagbo et al. 2008).
This is the first record of this species for La Réunion Island.
Specimens examined: 15 ♀♀ + 3 ♂♂ in total, 8 ♀♀ measured + 3 ♂♂ measured. Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 1 ♀ on D. ramularis, 6 ♀♀ on M. repens + 2 ♂♂, 3 ♀♀ + 1 ♂ on Cynodon dactylon (L.), 1 ♀ on Acanthospermum hispidum DC., 1 ♀ on L. leucocephala, 20/2/2017; 3 ♀♀ in plots CC and HM, 3/04/2017.
Remarks: measurements of characters of the eight female and 3 male specimens (Table 8) fit well those obtained for populations of other countries, with only slight variations.



Neoseiulus scapilatus (van der Merwe)
Amblyseius (Amblyseius) scapilatus van der Merwe, 1965: 71.
Amblyseius scapilatus, Meyer & Rodriguez, 1966: 28.
Neoseiulus scapilatus, Moraes et al. 1986: 95; McMurtry & Moraes 1991: 26; Chant & McMurtry 2003a: 37; Moraes et al. 2004a: 142; Chant & McMurtry 2007: 31; El-Banhawy & Knapp 2011: 12.
Like N. barkeri and N. longispinosus, N. scapilatus belongs to the barkeri species group (see above) (Chant and McMurtry 2003a).
Quilici et al. (2000) have collected before this species in La Réunion that is distributed in several countries of sub-Saharan Africa. Exact indications of locations were provided but without any measurements of specimens collected. Measurements of specimens collected during this study are provided in table 9. The biology of this species remains unknown.



Specimens examined: 11 ♀♀ + 1 ♂ in total, all measured. Le Tampon – Grand Tampon, Janick Bénard farm (aasl 861 m, Long 55°32'90'' E, Lat 21°12'80'' S), 1 ♀ on Lantana camara L., 9/12/2015; 1 ♀ on Ageratum conyzoides (L.) and 1 ♀ + 1 im. on Raphanus raphanistrum L.; 24/5/2016; 2 ♀♀ on Bromus catharticus Vahl, 1 ♀ on Conyza sumatrensis (S.F. Blake), 9/1/2017; Petite Île – Piton Bloc, Yébo Luguy farm (aasl 973 m, Long 55°34'64'' E, Lat 21°18'64'' S), 3 ♀♀ on B. catharticus, 18/10/2016; 1 ♀ + 1 ♂ on Pteridium aquilinum (L.), 9/1/2017; Le Tampon – Ligne des 400 (aasl 463 m, Long 55°30'36" E, Lat 21°17'24" S), 1 ♀ on Ipomoea sp., 10/1/2017.
Remarks: measurements of morphological characters of N. scapilatus female specimens from La Réunion (Table 9) are very close from measurements for specimens from neighbouring countries, except for specimens from South Africa that are larger (van der Merwe 1965). La Réunion specimens have slightly shorter macrosetae.
For the male (Table 9), some setae (j3, r3, S4, z5, JV5) or dimensions (st3-st3, st1-st5) are shorter.
Neoseiulus teke Pritchard & Baker
Amblyseius (Amblyseius) teke Pritchard & Baker, 1962: 239.
Amblyseius teke, Meyer & Rodrigues 1966: 30; Moraes et al. 1989a: 83; Moraes et al. 1989b: 97.
Neoseiulus teke, Moraes et al. 1986: 98; Chant & McMurtry 2003a: 37; Moraes et al. 2004a: 147; Chant & McMurtry 2007: 31.
Amblyseius (Amblyseius) bibens Blommers 1973: 111 (synonymy according to Ueckermann & Loots 1988).
Like N. barkeri, N. longispinosus and N. scapilatus, N. teke belongs to the barkeri species group and like N. longispinosus and N. scapilatus, it belongs to the womersleyi species subgroup (see above) (Chant and McMurtry 2003a).
This species is found in sub-Saharan Africa often associated with Mononychellus tanajoa (Bondar), the cassava green mite (CGM). It has been studied for its potential as BCA against the CGM. Nwilene and Nachman (1996) studied its reproduction characteristics on M. tanajoa. It was more efficient than Iphiseius degenerans (Berlese), but seems not efficient enough in field conditions (Nwilene and Nachman 1996). Quilici et al.(2000) have collected this species before in La Réunion. Exact indications of locations were provided in the paper but without any measurements of specimens collected. Measurements of specimens collected during this study are provided in table 10.
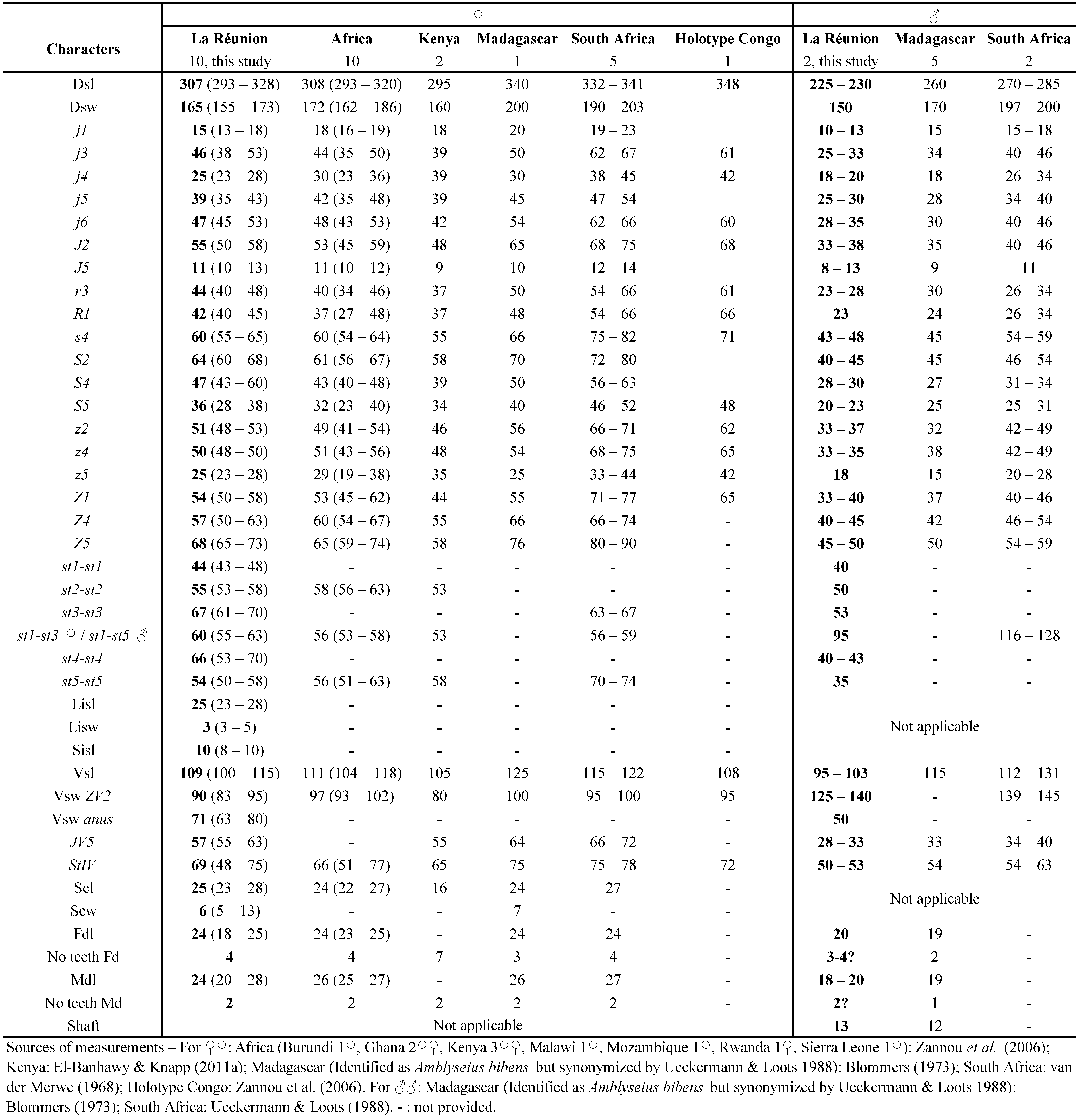


Specimens examined: 12 ♀♀ in total + 6 ♂♂ + 2 im., 10 ♀♀ + 2 ♂♂ measured. Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 1 ♀ + 1 im. on M. repens, 20/2/2017; 1 ♀ on A. hispidum, 1 ♀ + 1 im. on M. repens, 1 ♀ + 1 ♂ on P. maximum, 30/3/2017; 4 ♀♀ + 1 ♂ on M. repens, 3/4/2017; 2 ♀♀ + in plots HM, 1 ♀ + 1 ♂ in plot CC, 1 ♀ in plot H, 4/4/2017 and 6/4/2017.
Remarks: measurements of morphological characters of N. teke female and male specimens from La Réunion (Table 10) are very close from measurements for specimens from neighbouring countries, especially from specimens from various countries in Africa, except for the holotype (Zannou et al. 2006) and specimens from South Africa which are larger (van der Merwe 1965).
Tribe Kampimodromini Kolodochka
Kampimodromini Kolodochka 1998: 59; Chant & McMurtry, 2003b: 189; 2006b: 137; 2007: 33.
Subtribe Paraphytoseiina Chant & McMurtry
Paraphytoseiina Chant & McMurtry 2003b: 211.
Genus Paraphytoseius Swirskii & Shechter
Paraphytoseius Swirski & Shechter 1961: 113; Moraes et al. 1986:104; Chant & McMurtry 2003b: 216; Moraes et al. 2004a: 160; Chant & McMurtry 2007: 49.
Amblyseius (Paraphytoseius), Ueckermann & Loots 1987: 221.
Amblyseius (Ptenoseius), Pritchard & Baker1962: 295.
Proprioseius (Paraphytoseius), Karg 1983: 302.
Ptenoseius, Schuster & Pritchard 1963: 198.
Paraphytoseius horrifer (Pritchard & Baker)
Amblyseius (Ptenoseius) horrifer Pritchard & Baker, 1962: 295.
Amblyseius horrifer, Meyer & Rodrigues 1966: 30.
Amblyseius (Paraphytoseius) horrifer, van der Merwe 1968: 169.
Proprioseius (Paraphytoseius) horrifer, Karg 1983: 302.
Paraphytoseius horrifer, Moraes et al. 1986: 105; Beard 2001: 84; Chant & McMurtry 2003a: 37; Moraes et al. 2004a: 152; Chant & McMurtry 2007: 53.
In our specimens of this species, setae S5 are absent. So accordingly with Chant and McMurtry (2003b) all specimens belong to the orientalis species group.
Accordingly with these previous authors, and with Moraes et al. (2007), we consider that P. horrifer and P. orientalis are different valid species. Our specimens with longer setae s4, Z4, Z5, and lacking a distinctly short, thick, spatulate macroseta on genu I.
This species is widely distributed in Sub-Saharan Africa and Madagascar. The biology of P. horrifer remains totally unknown.
This is the first mention of this species from La Réunion Island.
Specimens examined: 13 ♀♀ + 1 ♂ + 1 im. in total, 12 ♀♀ + 1 ♂ measured. Le Tampon – Ligne des 400 (aasl 463 m, Long 55°30'36" E, Lat 21°17'24" S), 1 ♂ + 2 im. on Ipomoea purpurea (L.), 11/2/2017; Ravine Langevin – Grand-Galet Waterfall (aasl 850 m, Long 55°21'33'' E, Lat 21°17'47'' S), 7 ♀♀ on Desmodium incanum DC., 11/12/2016; Forêt de Bélouve – Gîte (aasl 1500 m, Long 55°33'36" E, Lat 21°6'0" S), 1 ♀ on Fuchsia boliviana Carrière, 20/12/16; Cilaos – Village (aasl 991 m, Long 55°27'0" E, Lat 21°8'24" S), 1 ♀ on Acalypha hispida Burm. f., 8/1/17; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 1 ♀ in plot BM, 23/8/2016; 1 ♀ on M. coromandelianum, 19/6/2017; 1 ♀ on A. viridis, 20/6/2017.
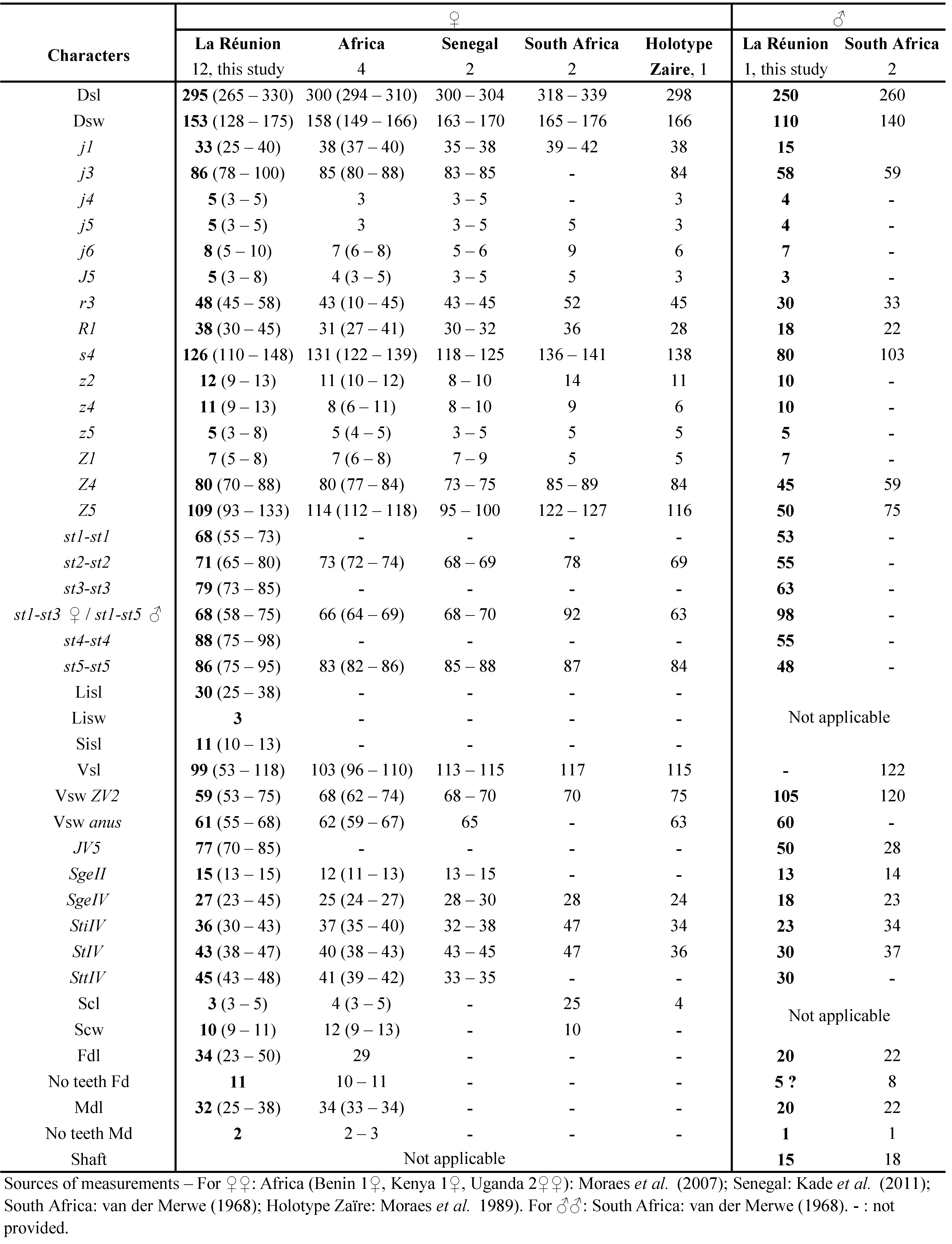
Remarks: even if lengths of setae seem very variable in P. horrifer in the literature (van der Merwe 1968; Moraes et al. 2007), measurements of morphological characters of La Réunion specimens (Table 11) agree well with measurements found in the literature, being however slightly shorter for almost all characters compared with female and male specimens of South Africa (van der Merwe 1968).


Paraphytoseius orientalis (Narayanan, Kaur & Ghai)
Typhlodromus (Amblyseius) orientalis Narayanan, Kaur & Ghai 1960: 394.
Paraphytoseius orientalis, Moraes et al. 1986: 105; Chant & McMurtry 2003b: 220; Moraes et al. 2004a: 162, Chant & McMurtry 2007: 53.
Amblyseius ipomeai, Narayanan, Kaur & Ghai 1960: 394 (synonymy according to El-Banhawy 1984); Paraphytoseius narayanani, Ehara 1967: 67 (synonymy according to Ehara & Ghai, in Ehara 1967: 77); Paraphytoseius multidentatus, Swirski & Shechter 1961: 114 (synonymy according to Matthysse & Denmark 1981 in Denmark et al. 1999: 11).
As mentioned earlier, in our specimens of Paraphytoseius spp., setae S5 are absent, all our specimens belong to the orientalis species group (Chant and McMurtry 2003b) and we consider that P. horrifer and P. orientalis are distinct, valid species.
Our specimens with shorter s4, Z4 and Z5 setae, having a distinctly short, thick, spatulate macroseta on genu I belong to P. orientalis.
This species is widely distributed in tropical and subtropical areas in South America, Africa and Asia. This species belongs to a genus included in the large polyphagous generalist group named type III phytoseiid mites (McMurtry and Croft 1997; McMurtry et al. 2013). Navasero and Navasero (2016) have studied the life history of P. orientalis on the broad mite (P. latus) as prey. The authors reported high predation rates on the eggs of P. latus, suggesting good potential for the control of this pest. Quilici et al. (2000) have collected before this species in La Réunion but provided no measurements. We herein provide measurements of specimens collected in La Réunion (Table 12).


Specimens examined: 20 ♀♀ + 1 ♂ + 1 im. in total, 5 ♀♀ + 1 ♂ measured. Saint-Pierre – Bassin Martin, Armefhor Station (aasl 450 m, Long 55°31'9'' E, Lat 21°18'14'' S), 8 ♀♀ + 1 ♂ on Borago officinalis L., 17/12/2015; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 4 ♀♀ + 1 im. in plot BM, 2 ♀♀ in plot F, and 2 ♀♀ in plot H, 23/8/2016; 1 ♀ on M. coromandelianum, 19/6/2017; 1 ♀ on A. viridis, 20/6/2017; Ravine Langevin – Grand-Galet Waterfall (aasl 850 m, Long 55°21'33'' E, Lat 21°17'47'' S), 2 ♀♀ on D. incanum, 11/12/2016.
Remarks: measurements of morphological characters of La Réunion female and male specimens (Table 12) agree well with measurements of specimens from other countries found in the literature.
Tribe Phytoseiulini Chant & McMurtry
Phytoseiulini Chant & McMurtry 2006: 7.
Genus Phytoseiulus Evans
Phytoseiulus Evans 1952: 397.
Phytoseiulus persimilis Athias-Henriot
Phytoseiulus persimilis Athias-Henriot 1957: 347; Moraes et al. 1986: 109; Moraes et al. 2004a: 169; Chant & McMurtry 2006a: 20; 2007: 55.
Phytoseiulus (Phytoseiulus) persimilis, Wainstein 1962: 17.
Typhlodromus persimilis, Hirschmann 1962: 75.
Phytoseiulus riegeli Dosse 1958: 48 (synonymy according to Chant 1959); Phytoseiulus tardi Lombardini 1959: 166 (synonymy according to Kennett & Caltagirone 1968).
Phytoseiulus persimilis is a Mediterranean/subtropical predatory mite, a type I species, i.e. a specialist predator of the urticae species group of the genus Tetranychus (McMurtry and Croft 1997; McMurtry et al. 2013). Considerable research has been conducted on this predator-prey interaction (see review by Kostiainen and Hoy 1996), and numerous biological control programs have used P. persimilis against T. urticae on a wide range of ornamental and vegetable crops. Phytoseiulus persimilis was the first greenhouse biological control agents available commercially and it is one of the most successful BCA in the world. It can also be used in temperate climates on open-field crops such as strawberries. Optimum conditions are 20-27 °C and relative humidity of 60-90 %. Cooler or warmer temperatures may have a negative effect on reproduction, development and efficiency of this predatory mite. This species is present in Mauritius (Kreiter et al. 2018a) and La Réunion probably because of its commercial introduction and uses in vegetable and ornamental greenhouses, dispersion of some specimens released and establishment in the environment. This species is actually reared and sold in La Réunion and commercialised in Mascareignes since a long time (Quilici, personal communication). Phytoseiulus persimilis was already known from La Réunion (Quilici et al. 1997, 2000). Exact indications of locations were provided in these papers but without any measurements of specimens collected. Measurements of specimens collected during this study are provided in Table 13.



Specimens examined: 5 ♀♀ in total, all measured. Montvert-les-Hauts – EARL Le Mont Vert farm (aasl 582 m, Long 55°32'19'' E, Lat 21°19'42'' S), 1 ♀ on Fragaria sp., 3 ♀♀ on E. sonchifolia, 4/8/2015; Le 19e – Plaine des Caffres, JL Robert farm (aasl 1000 m, Long 55°32'9'' E, Lat 21°14'16'' S), 1 ♀ on Phytolacca americana L., 15/12/2015.
Remarks: measurements of morphological characters (Table 13) of the 5 females fit well with measurements found in the literature, especially with those of specimens collected recently in Mauritius (Kreiter et al. 2018a), and particularly for setae j4, J5, z2, z5 and r3. Some setae are slightly shorter in La Réunion specimens compared to specimens from other countries, mainly for the long setae of this species (j6, s4, Z1, Z4, and Z5). Some other shorter setae of this species, for example R1 and macrosetae of the leg IV are also shorter in La Réunion specimens. Nevertheless, these findings are questionable given that only five females of P. persimilis have been measured compared to measurements of 14 females collected in Mauritius and to large numbers of specimens from other countries.
Tribe Typhlodromipsini Chant & McMurtry
Typhlodromipsini Chant & McMurtry 2005c: 318.
Genus Typhlodromips De Leon
Typhlodromipsini Chant & McMurtry 2005c: 318; 2006b: 137; 2007: 55.
Typhlodromips culmulus (van der Merwe)
Amblyseius (Amblyseius) culmulus van der Merwe 1968: 132; Ueckermann & Loots 1988: 157.
Typhlodromips culmulus, Moraes et al. 1986: 139; 2004a: 210; Chant & McMurtry 2005c: 327; Chant & McMurtry 2007: 61.
This species belongs to the culmulus species group as the spermatheca has a calyx shallow dish-shaped. This species group contains only 10 species (Chant and McMurtry 2005c).
Typhlodromips culmulus is mentioned from western and southern Africa (Demite et al. 2019). It was found recently in Mauritius (Kreiter et al. 2018a). Species of this quite large genus are supposed to all belong to the type III (McMurtry and Croft 1997; McMurtry et al. 2013), i.e. a polyphagous generalist predator. However, the biology of T. culmulus remains totally unknown. This is the first mention of this species from La Réunion Island.
Specimens examined: 105 ♀♀ + 18 ♂♂ + 6 im., 10 ♀♀ + 3 ♂♂ measured. Petite Île – Piton Bloc, Yébo Luguy farm (aasl 973 m, Long 55°34'64'' E, Lat 21°18'64'' S), 1 ♀ on I. obscura, 28/4/2016; 6 ♀♀ + 1 im. on P. lanceolata, 2 ♀♀ on R. raphanistrum, 1 ♀ on I. obscura, 18/10/2016; Le Tampon – Grand Tampon, Janick Bénard farm (aasl 861 m, Long 55°32'90'' E, Lat 21°12'80'' S), 1 ♀ on Begonia cucullata, 7/1/2016; 4 ♀♀ + 2 ♂♂ + 1 im. on R. raphanistrum, 1 ♀ on B. pilosa, 24/5/2016; 8 ♀♀ + 1 im. on R. raphanistrum, 2 ♀♀ on B. pilosa, 20/9/2016; Saint-Pierre – Bassin Martin, Armefhor Station (aasl 450 m, Long 55°31'9'' E, Lat 21°18'14'' S), 18 ♀♀ + 2 ♂♂ + 1 im. on various weeds and 62 ♀♀ + 13 ♂♂ + 2 im. on C. rotundus, 3/8/2017; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 1 ♀ in plot BM, 23/8/2016; 1 ♀ on A. viridis, 20/6/2017.
Remarks: measurements of morphological characters of T. culmulus female and male specimens from La Réunion (Table 14) are very close from measurements for specimens from neighbouring countries, especially for specimens from Kenya and Mauritius and except for specimens from South Africa which are larger in van der Merwe (1968) but very close for the measurements of type material provided by Moraes et al. (2007).



Genus Scapulaseius Karg & Oomen-Kalsbeek
Scapulaseius Karg & Oomen-Kalsbeek 1987: 132.
Amblyseius (Scapulaseius) Karg & Oomen-Kalsbeek 1987: 132.
newsami group of Typhlodromus (Amblyseius), Chant 1959: 95.
markwelli species group of Amblyseius, Schicha 1987: 25.
japonicus species group of Amblyseius, Schicha 1987: 26.
oguroi species group of Amblyseius, Wu & Ou 1999: 103.
Scapulaseius, Chant & McMurtry 2005c: 331; 2007: 65.
Scapulaseius reptans (Blommers)
Amblyseius (Amblyseius) reptans Blommers 1974: 145.
Typhlodromips reptans, Moraes et al. 1986: 146; Moraes et al. 2004a: 222.
Scapulaseius reptans, Chant & McMurtry 2005c: 335; Chant & McMurtry 2007: 68.
This species belongs to the ficilocus species group of the genus Scapulaseius as the setae R1 are inserted on lateral integument of adult female (Chant and McMurtry 2005c). This species group contains 40 species with setae R1 inserted on the lateral integument of the adult female.
This species is mentioned only from the Indian Ocean area, Madagascar (Blommers 1974), La Réunion (Quilici et al. 2000) and recently Mauritius (Kreiter et al. 2018a). Species of this genus Scapulaseius are supposed to be of type III (McMurtry and Croft 1997; McMurtry et al. 2013), i.e. polyphagous generalist predators. However, the biology of S. reptans remains unknown. S. reptans was already mentioned from La Réunion by Quilici et al.(2000). Exact indications of locations were provided in this paper but without any measurements of specimens collected. They are provided for specimens collected during this study and listed in table 15.



Specimens examined: 2 ♀♀ + 1 ♂ in total, all measured. Ravine Langevin – Grand-Galet Waterfall (aasl 850 m, Long 55°21'33'' E, Lat 21°17'47'' S), 1 ♀ + 1 ♂ on D. incanum, 11/12/2016; Petite Île – Piton Bloc, Yébo Luguy farm (aasl 973 m, Long 55°34'64'' E, Lat 21°18'64'' S), 1 ♀ + 1 im. on L. camara, 9/1/2017.
Remarks: measurements of the two female and the male specimens in the table 15 show a great overlap with measurements mentioned in Kreiter et al. (2018a) on specimens from Mauritius, except for setae S2, S4 and S5 which are longer in La Réunion specimens. Setae of specimens from La Réunion are generally longer, except for j1 and the macroseta of genu I that are nearly the same as for specimens from Mauritius. However, sternogenital shield is longer in Mauritius specimens. Both specimens from La Réunion and Mauritius have globally greater dimensions than type specimens from Madagascar, especially setae.
Ferragut and Baumann (2019) discussed a possible synonymy between S. reptans and S. asiaticus (Evans) recently. Our specimens of La Réunion Island markedly differ from specimens of S. asiaticus collected recently in Vietnam (Kreiter et al. in prep.) and we therefore disagree with this possible synonymy with arguments that will be developed in a future paper.
Tribe Amblyseiini Muma
Amblyseiini, Muma, 1961: 68.
Subtribe Amblyseiina Muma
Amblyseiina Muma, 1961: 69.
Genus Transeius Chant & McMurtry
Transeius Chant & McMurtry, 2004a: 181.
Transeius maelliae Kreiter n. sp.
ZOOBANK: C6EA34F5-9601-4208-8201-B14CE6C6FC5D ![]()
Diagnosis — Transeius maelliae Kreiter n. sp. belongs to the subfamily Amblyseiinae (absence of dorsolateral setae z3 and s6 and caudoventral seta JV3), to the tribe Amblyseiini (setae j3, s4, Z4 and Z5 longer than other setae, ratio s4/Z1 \textgreater 3.1, many teeth on the fixed cheliceral digit and macrosetae on several legs in addition of the three on leg IV), to the subtribe Amblyseiina (sternal shield as long as wide, ventrianal shield longer than wide, seta J2 present, genital shield almost as wide as ventrianal shield, ventral shields generally smooth, macrosetae on all legs, setae j5, J2, S2, S4, S5 and Z1 present), to the genus Transeius (ratio s4/S2 < 2.7, Setae S5 present, spermathecal with atrium not bifurcate) (Chant and McMurtry 2007). Seta z4 is not as long as 2/3 the distance between its base and that of seta s4 that allows to classify this new species in the species group bellottii (Chant and McMurtry 2004a). The bell-shaped spermatheca keys to the species subgroup bellottii.
Transeius maelliae Kreiter n. sp. is quite similar to the other new species described further, T. mickaeli Kreiter n. sp. with which it was at first confused early on during the identification process. T. maelliae n. sp. is different from T. mickaeli n. sp. by: having only five solenostomes instead of seven, a longer seta z4, almost double, the shape of the spermatheca also bell-shaped but with an undistinct atrium, longer setae JV5, less teeth on both digits of chelicerae and very slightly reticulated ventral shields (see table 27).
In the species subgroup bellottii, the species closest to T. maelliae n. sp. is Transeius jujae El-Banhawy and Knapp. T. maelliae n. sp. resembles to T. jujae, having similar lengths for setae j3, s4, Z4, Z5, and JV5, and the dimensions of the spermatheca. The new species can be distinguished however by the longer length of setae z2, above all by the length of setae z4 which is 2.5 as long as that of T. jujae, and by the shape of the spermatheca with an undifferentiated atrium and walls of calyx slightly converging apically in the new species opposed to a distinct atrium and walls of calyx strictly parallel in T. jujae. In addition, a macroseta is existing in the genu of leg I of T. maelliae n. sp. and lacking in specimens of T. jujae, the ventrianal shield is wider and the dorsal shied is totally smooth in that species.
Description of the adult female
n = 4 (Figs. 2a – d)
Dorsum — (Fig. 2a). Dorsal shield fused with peritremal shield in the level of j1 position, smooth except some slight reticulation near the anterolateral margins of the dorsal shield, 393 (368 – 418) long and 221 (203 – 243) wide, with five solenostomes (gd1, gd5, gd6, gd8, and gd9), 13 pairs of poroids or lyrifissures, 17 pairs of dorsal setae and two pairs of sub-lateral setae off the dorsal shield: j1 30 (25 – 35), j3 39 (38 – 40), j4 8, j5 8, j6 11 (10 – 13), J2 9 (8 – 10), J5 10, z2 16 (14 – 18), z4 30 (28 – 33), z5 7 (6 – 8), Z1 11 (10 – 11), Z4 53 (50 – 55), Z5 75 (70 – 81), s4 47 (43 – 51), S2 21 (20 – 21), S4 15 (13 – 15), S5 13 (10 – 15), r3 18 (18 – 20), R1 19 (18 – 20). All setae smooth except Z5 which is very slightly serrated (with few barbs) in only two of the four specimens collected.
Peritreme — (Fig. 2a). Reaching the level of j1.
Venter — (Fig. 2b). All ventral shields smooth. Sternal shield with three pairs of setae and two pairs of lyrifissures; one pair of sternal setae on a small metasternal shield with one poroid; posterior margin of the sternal shield straight to slightly convex. Distances between st1-st1 66 (65 – 70), st2-st2 78, st3-st3 84 (80 – 85), st1-st3 73 (70-75), st4-st4 84 (80-95). Genital shield length 122 (113 – 128), width at the level of st5 83 (78 – 86), width at the level of the posterior corners 88 (83 – 93), distance st5-st5 75 (73 – 78). Two pairs of metapodal shields 22 (20 – 23) long and 5 (4 – 6) wide for the larger and 11 (10 – 11) long the slender shield. Ventrianal shield with three pairs of preanal setae (JV1, JV2 and ZV2), two small oblong pre-anal solenostomes 37-40 apart. Cribrum spicules on three lines. Membrane surrounding ventrianal shield with four pairs of setae (ZV1, ZV3, JV4 and JV5), and seven pairs of round to oblong poroids around genital/ventrianal shields; ventrianal shield 133 (125 – 145) long, 99 (93 – 103) wide at level of anterior corners (ZV2), and 87 (83 – 90) wide at level of anus. JV5 smooth 51 (48 – 53) long.
Chelicera — Chelicerae visible but dorsoventrally oriented; therefore, they are not drawn. Fixed digit 36 (35 – 38) long with no discernible tooth on the four females; movable digit 39 (38 – 40) long with putatively 2 teeth.
Spermatheca — (Fig. 2c). Spermatheca pocular (Denmark et al. 1999), with a moderately elongate calyx 18 (13 – 20) long and 8 (6 – 9) wide, without neck and an undifferentiated atrium. The walls of calyx are slightly converging apically in the four specimens examined. Long ductus major membranous visible and short ductus minor not well discerned.
Legs — (Fig. 2d). Macrosetae on all legs, all pointed except on leg IV slightly knobbed, one on genu of leg I, II and III, one on tibia III and three on leg IV, with one on each of genu, tibia and basitarsus: SgeI 27 (25 – 28), SgeII 32 (31 – 33), SgeIII 33 (31 – 35), Sti III 23 (20 – 25), SgeIV 60 (58 – 63), StiIV 48 (43 – 50), StIV 83 (80 – 87). Genu II and III with seven setae each, chaetotactic formula of genu II: 1-2/0, 2/0-1; genu III: 1-2/0, 2/0-1.



Male — Unknown.
Specimens examined — 4 ♀♀ + 2 im. in total, 4 ♀♀ measured and 4 ♀♀ + 2 im. as type material (see below Type material for the deposit). Forêt de Bélouve – Trou de fer (aasl 1300 m, Long 55°33'36" E, Lat 21°2'24" S), 2 ♀♀ + 1 im. on Erica arborescens (Willd.), 28/1/2017; Forêt de Bélouve – Gîte (aasl 1500 m, Long 55°33'36" E, Lat 21°6'0" S), 1 ♀ + 1 im. on Weinmannia macrostachya DC., 28/1/2017; Forêt de Sans Souci – Ilet Alcide, (aasl 1452 m, Long 55°22'07'' E, Lat 21°01'17'' S), 1 ♀ on Eriobotrya japonica (Thumb.) Lindl., 18/11/2018.
Type material — The holotype female, three paratype females and two paratype immatures deposited in Montpellier SupAgro – INRA Acarology collection, Montpellier.
Etymology — The name ''maelliae'' refers to the stepdaughter of Serge Kreiter, Maëllia Gaultier, to whom the new species is dedicated.
Remarks — Table 16 shows comparison of T. maelliae n. sp. and the closest species in the whole genus Transeius, T. jujae (see diagnosis above) and the next new species described below.



All specimens of T. maelliae n. sp. were found between 1300 and 1500 m aasl and in humid tropical forests, along with two other species, the next new species described below and Amblyseius neoankaratrae Ueckermann and Loots.
Transeius mickaeli Kreiter n. sp.
ZOOBANK: 2C86EAF9-4708-4674-88BA-B76BDD38B95F ![]()
Diagnosis — Transeius mickaeli Kreiter n. sp. belongs also to the subfamily Amblyseiinae (see above description of T. maelliae n. sp.), to the tribe Amblyseiini (see above), to the subtribe Amblyseiina (see above), to the genus Transeius (see above) (Chant and McMurtry 2007) and to the species group bellottii and to the species subgroup bellottii (Chant and McMurtry 2004a).
Transeius mickaeli n. sp. is different from T. maelliae n. sp. described above, by several characters indicated above in the description of T. maelliae (see diagnosis of T. maelliae).
In this subgroup bellottii, the closest species of T. mickaeli n. sp. is Transeius quichua (McMurtry and Moraes). Transeius mickaeli n. sp. differs however from T. quichua in having 7 solenostomes instead of 6, longer setae j1, s4, S2, Z4, and StIV and shorter setae Z5, peritreme reaching j1 and not between j1 and j3, reticulations of the dorsal shield, slight reticulations of the sternal and moreover of the ventrianal shields in the female and male, shape of the spermatheca which is saccular and bell-shaped in the new species and more cup shaped, pocular and open (Denmark and Evans 2011) in T. quichua and the insertion of gv3 further to JV2 insertion in the new species (gv3 are very close to setae JV2 positions in T. quichua).
Description of the adult female
n = 6 (Figs. 3 a – d)
Dorsum — (Fig. 3a). Dorsal shield fused with peritremal shield close to j1 position, 403 (333 – 512) long and 221 (200 – 295) wide, slightly reticulated anterolateraly, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8, gd9), 11 pairs of poroids, 17 pairs of dorsal setae and two pairs of sub-lateral setae: j1 29 (25 – 35), j3 34 (33 – 36), j4 11 (10 – 13), j5 10 (8 – 11), j6 11 (10 – 13), J2 10, J5 8 (8 – 10), z2 16 (13 – 19), z4 16 (15 – 18), z5 10, Z1 13 (13 – 15), Z4 47 (38 – 59), Z5 63 (58 – 68), s4 41 (38 – 45), S2 24 (23 – 28), S4 16 (10 – 23), S5 13 (10 – 16), r3 19 (18 – 20), R1 19 (18 – 23). All setae smooth except Z5 that is often smooth and sometimes very slightly barbed.
Peritreme — (Fig. 3a). Extending to the level j1.
Venter — (Fig. 3b). All shields very slightly reticulated. Sternal shield with three pairs of setae and two pairs of lyrifissures; one pair of sternal setae on elongate metasternal shields with a pair of pores; posterior margin straight to very slightly convex. Distances between st1-st1 62 (55 – 66), st2-st2 75 (73 – 78), st3-st3 78 (75 – 80), st1-st3 73 (68 – 78), st4-st4 80 (73 – 88). Genital shield length 135 (130 – 143), width at the level of st5 82 (78 – 85), width at the level of the posterior corners 87 (83 – 93), distance st5-st5 77 (68 – 90). Two pairs of metapodal shields 24 (18 – 28) long and 5 (4 – 8) wide for the larger and 11 (8 – 13) long for the slender shield. Ventrianal shield with three pairs of preanal setae (JV1, JV2, and ZV2), small rounded oblong gv3 43-45 apart, almost directly posterior in straight line to setae JV2. Ratio JV2-JV2 / gv3-gv3 between 1.1 and 1.3. Membrane surrounding ventrianal shield with four pairs of setae (ZV1, ZV3, JV4 and JV5), and six pairs of round to oblong poroids; ventrianal shield 121 (105 – 143) long, 100 (93 – 105) wide at level of anterior corners (ZV2), and 81 (65 – 93) wide at level of anus. JV5 smooth, 32 (23 – 40) long.
Chelicera — Chelicerae are visible but not by the side with digits open. Consequently, they are not drawn. Fixed digit 34 (28 – 38) long with 7 teeth; and movable digit 40 (30 – 45) long with 2 teeth.
Spermatheca — (Fig. 3c). Spermatheca pocular (Denmark et al. 1999), with an unelongate calyx 15 (14 – 15) long and 8 (5 – 10) wide, a differentiated atrium at the basis of the calyx. Visible short ductus minor and a long ductus major.
Legs — (Fig. 3d). Macrosetae on all legs, all pointed, one on genua of Legs I, II and III, and three on genu, tibia and basitarsus of leg IV: SgeI 23 (19 – 25), SgeII 23 (20 – 28), SgeIII 25 (23 – 28), StiIII 21 (20 – 23), SgeIV 38 (35 – 48), StiIV 34 (30 – 38), StIV 77 (70 – 82). Genu II and III with 7 setae each, chaetotactic formula of genu II: 2-2/0, 2/0-1; genu III: 1-2/1, 2/0-1.
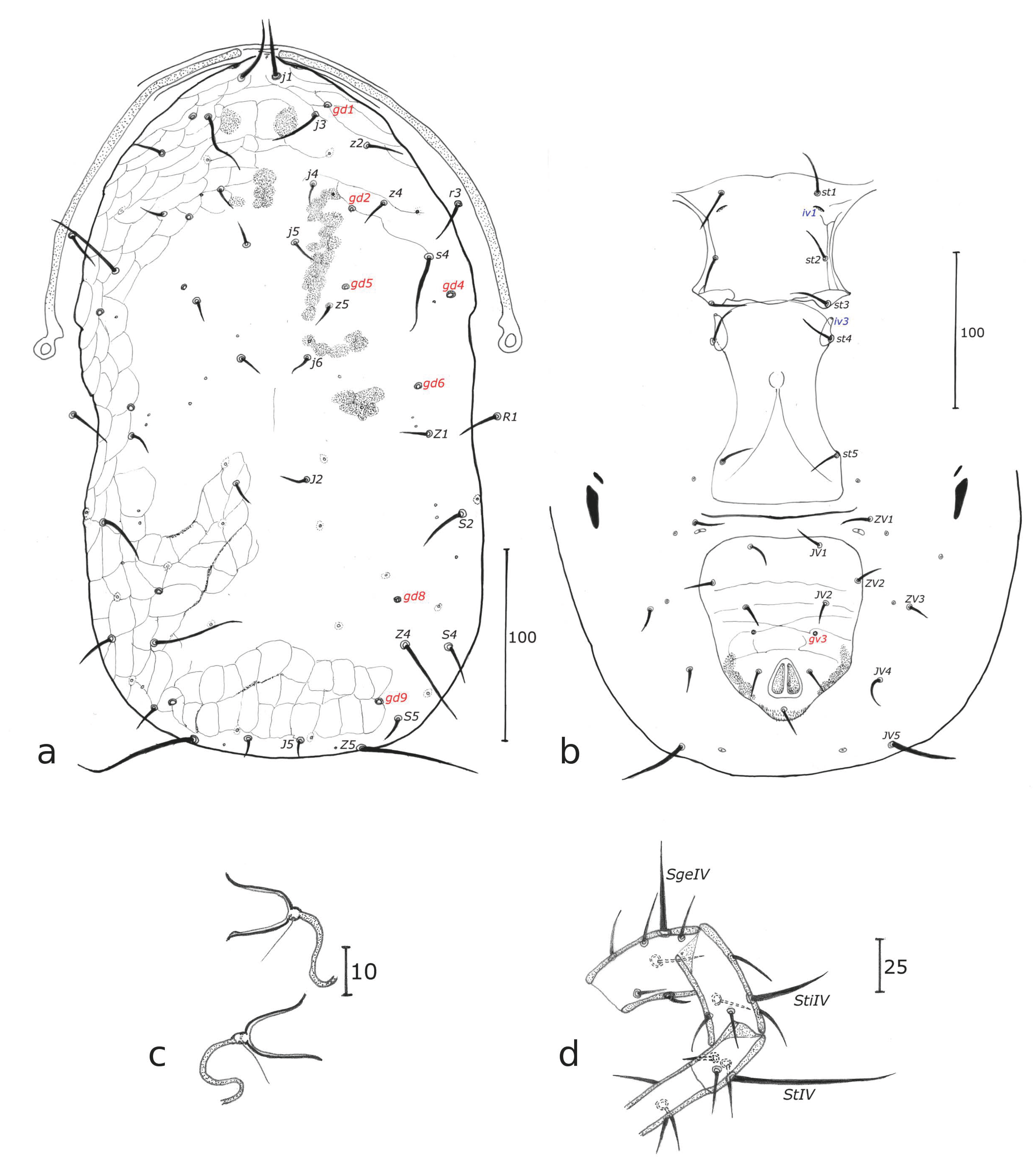


Description of the adult male
n = 2 (Figs 4 a – c)
Dorsum — (Fig. 4a). Dorsal shield fused with peritremal shield close to j1 position, 300 – 360 long and 195 – 238 wide, with 7 solenostomes like in the female but gd5 having migrate just behind s4. The dorsal shield bears 17 pairs of dorsal setae and 2 pairs of sub-lateral setae on the dorsal shield: j1 23, j3 23 – 28, j4 9 – 10, j5 9 – 10, j6 10 – 12, J2 15, J5 8, z2 15, z4 15, z5 9 – 10, Z1 15, Z4 35 – 40, Z5 55, s4 28, S2 33, S4 18, S5 15, r3 18, R1 18. All setae smooth except Z5 which is very slightly serrated with only few barbs.
Peritreme — (Fig. 4a). Extending to the level of j1. Peritremal shield fused with dorsal shield.
Venter — (Fig. 4b). All ventral shields very lightly reticulated. Distances between st1 – st1 50 – 54, st2 – st2 58 – 65, st3 - st3 58 – 63, st1 – st5 118 – 128, st4 - st4 45 – 50, st5 – st5 35 – 43. Ventrianal shield with three pairs of pre-anal setae, JV1, JV2, and ZV2, five pairs of pre-anal poroids and a pair of small oblong solenostomes 30 – 33 apart, almost directly posterior in straight line to setae JV2. Membrane surrounding ventrianal shield with one pair of setae JV5 smooth; ventrianal shield 105 – 115 long, 135 – 165 wide at anterior corners and 63 – 75 wide at level of anus. JV5 15 – 20 long, smooth. A pair of lyrifissures near JV5.
Chelicera — Fixed digit 23 – 25 long, with 4 (?) teeth and movable digit 15 – 18 long with 1 (?) tooth. Spermatodactyl L-shaped, shaft (Fig. 4c) 20 – 25.
Legs — Legs and macrosetae like in females with shorter dimensions. Macrosetae on all legs like in females: SgeI 20, SgeII 15 – 18, SgeIII 20, StiIII 20, SgeIV 23, StiIV 20 – 25, and StIV 55 – 69. Chaetotactic formula of genu II and III similar to females.



Specimens examined — Six ♀♀ + 2 ♂♂ + 3 im. in total, 6 ♀♀ + 2 ♂♂ measured, and 6 ♀♀ + 2 ♂♂ + 3 im. as type material (see below Type material for the deposit). Forêt de Bélouve – Gîte (aasl 1500 m, Long 55°33'36" E, Lat 21°6' S), 1 ♂ + 1 im. on Cyathea borbonica Desv., 28/1/2017; Forêt de Bélouve – Trou de fer (aasl 1300 m, Long 55°33'36" E, Lat 21°2'24" S), 4 ♀♀ + 1 ♂ + 1 im. on E. arborescens, 2 ♀♀ + 1 im. on Acacia heterophylla (Lam.) Willd., 28/1/2017.
Type material — The holotype female, five paratype females, 2 paratype males and 4 paratype immatures in 4 slides deposited in CBGP, in Montpellier SupAgro Acarology collection, France.
Etymology — The name ''mickaeli'' refers to the stepson of Serge Kreiter, Mickaël Gaultier, to whom this new species is dedicated.
Remarks — Table 16 shows comparison of T. mickaeli n. sp. and the closest species, T. maelliae n. sp. and T. quichua (McMurtry and Moraes).
All specimens of T. mickaeli n. sp. were found between 1300 and 1500 m aasl and in humid tropical forests, sometimes in mixed populations with T. maelliae n. sp. and/or with A. neoankaratrae.
Transeius soniae Zannou, Moraes & Oliveira
Transeius soniae Zannou, Moraes & Oliveira in Zannou et al. 2007: 38; El-Banhawy & Knapp 2011: 22.
Just like the two previous new species, T. soniae belongs to the bellottii species group. It belongs to the namurensis species subgroup as the spermatheca has the calyx swollen basally, then narrowing and flaring distally. This species subgroup contains only six species (Chant and McMurtry 2004a, Zannou et al. 2007, Tixier et al. 2016).
This species was described and mentioned only from Kenya. Although species of Transeius are considered as type III-b (generalist predators living on glabrous leaves) (McMurtry et al. 2013) phytoseiids based on the only studied species, Transeius montdorensis (Schicha) (McMurtry and Croft 1997, McMurtry et al. 2013), the biology of T. soniae remains totally unknown, just like many other species of Transeius, including the two new species above described.
This is the first mention of this species in another country than Kenya and the first mention for La Réunion Island.
Specimens examined: 3 ♀♀ in total, all measured. Le Tampon – Grand Tampon (aasl 1100 m, Long 55°34'12" E, Lat 21°16'48" S), 3 ♀♀ on S. mauritianum, 18/1/2017.
Remarks: measurements of specimens of La Réunion (Table 17) fit well with those already published in the literature, especially with those of the original description of Zannou et al.(2007). Some setae such as j4 and j5 are longer in La Réunion specimens than those in Kenya specimens. Setae s4, S2, and Z4 (between 14 and \textgreater to 22 %) and in a lesser extent Z1 are however shorter than those collected in Kenya (Zannou et al. 2007; El-Banhawy and Knapp 2011).
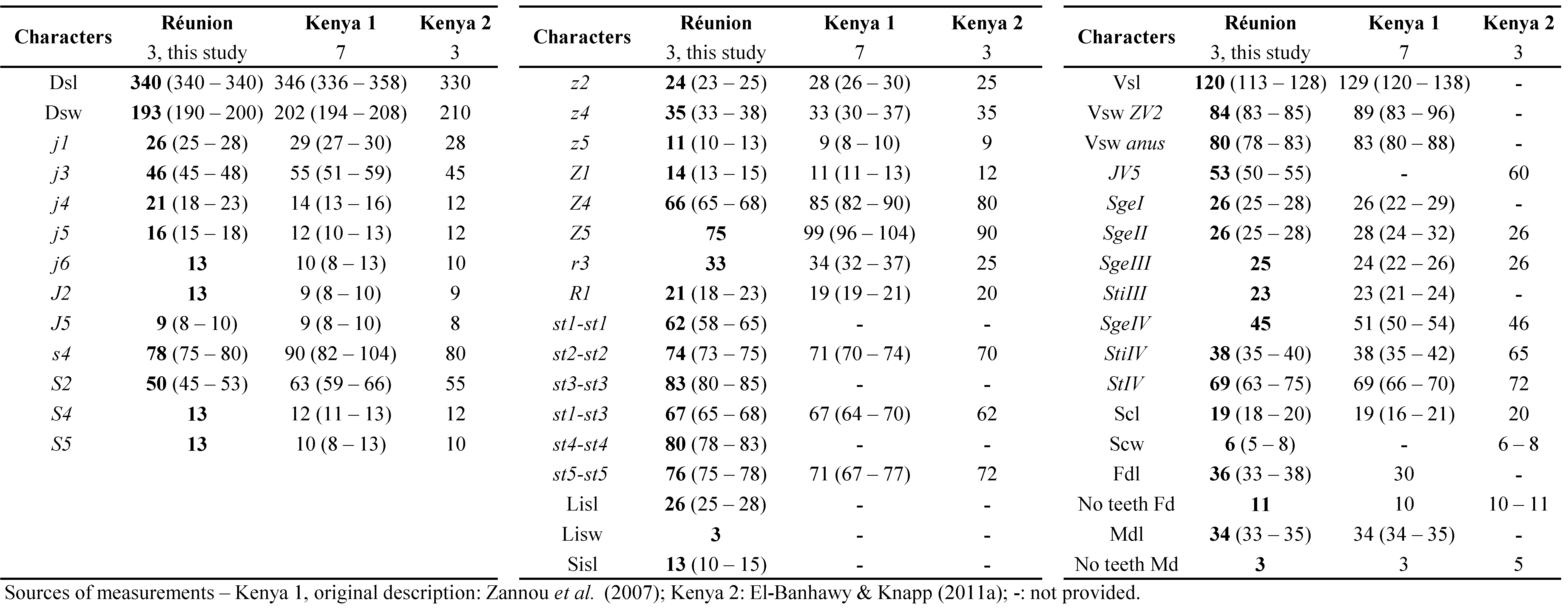


Genus Amblyseius Berlese
Amblyseius Berlese, 1914: 143.
Amblyseius djenaeli Kreiter n. sp.
ZOOBANK: F3A10C4E-9A70-457F-A76D-939A73A17CFF ![]()
Diagnosis — Amblyseius djenaeli Kreiter n. sp. belongs to the subfamily Amblyseiinae (absence of dorsolateral setae z3 and s6 and the caudoventral setae JV3), to the tribe Amblyseiini (setae j3, s4, Z4 and Z5 longer than other setae, ratio s4 / Z1 \textgreater 3.1, many teeth on the fixed cheliceral digits and macrosetae on legs I, II and/or III[?] in addition of macrosetae on leg IV), to the subtribe Amblyseiina (sternal shield as long as wide, ventrianal shield longer than wide, seta J2 present, genital shield almost as wide as ventrianal shield, ventral shields generally smooth, macrosetae on all legs, setae j5, J2, S2, S4, S5 and Z1 present), to the genus Amblyseius (ratio s4 / S2 \textgreater 3.0, chelicerae of normal size with fixed digit of the same size as movable digit, seta JV2 present, without incision in lateral margin of dorsal shield at the level of seta s4, ventrianal shield not reduced to a simple anal shield, Ge III and Ti III each generally with a macroseta) (Chant and McMurtry 2007).
Setae J2 and Z1 are present, dorsocentral setae and setae z2, z4, Z1, S2, S4, and S5 are minute, setae s4, Z4 and Z5 are prominent, elongate and whip-like, female ventrianal shield usually pentagonal, as wide at level of anus than at level of setae ZV2 or wider at this later level, which allows to classify this new species in the species group obtusus(Chant and McMurtry 2004a).
Like A. tamatavensis, A. djenaeli n. sp. belongs to the species subgroup aerialis with spermatheca tubular. This subgroup contains 46 species (Chant and McMurtry 2004a). Many of those species are very different from the new species (Table 18).



The type material of the more similar species, A. solani, was requested in Cuba for comparison with A. djenaeli n. sp. without any success.
Description of the adult female
n = 5 (Figs. 5 a – e)
Dorsum — (Fig. 5a). Dorsal shield fused with peritremal shield at the level of j1 position, 347 (325 – 378) long and 244 (233 – 250) wide at the level of waist, smooth, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9), 11 pairs of poroids, 17 pairs of dorsal setae and two pairs of sub-lateral setae: j1 33 (30 – 38), j3 52 (48 – 56), j4 4 (3 – 4), j5 4 (3 – 4), j6 5 (4 – 5), J2 5, J5 4 (4 – 5), z2 6 (6 – 8), z4 6 (5 – 8), z5 5, Z1 6 (6 – 8), Z4 109 (107 – 113), Z5 259 (245 – 268), s4 91 (89 – 95), S2 6 (5 – 8), S4 7 (6 – 8), S5 6 (5 – 6), r3 15 (13 – 16), R1 6 (5 – 8). All setae smooth.
Peritreme — (Fig. 5a). Extending to the level of j1.
Venter — (Fig. 5b). All shields smooth. Sternal shield with three pairs of setae (st1, st2, st3) and two pairs of poroids; one pair of st4 and one pair of pores on a small metasternal shield; posterior margin of the sternal shield straight. Distances between st1-st1 62 (60 – 63), st2-st2 69 (65 – 73), st3-st3 77 (75 – 80), st1-st3 70 (59 – 93), st4-st4 81 (65 – 93). Genital shield length 125 (118 – 130), width at the level of st5 77 (75 – 80), width at the level of the posterior corners 85 (83 – 88), distance st5-st5 77 (75 – 80). Two pairs of metapodal shields 22 (20 – 23) long and 6 (5 – 8) wide for the larger and 12 (9 – 13) long for the slender. Ventrianal shield with three pairs of preanal setae (JV1, JV2, and ZV2), and one pair of evolved and crateriform gv3, with 15 distance. Membrane surrounding ventrianal shield with four pairs of setae (ZV1, ZV3, JV4 and JV5), and five pairs of round to oblong poroids; ventrianal shield 118 (118 – 120) long, 95 (93 – 98) wide at level of anterior corners (ZV2), and 88 (80 – 95) wide at level of paranals setae. JV5 smooth, 90 (88 – 93) long.
Chelicera — (Fig. 5c). Fixed digit 31 (29 – 33) long with 9 strong teeth; and movable digit 36 (35 – 38) long with 4 strong teeth.
Spermatheca — (Fig. 5d). Spermatheca tubular (Denmark et al. 1999), with an elongate calyx with parallel margin 24 (23 – 25) long and 3 wide, an atrium included in the basis of the calyx. Visible small ductus minor and large membranous ductus major.
Legs (Fig. 5e) — Pointed whip-like macrosetae on genua I, II and III, on the tibia III, and on the basitarsus, tibia and genu IV. Measurements: SgeI 43 (40 – 45), SgeII 41 (38 – 45), SgeIII 58 (54 – 60), StiIII 49 (43 – 53), SgeIV 105 (103 – 110), StiIV 80 (75 –85), StIV 80 (75 – 83). Genu II and III with seven and six setae, respectively. Chaetotactic formula of genu II: 2-2/0, 2/0-1; genu III: 1-2/0, 2/0-1.



Male — Unknown.
Material examined — 5 ♀♀ in total, all measured, 5 ♀♀ as type material. LeTampon – Grand Tampon, Janick Bénard farm (aasl 861 m, Long 55°32'90'' E, Lat 21°12'80'' S), 2 ♀♀ on A. conyzoides, 1 ♀ on B. pilosa, 2 ♀♀ on R. raphanistrum, 24/5/2016.
Type material — The holotype female and three paratype females deposited in Montpellier SupAgro – INRA Acarology collection, Montpellier, France; 1 paratype female in Bassin-Plat CIRAD Research Station collection.
Etymology — The name ''djenaeli'' refers to the first name of the stepson of the senior author, Djénaël Gaultier. The species is named in his honour.
Amblyseius herbicolus (Chant)
Typhlodromus (Amblyseius) herbicolus Chant 1959: 84.
Amblyseius (Amblyseius) herbicolus, Muma 1961: 287.
Typhlodromus herbicolus, Hirschmann 1962: 23.
Amblyseius herbicolus, Moraes et al. 1986: 14; 1989a: 79; Chant & McMurtry 2004a: 208; Moraes et al. 2004a: 27; Chant & McMurtry 2007: 78.
Amblyseius deleoni, Muma & Denmark 1970: 68 (synonymy according to Daneshvar & Denmark 1982; Denmark & Muma 1989).
Amblyseius giganticus Gupta 1981: 33 (synonymy according to Gupta 1986).
Amblyseius impactus Chaudhri 1968: 553 (synonymy according to Daneshvar & Denmark 1982; Denmark & Muma 1989); Amblyseius (Amblyseialus) thermophilus Karg 1991: 12 (synonymy according to El-Banhawy & Knapp 2011; Demite et al. 2019); Typhlodromus (Amblyseius) amitae Bhattacharyya 1968: 677 (synonymy according to Denmark & Muma 1989).
Amblyseius herbicolus belongs to the largoensis species group as setae J2 and Z1 are present, setae s4 are minute and the ventrianal shield of the female is vase-shaped. It belongs to the largoensis species subgroup as setae Z4 are long, spermatheca has the calyx elongate and the female ventrianal shield is entire (Chant and McMurtry 2004a).
Amblyseius herbicolus is widespread in all tropical and subtropical regions of the world. It is the second most abundant phytoseiid mites on Coffea arabica L. in Brazil, associated with Brevipalpus phoenicis (Geijskes), vector of the coffee ring spot virus and it was found to be an efficient predator (Reis et al. 2007). A. herbicolus is also found associated with the broad mite, P. latus in crops such as chili pepper (C. annuum) in Brazil and has also a good potential for controlling the pest. Rodriguez-Cruz et al.(2013) have studied biological, reproductive and life table parameters of A. herbicolus on three different diets: broad mites, castor bean pollen (R. communis) and sun hemp pollen (Crotalaria juncea L.). The predator was able to develop and reproduce on all these three diets. However, its intrinsic growth rate was higher on broad mites and castor bean pollen. Feeding on alternative food such as pollen can facilitate the predator's mass rearing and maintain its population on crops when prey is absent or scarce. Many polyphagous generalist phytoseiid mites are important natural enemies because they can feed on plant provided pollen and various prey species, and thus persist in crops even in the absence of target pests (McMurtry et al. 2013). Hence, populations of these predators can be established in a crop by providing alternative food, thus increasing biological control. Alternative food affects P. latus control on chilli pepper plants by predatory mites (Duarte et al. 2015). A. herbicolus had high oviposition and population growth rates when fed with cattail pollen (Typha latifolia L.), chilli pepper pollen and bee-collected pollen, and a low rate on the alternative prey T. urticae. Supplementing pepper plants with pollen resulted in better control of broad mite populations (Duarte et al. 2015). Release of A. herbicolus on young plants with weekly addition of honeybee pollen or cattail pollen until plants produce flowers seems a viable strategy to sustain populations of this predator (Duarte et al. 2015).
Amblyseius herbicolus was collected recently in Comoros archipelago (Kreiter et al. 2018b). It was already well known from La Réunion since previous studies (Quilici et al. 1997, 2000). All details of collections were provided in the paper but without any measurements of specimens collected given. Measurements of specimens collected during this study are provided in table 19.
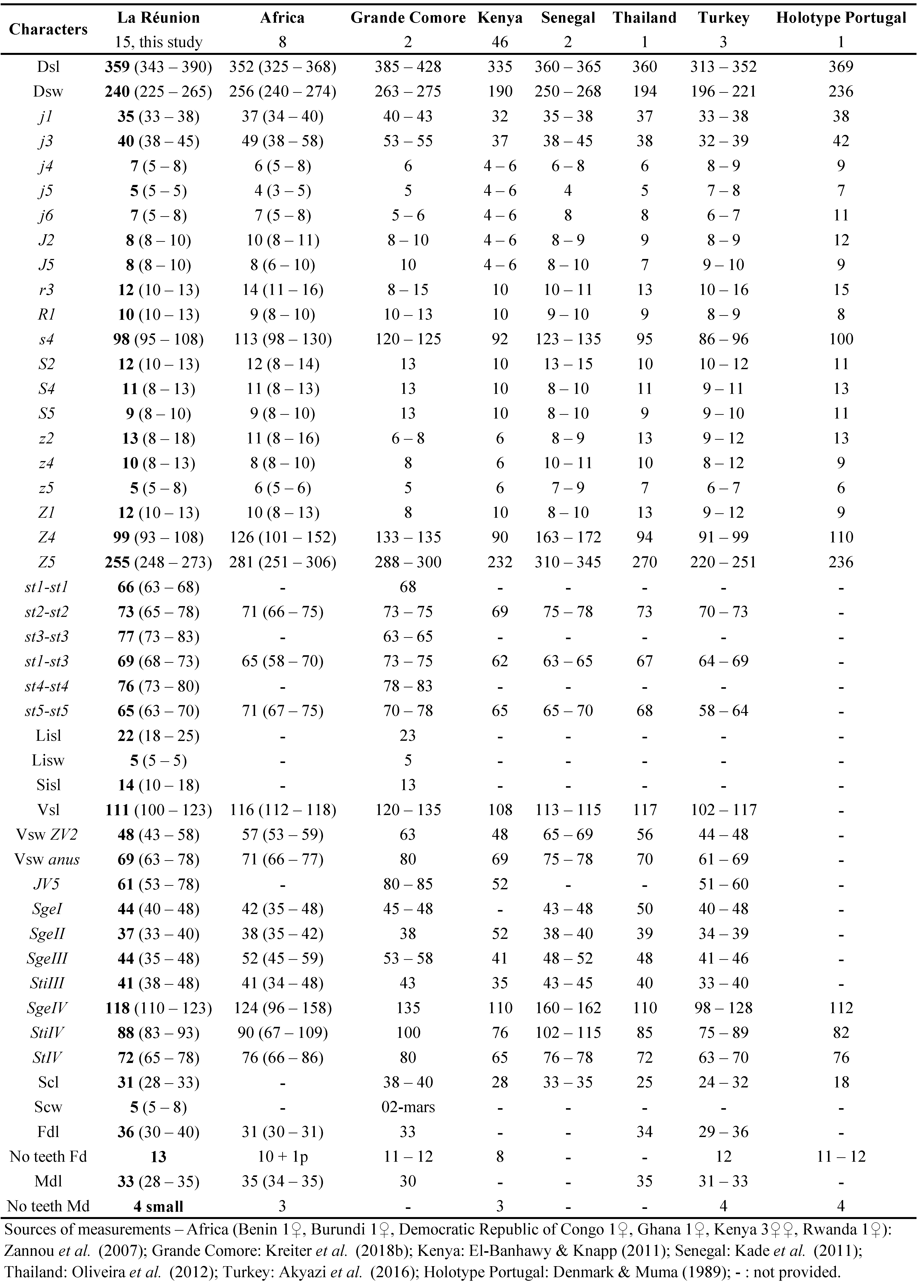


Specimens examined — 114 ♀♀ + 8 im. in total, 15 ♀♀ measured. Saint-Paul – Savannah (aasl 61 m, Long 55°29'43'' E, Lat 21°20'41'' S), 2 ♀♀ in P. vulgaris flowers, 28/7/2015; Les Avirons – Tévelave (aasl 1328 m, Long 55°21'23'' E, Lat 21°12'9'' S), 1 ♀ on P. persica, 11 ♀♀ on Citrus reticulata Blanco, 12 ♀♀ on A. conyzoides, 2 ♀ on Psidium guajava L., 8/12/2015; Le Tampon – Grand Tampon, Janick Bénard farm (aasl 861 m, Long 55°32'90'' E, Lat 21°12'80'' S), 3 ♀ on R. raphanistrum, 24/5/2016, and 1 ♀ on R. raphanistrum, 20/9/2016; Petite Île – Piton Bloc, Yébo Luguy farm (aasl 973 m, Long 55°34'6'' E, Lat 21°18'64'' S), 6 ♀♀ on Citrus limon (L.), and 1 ♀ on P. lanceolata, 9/12/2015, 1 ♀ on B. catharticus, 10 ♀♀ + 1 im. on P. lanceolata, 9 ♀♀ + 1 im. on R. raphanistrum, 18/10/2016; 14 ♀♀ + 2 im. on Citrus sp., and 6 ♀♀ + 2 im. on S. mauritianum, 29/11/2016, 4 ♀♀ + 3 im. on L. camara, 5 ♀♀ on Acacia dealbata Link, and 5 ♀♀ on P. aquilinum, 9/1/2017; Montvert-les-Hauts – EARL Le Mont Vert farm (aasl 582 m, Long 55°32'19'' E, Lat 21°19'42'' S), 1 ♀ on C. annuum, 14/12/2015; Ravine Langevin – Grand-Galet Waterfall (aasl 850 m, Long 55°21'33'' E, Lat 21°17'47'' S), 8 ♀♀ on Syzigium jambos L., and 1 ♀ on D. incanum, 11/12/2016; Forêt de Bélouve – Gîte (aasl 1500 m, Long 55°33'36" E, Lat 21°6'0" S), 1 ♀ on Fuchsia boliviana Carrière, 20/12/2016; Cap Blanc – Waterfall (aasl 1400 m, Long 55°38'24" E, Lat 21°16'48" S), 7 ♀♀ + 1 im. on Bohemeria macrophylla Jacq., 3 ♀♀ + 1 im. on S. jambos, and 1 ♀ on Polyscias bernieri (Baill.), 25/12/2016; Salazie – Voile de la Mariée Road (aasl 798 m, Long 55°32'24'' E, Lat 21°2'24'' S), 2 ♀♀ on Morus alba L., 7/1/2017; Sainte-Rose – Anse des Cascades (aasl 2 m, Long 55°49'34'' E, Lat 21°11'6'' S), 1 ♀ on Brillantaisia owariensis P. Beauv., 21/1/2017.
Remarks — Measurements of 15 females collected in La Réunion (Table 19) agree well with those of females from Grande Comore (Kreiter et al. 2018b) and females of Kenya (El-Banhawy and Knapp 2011).
The species was described as Amblyseius deleoni Muma and Denmark and synonymized after by Daneshvar and Denmark (1982) and Denmark and Muma (1989) with A. herbicolus. Although Muma and Denmark (1970) reported males of A. herbicolus from Florida, there are no other records of males from other regions of the world in the whole literature. Whereas the male of A. deleoni has been described from Florida, it has not been found on leaf samples taken on a regular basis over five years from citrus leaves in New South Wales, Australia (Schicha 1981c). Blommers (1976) failed also to observe even a single male in the mass rearing of the species in Madagascar. Van der Merwe (1968) and McMurtry (unpublished obs. but mentioned in McMurtry and Moraes 1984) confirmed thelytoky in populations of A. herbicolus from South Africa and California, respectively, by rearing isolated immatures to adult females and observing reproduction of these females.
Males collected in this study appear to be compatible with the description of adult males of A. herbicolus. Unfortunately, they were collected alone, without females or any association link with a nearby (female) population of A. herbicolus. So they cannot be reliably identified as males of A. herbicolus. We have collected 114 females from different localities, habitats and plants of the Island, with and no sign of males despite females sometimes collected in high densities. The thelytoky of this species is thus strongly suspected.
Amblyseius longipilus (Kreiter & Ueckermann)
Proprioseiopsis longipilus Kreiter & Ueckermann in Kreiter et al. 2002: 341.
Amblyseius longipilus, Chant & McMurtry 2004a: 205; 2007: 78.
Amblyseius longipilus belongs to the pusillus species group as setae J2 are absent. It was placed in the original description in the genus Proprioseiopsis but the female ventrianal shield is narrow and they are more lightly sclerotized. However, specimens of A. longipilus are brownish in colour (Chant and McMurtry 2004a).
The biology of this species remains totally unknown. A. longipilus has been described previously by Kreiter et al.(2002) from La Réunion but have never been recorded from other countries or record again in La Réunion since its description. So this is the first new record since the original description.
Specimens examined: 3 ♀♀ in total, all measured. Le Tampon – Grand Tampon, Janick Bénard farm (aasl 861 m, Long 55°32'90'' E, Lat 21°12'80'' S), 1 ♀ on Rubus alceifolius poiret, 7/1/2016; Saint-Pierre – Domaine Vidot (aasl 826 m, Long 55°34'12" E, Lat 21°18'45" S), 1 ♀ on Viburnum tinus L., 18/1/2017; Le Tampon – Grand Tampon (aasl 1100 m, Long 55°32'49" E, Lat 21°16'48" S), 1 ♀ on S. mauritianum, 18/1/2017.
Remarks: the single description available in the literature is the original description of Kreiter et al.(2002). Measurements obtained in this study (Table 20) fit well with those of the original description, except for JV5 that is a far greater in the new specimens collected in this study. JV5 setae were measured 16 (13 – 18) in the original description and this very small size is quite surprising for setae belonging to an Amblyseius species.


We have measured again these setae in 11 specimens, 1 holotype and 10 paratype females and we found 69 (63 – 75) (11), very close to the value for the three specimens found in this study.
The first values published were a mistake of measurements and they must be replaced by value of the table 20.
Amblyseius neoankaratrae Ueckermann & Loots
Amblyseius neoankaratrae Ueckermann & Loots 1988: 92.
Amblyseius neoankaratrae Ferragut & Baumann 2019: 816.
This brown-reddish species was collected in numbers in high altitude \textgreater 1000 m aasl. This species is peculiar in the genus Amblyseius by having ratios s4 / Z1 and s4 / S2 <3.1, by the shortness of setae Z4 and by the absence of macroseta on tarsus IV. Like A. herbicolus, it belongs to the largoensis species group as setae J2 and Z1 are present, setae s4 are minute and the ventrianal shield of the female is vase-shaped. It belongs to the species subgroup ankaratrae as setae Z4 are short. Only two species are in the subgroup ankaratrae and another one in the close species subgroup nahatius with only one species. Amblyseius nahatius is very different from the two first with its divided ventrianal shield, its shorter setae, and very different insemination apparatus.
This species seems to live in high altitude habitats, such as T. maelliae n. sp. and T. mickaeli n. sp. with which it makes apparently mixed colonies. The biology of that species is totally unknown.
The male of that species was unknown until now and is described thereafter.
This is the first mention of this species in another country than South Africa and Mauritius and the first mention from La Réunion Island.
Specimens examined: 72 ♀♀ + 20 ♂♂ + 12 im. in total, 20 ♀♀ + 12 ♂♂ measured. Le Tampon – Notre-Dame-de-la-Paix Forest (aasl 1600 m, Long 55°35'50'' E, Lat 21°15'50'' S), 2 ♀♀ + 1 ♂ on Monimia rotundifolia Pet.-Th., 19/12/2016; Cap Blanc – Waterfall (aasl 1400 m, Long 55°38'24" E, Lat 21°10'48" S), 1 ♀ on P. bernieri, 25/12/2016; Bras noir – Waterfall (aasl 1515 m, Long 55°32'60" E, Lat 21°06'36" S), 4 ♀♀ on Tambourissa elliptica A. DC., 26/12/2016; Forêt de Bélouve – Gîte (aasl 1500 m, Long 55°33'36" E, Lat 21°6'0" S), 13 ♀♀ on Claoxylon glandulosum Boivin ex Baill., 20/12/2016, and 3 ♀♀ + 1 ♂ + 1 im. on C. borbonica, 1 ♀ on W. macrostachya, 4 ♀♀ + 1 ♂ + 2 im. on Cryptomeria japonica (Thunb. ex L.f.), 2 ♀♀ + 2 ♂♂, on E. japonica, 28/1/2017; Forêt de Bélouve – Trou de fer (aasl 1300 m, Long 55°33'36" E, Lat 21°2'24" S), 1 ♀ on Erica arborescens, 2 ♀♀ on A. heterophylla, 1 ♀ + 1 ♂ on Passiflora tripartita (Juss.) Poir., 28/1/2017; Le Maïdo – Summit (aasl 2205 m, Long 55°23'16'' E, Lat 21°04'08'' S), 1 ♀ on C. japonica, 18/2/2017; Forêt de Sans Souci – Ilet Alcide, (aasl 1452 m, Long 55°22'07'' E, Lat 21°01'17'' S), 8 ♀♀ on Coffea mauritiana Lam., 6 ♀♀ + 4 ♂♂ on Cyathea glauca Bory, 1 ♀ on Aphloia theiformis (Vahl) Benn., 16 ♀♀ + 4 ♂♂ + 4 im. on Boehmeria macrophylla Hornem., 5 ♀♀ + 6 ♂♂ + 3 im. on Eriobotrya E. japonica, 1 ♀ + 2 im. on Psiadia montana (Cordem.) Baill., 18/11/2018.
Remarks: measurements values (Table 21) are very close from those of the literature, especially from those recently published by Ferragut and Baumann (2019) concerning specimens from Mauritius.


In La Réunion Island, it was collected in altitude \textgreater 1,000 m aasl. In Mauritius, it was collected at 678 m aasl, which is amongst the highest elevation in that island.
We have no indications of the altitude of collection for the holotype from South Africa but it seems that this species is only met in the highest part of regions in which it is present.
Description of the adult male of Amblyseius neoankaratrae
n = 20 (Figs 6 a – c)
Body brown-reddish. See at the end of this description, in remarks paragraph for diagnosis.
Dorsum — (Fig. 6a). Dorsal shield fused with peritremal shield at the level position of j1 position, smooth, 278 (258 – 293) long and 197 (178 – 215) wide, with 7 solenostomes as in females. The dorsal shield bears 17 pairs of dorsal setae and 2 pairs of sub-lateral setae: j1 19 (18 – 20), j3 30 (28 – 33), j4 8, j5 8 (8 – 10), j6 8 (8 – 9), J2 10 (8 – 10), J5 6 (5 – 8), z2 10 (5 – 13), z4 10 (8 – 13), z5 8, Z1 10, Z4 19 (15 – 20), Z5 122 (109 – 125), s4 30 (10 – 35), S2 12 (10 – 13), S4 8 (5 – 10), S5 8, r3 10 (5 – 12), R1 8 (5 – 10). All setae smooth except Z5 that is very slightly serrated.
Peritreme — (Fig. 6a). Extending to the level of j1.
Venter — (Fig. 6b). All shields are slightly reticulated: the sternogenital shield is smooth except for areas near margin and the posterior fourth of the shield; in contrast, the ventrianal shield is lineate throughout. Distances between st1 – st1 46 (43 – 48), st2 – st2 54 (50 – 55), st3 - st3 46 (43 – 48), st1 – st5 114 (110 – 118), st4 - st4 38 (35 – 40), st5 – st5 29 (25 – 33). Two pairs of lyrifissures and one pair of poroids on the sternal shield. Ventrianal shield with three pairs of pre-anal setae, JV1, JV2, and VZ2, a pair of conspicuous crateriform gv3 very close to and just behind setae JV2 (ratio JV2-JV2 / gv3-gv3 = 1 JV1, JV2 and ZV2 are inserted in the center of ventrianal shield particularly close to each other. Four pairs of poroids. Membrane surrounding ventrianal shield with one pair of setae JV5; ventrianal shield 108 (100 – 113) long, 128 (123 – 138) wide at anterior corners and 50 (45 – 60) wide at level of anus. JV5 smooth, 16 (10 – 20) long. A pair of lyrifissures near JV5.
Chelicera — Fixed digit 23 (20 – 25) long, with 6 teeth and movable digit 23 (23 – 25) long with 3 teeth. Spermatodactyl L-shaped, with a moderately elongate shaft (Fig. 6c) 15 (8 – 23) with a wide toe 10 (10 – 12) long.
Legs — Like in female, a pair of macrosetae on the genu of the Leg I and II, two pairs of macrosetae on genua and tibia of Leg III, three macrosetae slightly knobbed on the leg IV, on the basitarsus, tibia and genu. SgeI 26 (25 – 28), SgeII 25 (25 – 26), SgeIII 24 (23 – 25), StiIII 18 (16 – 23), SgeIV 50 (48 – 53), StiIV 55 (50 – 63), StIV 20 (20 – 23). Chaetotactic formula of genua II and III similar to females.
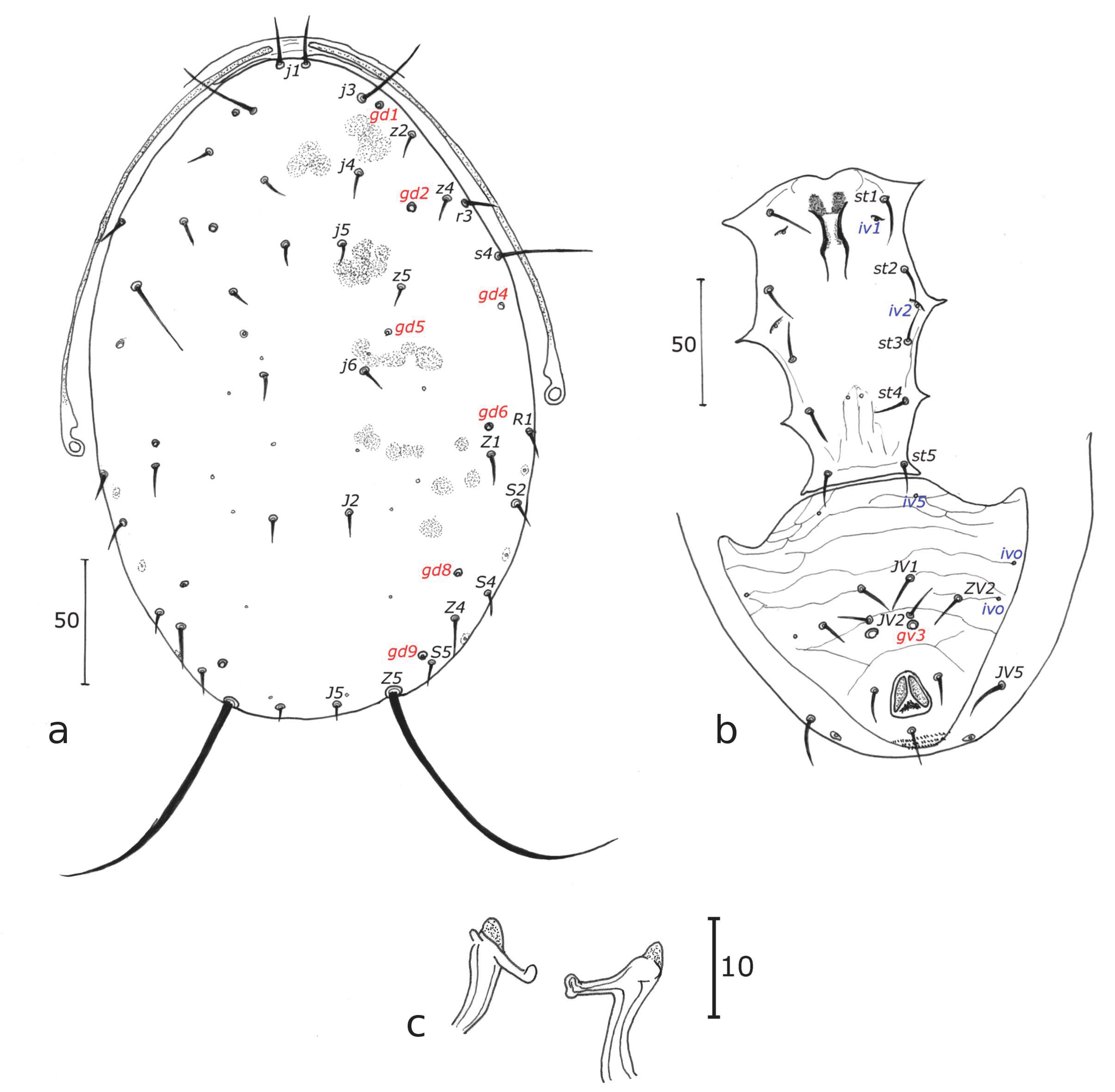


Specimens examined — (males): 20 ♂♂ in total, 12 ♂♂ measured, 20 ♂♂ as type material (see below). Le Tampon – Notre-Dame-de-la-Paix Forest (aasl 1600 m, Long 55°35'50'' E, Lat 21°15'50'' S), 1 ♂ on M. rotundifolia, 19/12/2016; Forêt de Bélouve – Gîte (aasl 1500 m, Long 55°33'36" E, Lat 21°6'0" S), 1 ♂ on C. borbonica, 1 ♂ on C. japonica, 2 ♂♂ on E. japonica, 28/1/2017; Forêt de Bélouve – Trou de fer (aasl 1300 m, Long 55°33'36" E, Lat 21°2'24" S), 1 ♂ on P. tripartita, 28/1/2017; Forêt de Sans Souci – Ilet Alcide, (aasl 1452 m, Long 55°22'07'' E, Lat 21°01'17'' S), 4 ♂♂ on C. glauca, 4 ♂♂ on B. macrophylla, 6 ♂♂ on E. japonica, 18/11/2018.
Type material — 20 paratype males and 3 paratype immatures deposited in Montpellier SupAgro – INRA Acarology collection, Montpellier, France.
Remarks — The male of A. neoankaratrae has relatively unique combination of characters that can allow to distinguish easily it from males of other species of Amblyseius, even in species subgroups ankaratrae and nahatius. It has very short setae Z4 permitting to distinguish it from any other males of all other species group of Amblyseius. Concerning the species subgroup ankaratrae and nahatius, with two and one species described in these subgroups, respectively, the only male described is for Amblyseius ankaratrae Blommers. The male of A. neoankaratrae can be distinguish from the male of A. ankaratrae by longer Z4 (19 vs 9) and s4 (30 vs 13), shorter Z5 (122 vs 170) and macrosetae of leg IV (50 and 55 vs 70 and 64 for genu and tibia, respectively), the presence of a macroseta on the basitarsus IV, less teeth on the fixed digit (6 vs 7) and a longer toe in the spermatodactyl (10 vs 7).
Amblyseius tamatavensis Blommers
Amblyseius tamatavensis Blommers 1974: 144; Moraes et al. 1986: 31; Denmark & Muma 1989: 13; Chant & McMurtry 2004a: 203; Ehara & Amano 2004: 17; Moraes et al. 2004a: 52; Chant & McMurtry 2007: 81.
Amblyseius (Amblyseius) tamatavensis, Ehara 2002: 33; Ehara & Amano 2002: 322.
Amblyseius aegyptiacus, Denmark & Matthysse in Matthysse & Denmark 1981: 343 (synonymy according to Denmark & Muma 1989)
Amblyseius maai Tseng 1976: 123 (synonymy according to Denmark & Muma 1989).
Amblyseius tamatavensis belongs to the obtusus species group as setae J2 and Z1 are present, setae z4 are minute and the female ventrianal shield is not vase-shaped or divided. It belongs to the aerialis species subgroup (46 species) as the calyx of the spermatheca is tubular (Chant and McMurtry 2004a).
It seems to fit the functional type III-b (generalist predators living on glabrous leaves) group defined by McMurtry et al.(2013). Cavalcante et al. (2017) reported this species as a promising natural enemy of B. tabaci. Experimental releases of this predator on caged plants in a screenhouse caused the reduction of the density of B. tabaci on pepper plants by up to 60-80 % (Massaro and Moraes 2019). It can be easily produced in large numbers (Massaro et al. 2018) when fed with astigmatine mites, which could allow the mass production for augmentative biological control. This species is reported in tropical areas from over 20 countries around the world (Africa, Asia, America and Oceania). It was already well known from La Réunion since previous studies (Quilici et al. 2000). All details of collections were provided in this paper but without any measurements of specimens collected given. Measurements of specimens collected during this study are provided in table 22.


Specimens examined: 212 ♀♀ + 44 ♂♂ + 30 im. in total, 11 ♀♀ + 5 ♂♂ measured. Petite Île – Les bas, Doris Morel farm (aasl 230 m, Long 55°34'1" E, Lat 21°21'22" S), 1 ♀ on A. viridis, 3 ♀♀ on M. coromandelianum, 5 ♀♀ on A. conyzoides, and 1 ♀ on Synedrella nodiflora (L.) Gaertn., 10/12/2015; 8 ♀♀ on L. camara, 2 ♀♀ on Solanum nigrum L., 3 ♀♀ on P. americana, 5 ♀♀ on Convolvulus farinosus L., 4 ♀♀ + 1 im. on P. lanceolata, 12/12/2015; 3 ♀♀ + 2 ♂♂ + 1 im. on P. lanceolata, 3 ♀♀ + 1 ♂ S. nodiflora, 31/05/2016; 1 ♀ + 1 ♂ + 1 im. on Oxalis corniculata L., 7 ♀♀ + 1 ♂ on D. incanum, 1 ♀ + 1 im. on S. nodiflora, 1 ♀ on B. pilosa, 1 ♂ on P. lanceolata, 6 ♀♀ + 1 ♂ + 1 im. on Citrus limon (L.) Osbeck, 1/12/2016; Le Tampon – Grand Tampon, Janick Bénard farm (aasl 861 m, Long 55°32'90'' E, Lat 21°12'80'' S), 3 ♀♀ + 1 im. on B. pilosa, and 1 ♀ on P. lanceolata, 20/9/2016; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 2 ♀♀ on E. hypericifolia, 1/11/2015; 56 ♀♀ + 10 ♂♂ + 10 im. in plot H; 6 ♀♀ + 10 ♂♂ in plot HM, 23 and 25/8/2016; 88 ♀♀ + + 17 ♂♂ + 14 im. in plot M; 2 ♀♀ + 1 ♂ in plot CC, 15/12/2016.
Remarks: measurements values (Table 22) are very close from that of the literature, especially from those concerning specimens from South-East Asia.
Sub-tribe Proprioseiopsina Chant & McMurtry
Proprioseiopsina Chant & McMurtry, 2004a: 219.
Genus Proprioseiopsis Muma
Proprioseiopsis Muma, 1961: 277.
Proprioseiopsis mexicanus (Garman)
Amblyseiopsis mexicanus Garman 1958: 75.
Amblyseius mexicanus, Moraes & McMurtry 1983: 134.
Proprioseiopsis mexicanus, Muma & Denmark 1970: 48; Denmark & Muma 1973: 237; Moraes et al. 1986: 118; Kreiter & Moraes 1997: 379; Moraes et al. 2004a: 181; Chant & McMurtry 2005a: 13, 2007: 89.
Proprioseiopsis amotus (Zack) (Synonymy according to Denmark & Evans 2011).
Proprioseiopsis asetus (Chant) (Synonymy according to Denmark & Evans 2011).
Proprioseiopsis clausae (Muma) (Synonymy according to Denmark & Evans 2011).
Proprioseiopsis kogi (Chant & Hansell) (Synonymy according to Denmark & Evans 2011).
Proprioseiopsis putmani (Synonymy according to Denmark & Evans 2011).
Proprioseiopsis temperellus (Denmark & Muma) (Synonymy according to Denmark & Evans 2011); Proprioseiopsis tropicanus (Garman) (Synonymy according to Denmark & Evans 2011).
Proprioseiopsis tulearensis (Blommers) (Synonymy according to Denmark & Evans 2011).
Proprioseiopsis versutus (Zack) (Synonymy according to Denmark & Evans 2011).
Proprioseiopsis mexicanus belongs to the belizensis species group as genu I have no macrosetae. As the spermathecal of that species has a short calyx, cup-shaped, it belongs to the asetus species subgroup (Chant and McMurtry 2005a).
This species is known from all islands of French West Indies (Kreiter and Moraes 1997; Moraes et al. 2000, Kreiter et al. 2006; Mailloux et al. 2010; Kreiter et al. 2018c) but it was found only in very large number during a previous study on companion plant in Guadeloupe (Mailloux et al. 2010) and in an actual study in La Réunion (Le Bellec, unpub. data). This species seems to be very abundant on weeds in the lower vegetation. Phytoseiid mites of the genus Proprioseiopsis have been found mainly in ground surface, humus, litter, soil, moss or on grass (Muma and Denmark 1970; McMurtry et al. 2015).
Proprioseiopsis mexicanus population increase when fed T. urticae eggs (Megevand et al. 1993) and this species seems to be a good predator of thrips (Kreiter, unpub. data). It is one of the prevailing phytoseiid species on citrus orchards in Alabama (Fadamiro et al. 2009). Denmark and Evans (2011) mentioned that the species can be reared on T. urticae and Oligonychus pratensis (Banks) and is associated with Bryobia praetiosa Koch, Bryobia sp. and P. ulmi. It was also found in association with Tetranychus evansi Baker and Pritchard (Furtado et al. 2014) but mentioned as a poor predator of that species. The biology of this species is however almost unknown.
Proprioseiopsis mexicanus was already recorded by Quilici et al.(2000) and all details of collections were provided but measurements of specimens collected and identified were not published. Measurements of specimens collected during this study are provided in table 23.
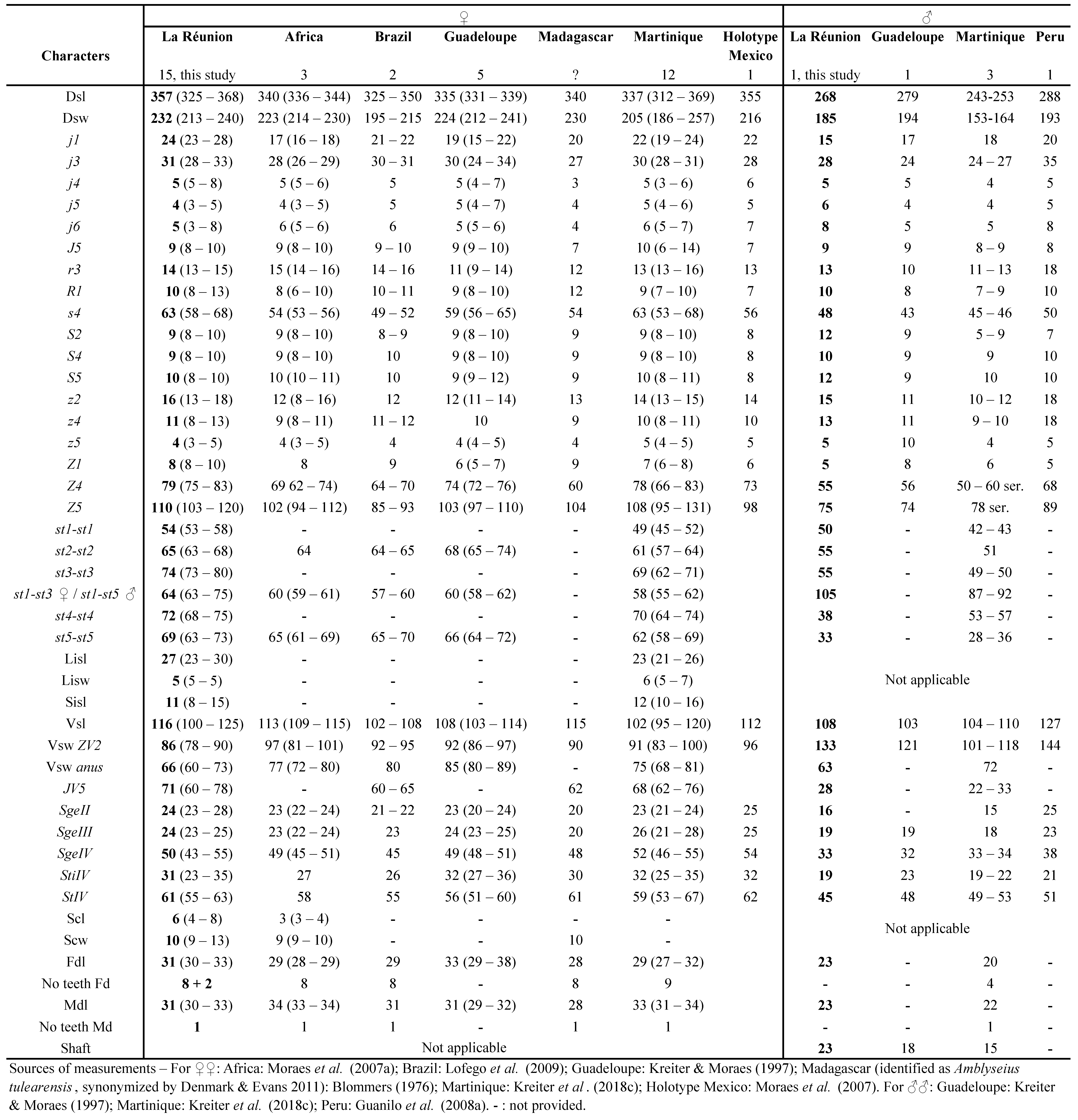


Specimens examined: 194 ♀♀ + + 2 ♂♂ + 9 im. in total, 13 ♀♀ + 1 ♂ measured (1 ♂ in bad shape). Petite Île – Les bas, Doris Morel farm (aasl 230 m, Long 55°34'1" E, Lat 21°21'22" S), 1 ♀ on C. farinosus, 12/12/2015; Montvert-les-Hauts – EARL Le Mont Vert farm (aasl 582 m, Long 55°32'19'' E, Lat 21°19'42'' S), 4 ♀♀ on Lobularia maritima (L.) Desv., 14/12/2015, 16 ♀♀ on Capsicum annuum L., 19/5/2015, 10 ♀♀ on C. annuum, 4/8/2015, 21 ♀♀ on C. annuum, 23/08/2016, 8 ♀♀ on C. annuum, 15/12/2016, 13 ♀♀ on C. annuum, 11/1/2017, and 5 ♀♀ C. annuum, 11/6/2017; Saint-Pierre – Armeflor Station (aasl 450 m, Long 55°31'9'' E, Lat 21°18'14'' S), 1 ♀ on Cosmos sulphureus Cav., 15/12/2015; Saint-Paul – Savannah (aasl 61 m, Long 55°29'43'' E, Lat 21°20'41'' S), 2 ♀♀ on C. annuum, 22/9/2016; Vincendo – Delaunay Jean-Max, Jacques Payet farm (aasl 110 m, Long 55°67'14'' E, Lat 21°38'' S), 10 ♀♀ + 2 ♂♂ on C. annuum, 15/12/2016; Petite Île – Les Bas, Doris Morel farm (aasl 230 m, Long 55°34'1" E, Lat 21°21'22" S), 1 ♀ on S. nodiflora, 31/5/2016; 5 ♀♀ + 1 im. on P. lanceolata, 2 ♀♀ on C. dactylon, and 1 ♀ on S. nodiflora, 1/12/2016; 8 ♀♀ on M. coromandelianum, 10/1/2017; Petite Île – Piton Bloc, Yébo Luguy farm (aasl 973 m, Long 55°34'6'' E, Lat 21°18'64'' S), 1 ♀ on A. conyzoides, 5 ♀♀ on R. raphanistrum, 3 ♀♀ on Ipomoea indica (Burm.) Merr., 1 ♀ on P. lanceolata, 1 ♀ on Veronica persica Poir., 28/4/2016; 1 ♀ on B. catharticus, 10 ♀♀ + 1 im. on P. lanceolata, 9 ♀♀ + 1 im. on R. raphanistrum, 18/10/2016; 14 ♀♀ + 2 im. on Citrus sp., 6 ♀♀ + 2 im. on S. mauritianum, 29/11/2016, 4 ♀♀ + 3 im. on L. camara, 5 ♀♀ on A. dealbata, 5 ♀♀ on P. aquilinum, 9/1/2017; Le Tampon – Grand Tampon, Janick Bénard farm (aasl 861 m, Long 55°32'90'' E, Lat 21°12'80'' S), 1 ♀ on P. lanceolata, 1 ♀ on A. conyzoides, 1 ♀ on Poa sp., 2 ♀♀ on B. pilosa, and 4 ♀♀ on R. raphanistrum, 24/5/2016; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 3 ♀♀ + 1 im. on A. hispidum, 17/2/2017; 2 ♀♀ on Datura innoxia Mill., 1 ♀ on Sorghum arundinaceum (Desv.) Stapf, 27/2/2017; 1 ♀ on P. hysterophorus, 30/3/2017; 5 ♀♀ in plot CC, 6/4/2017.
Remarks: measurement values of female and male specimens from La Réunion (Table 23) fit well with all those indicated in Kreiter et al.(2018c) for various countries. All setae and dimensions of specimens of this study have however slightly longer in females and males compared to specimens from other countries.
Proprioseiopsis ovatus (Garman)
Amblyseiopsis ovatus Garman 1958: 78.
Amblyseiulus ovatus, Muma 1961: 278.
Typhlodromus (Amblyseius) ovatus, Chant 1959: 90.
Typhlodromus ovatus, Hirschmann 1962: 19.
Proprioseiopsis (Proprioseiopsis) ovatus, Karg 1989: 208.
Proprioseiopsis ovatus, Moraes et al. 1986: 121; 2004a: 184; Chant & McMurtry 2005a: 15; 2007: 89.
Proprioseiopsis antonelli Congdon (Synonymy according to Denmark & Evans (2011).
Proprioseiopsis cannaensis (Muma) (Synonymy according to Denmark & Evans (2011).
Proprioseiopsis hundsonianus (Chant & Hansell) — (Synonymy according to Denmark & Evans (2011); Proprioseiopsis parapeltatus (synonymy according to Tseng 1983).
Proprioseiopsis peltatus (van der Merwe) (synonymy according to Tseng 1983).
Like P. mexicanus, P. ovatus belongs to the belizensis species (see above). As the spermatheca of that species is saccular, it belongs to the belizensis species subgroup (Chant and McMurtry 2005a).
This species is known from Guadeloupe, Marie-Galante and Martinique (Kreiter and Moraes 1997; Moraes et al. 2000; Mailloux et al. 2010; Kreiter et al. 2018c). This species was found in very large number only during a previous study on companion plant in Guadeloupe (Mailloux et al. 2010) and in a recent study in La Réunion (Le Bellec, unpub. data). In other habitats, this species seems to be rare. This species like P. mexicanus seems to be abundant on weeds in the lower vegetation. Denmark and Evans (2011) indicated that this species is associated with O. pratensis and Brevipalpus sp. It was also found in association with T. evansi (Furtado et al. 2014) but mentioned as poor predator of that species. Despite this information, the biology of this species remains unknown.
This is the first mention of this species from La Réunion.
Specimens examined: 58 ♀♀ in total, 12 ♀♀ measured. Petite Île – Piton Bloc, Yébo Luguy farm (aasl 973 m, Long 55°34'64'' E, Lat 21°18'64'' S), 1 ♀ on R. raphanistrum, 26/11/2015; 1 ♀ on P. lanceolata, 9/12/2015; 1 ♀ on A. conyzoides, 4 ♀♀ on R. raphanistrum, 1♀ on I. indica, 28/4/2016; 4 ♀♀ on R. raphanistrum, 1 ♀ on B. pilosa, 2 ♀♀ on Citrus lemon Eureka, 24/5/2016; Le Tampon – Grand Tampon, Janick Bénard farm (aasl 1100 m, Long 55°34'12" E, Lat 21°16'48" S), 1 ♀ on L. camara, 9/12/2015; Saint-Gilles – Pépinières du Théâtre (aasl 70 m, Long 56°13'58'' E, Lat 21°2'50'' S), 1 ♀ on Ipomoea nil (L.) Roth, 14/2/2017; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 40 ♀♀ in plot CC, 1 in plot F, 1 ♀ in plot H, 6/4/2017.
Remarks: measurements of female specimens of La Réunion (Table 24) fit well those obtained for populations of various countries. Despite the great number of females sampled, no male was recorded.



Tribe Euseiini Chant & McMurtry
Euseiini Chant & McMurtry 2005b: 191.
Sub-tribe Typhlodromalina Chant & McMurtry
Typhlodromalina Chant & McMurtry 2005b: 195.
Genus Typhlodromalus Muma
Amblyseius (Typhlodromalus) Muma, 1961: 288; Typhlodromalus, De Leon 1966: 87.
Typhlodromalus spinosus (Meyer & Rodrigues)
Amblyseius spinosus Meyer & Rodrigues 1966: 30.
Kampimodromus spinosus, Quilici et al. 2000: 100.
Typhlodromalus spinosus, Moraes et al. 186: 2004a: 204.
Typhlodromalus spinosus, Chant & McMurtry 2005a: 199; 2007: 111.
Typhlodromalus spinosus belongs to the athiasae species group as setae J1 and S5 are absent. This species group contains six species (Chant and McMurtry 2005b, Moraes et al. 2006).
Typhlodromalus spinosus was collected in eastern, western but mainly southern Africa and in La Réunion (Demite et al. 2019). The rapid multiplication of this species on the western flower thrips (WFT), F. occidentalis, was confirmed and clear evidence that T. spinosus predates on WFT under laboratory and field conditions but not on T. urticae was established (Mwangi et al. 2015). This species seems abundant in low vegetation as it was found in high populations in a study of companion plants in citrus orchard (Le Bellec et al. unpub. data). This species have never been record in Guadeloupe or Martinique in similar studies but it is interesting to notice that in those islands, another Typhlodromalus was collected, T. peregrinus (Muma) (Mailloux et al. 2010; Kreiter et al. 2013, 2018c). T. spinosus was already recorded by Quilici et al.(2000) and all details of collections were provided but measurements of specimens collected and identified were not published. Measurements of specimens collected during this study are provided in table 25.



Specimens examined: 113 ♀♀ + 18 ♂♂ + 10 im. in total, 13 ♀♀ + 4 ♂♂ measured. Petite Île – Piton Bloc, Yébo Luguy farm (aasl 973 m, Long 55°34'64'' E, Lat 21°18'64'' S), 1 ♀ on B. catharticus, 18/10/2016; 1 ♀ on P. lanceolata, 9/12/2015; 1 ♀ on A. conyzoides, 4 ♀♀ on R. raphanistrum, 1♀ on I. indica, 28/4/2016; 4 ♀♀ on R. raphanistrum, 1 ♀ on B. pilosa, 2 ♀♀ on C. limon, 24/5/2016; Le Tampon – Grand Tampon, Janick Bénard farm (aasl 1100 m, Long 55°34'12" E, Lat 21°16'48" S), 2 ♀♀ on P. americana, 1 ♂ on R. alceifolius, 7/1/2016; 3 ♀♀ + 1 ♂ on R. raphanistrum, 11 ♀♀ + 3 ♂♂ on B. pilosa, 12 ♀♀ on A. conyzoides, 24/5/2016; 6 ♀♀ on B. pilosa 16/9/2016; 41 ♀♀ + 8 ♂♂ + 6 im. on B. pilosa, 5 ♀♀ + 3 ♂♂ on P. lanceolata, 14 ♀♀ + 3 im. on R. raphanistrum, 2 ♀♀ + 1 ♂ on Trifolium repens L., 1 ♀ on Sonchus asper All., 1 ♂♂ + 1 im. on B. catharticus, 20/9/2016; Le Tampon – Bras de Pontho, Aldo Grace farm (aasl 661 m, Long 55°29'48" E, Lat 21°14'33" S), 1 ♀ on A. viridis, 21/2/2017.
Remarks: measurements of female and male specimens of La Réunion (Table 25) fit well those obtained for populations of various countries. Measurements are in general for all characters longer compared to those obtained on specimens of other countries in general.
Genus Ueckermannseius Chant & McMurtry
Ueckermannia Chant & McMurtry, 2005b: 201. Preoccupied by Ueckermannia Kazmierski, 1996 (Tydeidae).
Ueckermannseius Chant & McMurtry, 2005c: 337; 2007: 115.
Ueckermannseius nesiotus (Ueckermann & Kreiter)
Typhlodromalus nesiotus Ueckermann & Kreiter 2002: 343.
Ueckermannseius nesiotus Chant & McMurtry, 2005b: 203; 2007: 115.
This species was described as Typhlodromalus nesiotus Ueckermann and Kreiter in Kreiter et al.(2002) but as all setae are short to minute, the setae Z4 are not as long as distance between their base and that of setae S4 and the dorsal shield is smooth except some anterolateral striations, it belongs to the genus Ueckermannseius (Chant and McMurtry 2005b). This species has never been recorded from other countries or record again in La Réunion since its description. The biology of this species remains totally unknown.
This is the first new record since the original description in 2002.



Specimens examined: two ♀♀ in total, both measured. Petite Île – Piton Bloc, Yébo Luguy farm (aasl 973 m, Long 55°34'64'' E, Lat 21°18'64'' S), 1 ♀ on R. raphanistrum, 9/12/2015; Forêt de Sans Souci – Ilet Alcide, (aasl 1452 m, Long 55°22'07'' E, Lat 21°01'17'' S), 1 ♀ on Hypericum lanceolatum Lam., 18/11/2018.
Remarks: the two females collected and measured during this study (Table 26) slightly differ from females measured by Kreiter et al.(2002). Dorsal shield dimensions are greater by 15–26%, length and width of the sternal shield are greater by 25 and 20% respectively, and the length of JV5 is greater by 60%.
Ueckermannseius parahavu Moraes, Zannou & Oliveira
Ueckermannseius parahavu Moraes, Zannou & Oliveira, in Moraes et al. 2006: 36, Chant & Mcurtry 2007: 115.
All dorsal setae of this species are short to minute, the setae Z4 are not as long as distance between their base and that of setae S4 and the dorsal shield is smooth except some anterolateral striations. Thus, it belongs to the genus Ueckermannseius (Chant and McMurtry 2005b). This species is only known from Ghana (Moraes et al. 2006) and nothing is known on its biology.
This is the first new record of that species in another location and the first record of the species in La Réunion.
Specimens examined: 2 ♀♀ in total, both measured. Forêt de Bélouve – Gîte (aasl 1500 m, Long 55°33'36" E, Lat 21°6'0" S), 2 ♀♀ on C. borbonica, 28/1/2017.
Remarks: two females have been collected and compared (Table 27) to specimens of Ghana (Moraes et al. 2006). Measurements of morphological characters of specimen females from La Réunion (Table 27) fit well with those of specimens of Ghana, with slightly shorter setae, especially j3, s4, z4 and the macrosetae of the leg IV. Setae S4 and dorsum length dimensions are however slightly greater from those of specimens of Ghana (Moraes et al. 2006).
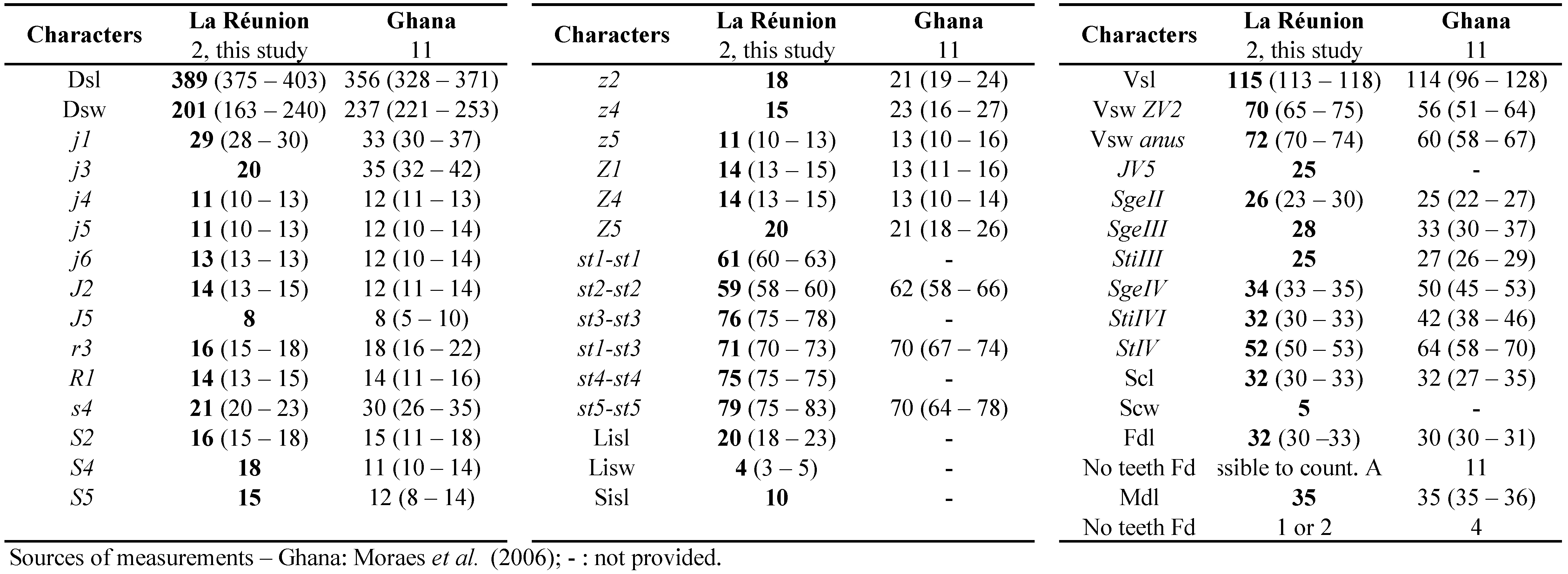


Genus Amblydromalus Chant & McMurtry
Amblydromalus Chant & McMurtry, 2005b: 203; 2007: 117.
Amblydromalus nakuruensis Moraes, Zannou & Oliveira
Amblydromalus nakuruensis Moraes, Zannou & Oliveira 2006, in Moraes et al.: 6; Chant & McMurtry 2007: 117.
This species has the setae Z4 much shorter than 40% of distance between its base and that of setae Z5 and thus belongs to the limonicus species group which contains 16 species (Chant and McMurtry 2005b).
This species was only known before from Kenya (Demite et al. 2019) and its biology remains totally unknown.
This is the first record of that species in another country and the first record in La Réunion.
Specimens examined: A single ♀ in total, measured. Saint-Pierre – Armeflhor Station (aasl 450 m, Long 55°31'9'' E, Lat 21°18'14'' S), 1 ♀ on Persea americana Mill., 15/12/2015.
Remarks: measurements of morphological characters of the single female found in La Réunion (Table 28) are 6–50% smaller than those obtained on specimen from Kenya, especially j1, j6, J2, s4, S2, z2, z4, Z5 and length of chelicerae. Measurements were however taken on a single specimen from each country, and so this data must be compared with caution.
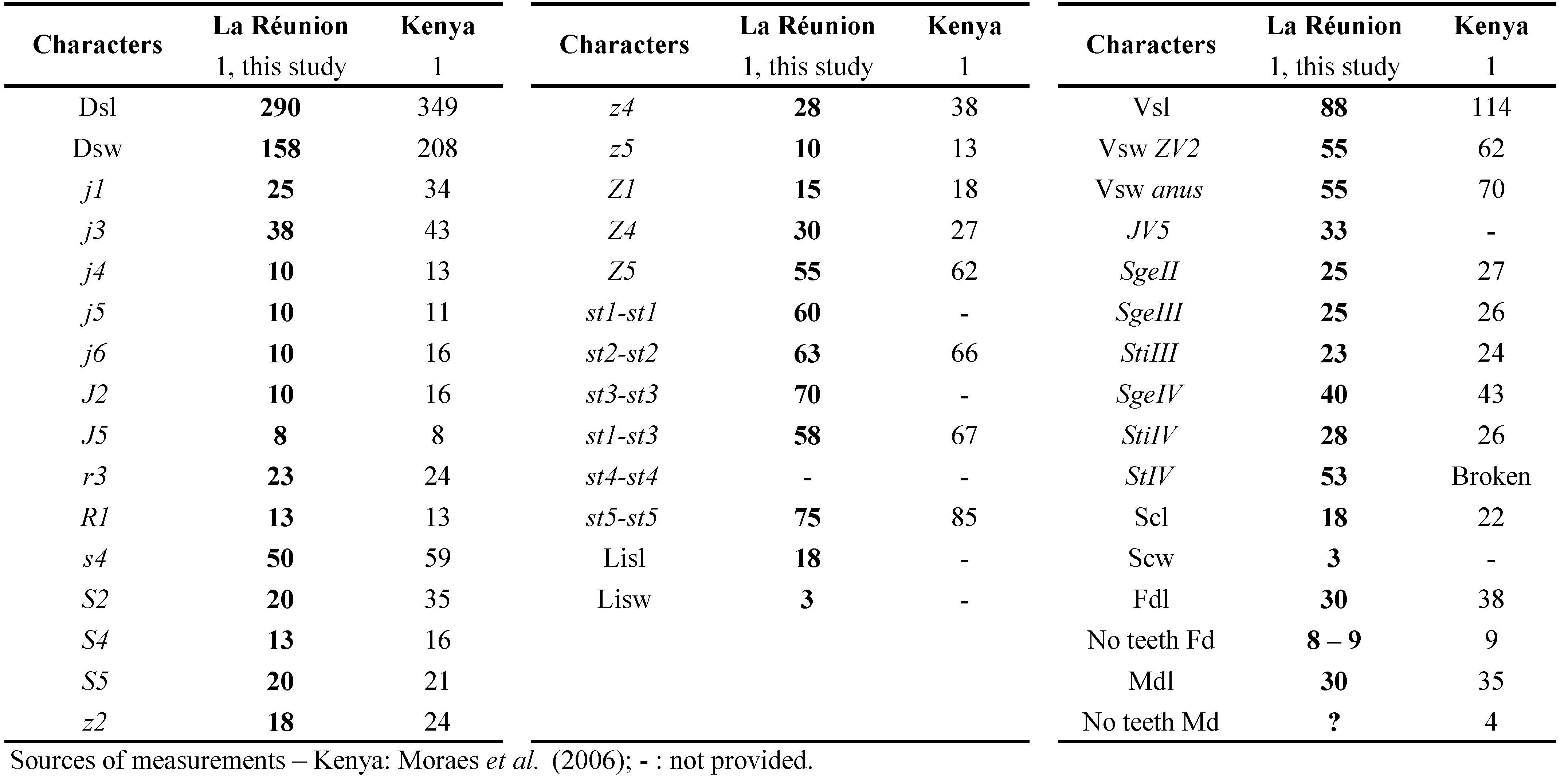


Subtribe Euseiina Chant & McMurtry
Euseiina Chant & McMurtry, 2005b: 209.
Genus Euseius Wainstein
Amblyseius (Amblyseius) section Euseius, Wainstein, 1962: 15; Euseius De Leon, 1967: 86.
Euseius hima (Pritchard & Baker)
Amblyseius (Amblyseius) hima Pritchard & Baker 1962: 257; Blommers 1976: 89.
Euseius hima, Moraes et al. 1986: 46, 2004a: 71; Quilici et al. 2000: 99; Chant & McMurtry 2005b: 215, 2007: 121.
This species was recorded from several countries of Sub-Saharan Africa but also from Madagascar, India (Demite et al. 2019) and La Réunion (Quilici et al. 2000; Demite et al. 2019). All details of collections were provided in the paper but measurements of specimens collected and identified were not published. Measurements of specimens collected during this study are provided in table 29. The biology of this species remains totally unknown.



Specimens examined: 26 ♀♀ + 4 ♂♂ + 16 im. in total, 15 ♀♀ + 4 ♂♂ measured. Le 19e – Plaine des Caffres, JL Robert farm (aasl 1000 m, Long 55°32'9'' E, Lat 21°14'16'' S), 1 ♀ on L. camara, 15/12/2015; Le Tampon – Bras creux (aasl 888 m, Long 55°32'39'' E, Lat 21°15'24'' S), 1 ♀ on Obetia ficifolia Gaudich., 18/12/2015; Le Tampon – Ligne des 400 (aasl 463 m, Long 55°30'36" E, Lat 21°17'24" S), 14 ♀♀ + 1 ♂ + 8 im. on Ipomoea sp., 17/12/2016 and 10/1/2017; 5 ♀♀ on Spathodea campanulata P. Beauv., 12/1/2017; 3 ♀♀ + 1 ♂ on Solanum wrightii Benth., 18/1/2017; Forêt de Sans Souci – Ilet Alcide, (aasl 1452 m, Long 55°22'07'' E, Lat 21°01'17'' S), 1 ♂ on C. glauca, 1 ♂ on A. theiformis, 18/11/2018; Saint-Pierre – Armeflhor Station (aasl 450 m, Long 55°31'9'' E, Lat 21°18'14'' S), 2 ♀♀ + 5 im. on Terminalia benzoe Pers., 15/12/2015.
Remarks: measurements of morphological characters of females of E. hima fit very well with those provided in the literature (Table 29), especially with those of specimens of various countries in Africa published in Moraes et al.(2001).
For the males (Table 29), setae Z5 is shorter and ventrianal shield is longer in specimens from La Réunion Island.
Sub-family Phytoseiinae Berlese
Phytoseiini Berlese 1913: 3; Phytoseiinae, Vitzthum 1941: 768.
Genus Phytoseius Ribaga
Phytoseius Ribaga 1904: 177
Phytoseius amba Pritchard & Baker
Phytoseius (Pennaseius) amba Pritchard & Baker 1962: 224; Blommers 1976: 85.
Phytoseius (Phytoseius) amba, Denmark 1966: 46.
Typhlodromus (Phytoseius) amba, van der Merwe 1968: 101.
Pennaseius amba, Matthysse & Denmark 1981: 352.
Phytoseius amba, Swirski & Ragusa 1978: 408; Moraes et al. 1986: 210, 2004a: 232; Chant & McMurtry 2007: 129; El-Banhawy & Knapp 2011: 47.
This species belongs to the plumifer species group as setae J2 and R1 are present (Chant and McMurtry 1994). It is widely distributed in sub-Saharan Africa, Madagascar (Demite et al. 2019), in La Réunion (Quilici et al., 2000; Demite et al. 2019) and recently from one island of the Comoros Archipelago, Grande Comore (Kreiter et al. 2018b).
The biology of this species remains totally unknown.
If all details of collections were provided in the previous paper concerning La Réunion (Quilici et al. 2000), measurements of specimens collected and identified were not published. Measurements of specimens collected during this study are provided in table 30.
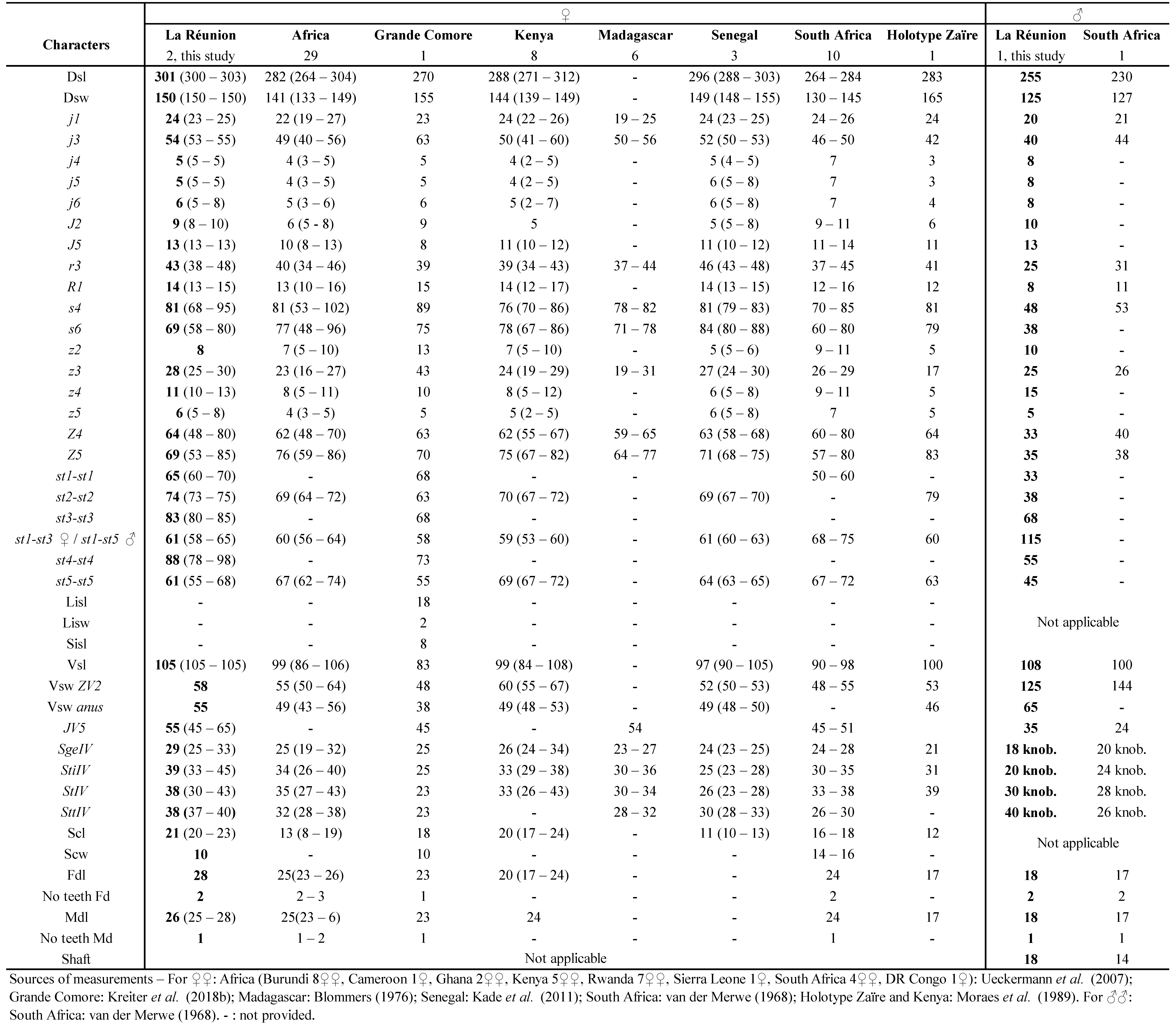


Specimens examined: 2 ♀♀ + 1 ♂ + 3 im. in total, 2 ♀♀ + 1 ♂ measured. Piton de la Fournaise – Pas de Bellecombe (aasl 2350 m, Long 55°41'20'' E, Lat 21°13'20'' S), 1 ♀ + 1 ♂ + 3 im. on Psiadia anchusifolia, 29/12/2016; Sainte-Rose – Forêt Mourouvin (aasl 871m, Long 55°46'12" E, Lat 21°10'48" S), 1 ♀ on Boehmeria penduliflora Wedd. ex D.G. Long, 21/1/2017.
Remarks: measurements of characters of the two females of P. amba from La Réunion (Table 30) compared to those of specimens from neighbouring countries show a slight variation of dimensions depending of the geographical origin. Measurements of characters of the two collected females are close from those of specimens from South Africa (Moraes et al. 1989), especially setae j1, J2, z3, Z5, S6, r3, JV5 and spermathecal width. Setae j3 and z3 are smaller and macrosetae of leg IV are longer in specimens from La Réunion compared to specimens from Comoros Archipelago but numbers of specimens (2 and 1, respectively) are very low in both cases.
For male specimens, measurements (Table 30) concern only one individual from La Réunion Island and one from South Africa. Values obtained for the male of La Réunion Island are slightly lower, except for some setae and for macrosetae of the leg IV, especially macrosetae of telotarsi of legs IV that are longer in the specimen of La Réunion Island.
Phytoseius crinitus Swirski & Shechter
Phytoseius (Dubininellus) crinitus Swirski & Shechter 1961: 102.
Phytoseius crinitus, Amitai & Swirski 1966: 21; Swirski & Amitai 1966: 11; Denmark 1966: 66; Moraes et al. 1986: 220, 2004a: 236; Chant & McMurtry 2007: 129.
This species belongs to the horridus species group as setae J2 and R1 are absent (Chant and McMurtry 1994). This species was recorded in several countries of Asia, in Burundi, Madagascar (Demite et al. 2019) and was already known from La Réunion (Quilici et al. 2000; Demite et al. 2019). If all details of collections were provided in the previous paper, measurements of specimens collected and identified were not given. Measurements of specimens collected during this study are provided in table 31. The biology of this species remains totally unknown.



Specimens examined: 8 ♀♀ in total, 5 ♀♀ measured. Saint-Pierre – Armeflhor Station (aasl 450 m, Long 55°31'9'' E, Lat 21°18'14'' S), 2 ♀♀ on P. americana, 15/12/2015; Sainte-Rose – Anse des Cascades (aasl 5 m, Long 55°49'48" E, Lat 21°10'48" S), 3 ♀♀ on Terminalia catappa L., 27/12/2016; Le Tampon – Ligne des 400 (aasl 463 m, Long 55°30'36" E, Lat 21°17'24" S), 2 ♀♀ on C. glauca, 10/1/2017; Saint-Anne – Bassin bleu (aasl 6 m, Long 55°45'0" E, Lat 21°4' 48" S), 1 ♀ on Ipomoea sp., 15/1/2017.
Remarks: specimens of this species have been recorded and measured from Burundi. Measurements of our specimens from La Réunion (Table 31) fit well with those of Burundi with sometimes slightly greater values, for example for setae s4 and Z4.
Values for both type of specimens are higher than those obtained for specimens from Asia (Table 31).
Phytoseius haroldi Ueckermann & Kreiter
Phytoseius haroldi Ueckermann & Kreiter, 2002: 339; Chant & McMurtry 2007: 129.
This species belongs to the horridus species group as setae J2 and R1 are absent (Chant and McMurtry 1994). This species was described Kreiter et al.(2002) but have never been recorded from other countries or recorded again in La Réunion since its description. This species was abundant on lower vegetation in a study of companion plants in citrus orchard. It seems that this species prefers low plants but despite this observation that has to be confirmed, the biology of this species remains totally unknown. Measurements of some additional females (9) and of some males are provided in table 32.



This is the first new record In La Réunion since the original description and the second record in Indian Ocean after recent records of Ferragut and Baumann (2019).
Specimens examined: 349 ♀♀ + 135 ♂♂ + 95 im. in total, 9 ♀♀ + 8 ♂♂ measured. Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 2 ♀♀ on D. incanum, 1/8/2015; 1 ♀ on Dombeya acutangula Cav., 1/11/2015; Saint-Joseph – Center of the city (aasl 37 m, Long 55°37'9'' E, Lat 21°22'44'' S), 4 ♀♀ + 1 ♂ on S. torvum, 16/12/2015; Saint-Pierre – Bassin-Plat CIRAD research station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 4 ♀♀ in plot CC, 1 ♀ + 2 ♂♂ in plot M, 5 ♀♀ in plot H, 12/4/2016; 1 ♀ + 1 ♂ + 1 im. on D. incanum, 23/6/2016; 1 ♀ on D. acutangula, 23/6/2016; 2 ♀♀ in plot HM, 1 ♀ in plot M, 1 ♀ in plot CC, 23/8/2016; 1 ♀ in plot CC, 2 ♀♀ + 1 ♂ + 1 im. in plot H, 15/12/2016; Petite Île – Les bas, Doris Morel farm (aasl 230 m, Long 55°34'1" E, Lat 21°21'22" S), 1 ♀ on D. incanum, 31/5/2016; Petite Île – Piton Bloc, Yébo Luguy farm (aasl 973 m, Long 55°34'64'' E, Lat 21°18'64'' S), 1 ♀ on Raphanus raphanistrum L., 29/11/2016; Le Tampon – Ligne des 400 (aasl 463 m, Long 55°30'36" E, Lat 21°17'24" S), 2 ♀♀ + 1 ♂ + 1 im. on Ipomoea sp., 17/12/2016; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 11 ♀♀ on P. guajava, 16/12/2016; La Saline – Jardin d'Eden (aasl 70 m, Long 55°13'46" E, Lat 21°20'25" S), 1 ♀ on Cananga odorata (Lam.) Hook. f. & Thomson, 22/1/2017; Langevin – Jacqueline Waterfall (aasl 5 m, Long 55°64'40'' E, Lat 21°38'9'' S), 1 ♀ on Scaevola taccada (Gaertn.) Roxb., 1 ♀ on I. purpurea, 19/2/2017; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 4 ♀♀ + 1 ♂ + 3 im. on B. pilosa, 2 ♂♂ + 1 im. on P. hysterophorus, 1 ♀ on E. hypericifolia, 1 ♀ on Cardiospermum halicacabum L., 5 ♀♀ + 1 ♂ + 1 im. on T. labialis, 20/2/2017; Montvert-les-Hauts – EARL Le Mont Vert farm (aasl 582 m, Long 55°32'19'' E, Lat 21°19'42'' S), 3 ♀♀ on L. camara, 22/2/2017; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 3 ♀♀ on Duranta serratifolia, 27/2/2017; 1 ♀ + 1 ♂ on Sida acuta, 1 ♀ on E. hypericifolia, 1 im. on Tephrosia purpurea (L.) Pers., 3 ♀♀ + 1 im. on I. obscura, 2 ♀♀ + 1 im. on A. hispidum, 1 ♀ + 3 ♂♂ on L. leucocephala, 1 ♀ on C. halicacabum, 3 ♀♀ + 2 ♂♂ + 1 im. on M. coromandelianum, 30/3/2017; 2 ♀♀ in plot H, 13 ♀♀ + 1 im. in plot M, 6/4/2017; 1 ♀ on E. hypericifolia, 8 ♀♀ + 1 ♂ + 3 im. on T. purpurea, 9 ♀♀ + 3 ♂♂ + 2 im. on I. obscura, 3 ♀♀ on B. pilosa, 1 ♀ on T. labialis, 1 ♀ on A. viridis, 4 ♀♀ + 2 ♂♂ + 1 im. on P. hysterophorus, 2 ♀♀ on Digitaria radicosa (J.Presl) Miq., 19/6/2017; 1 ♀ on D. radicosa, 20/6/2017; 246 ♀♀ + 113 ♂♂ + 76 im. on D. incanum, 3 ♀♀ on D. acutangula, 3/7/2017.
Remarks: measurements of characters of nine females collected and measured during this study (Table 32) are similar to those obtained from type material published in Kreiter et al.(2002) and from specimen females collected by Ferragut and Baumann (2019). Several males have been collected during this study but a male collected for the first time by Ferragut and Baumann (2019) in Mauritius was described before submission of this paper and the description of males collected during this study. Dimensions of eight additional male specimens (Table 32) agree well with those of the single specimen male collected by Ferragut and Baumann (2019), except slightly shorter z3, st1-st5 length, st5-st5 length, and both fixed and movable digit lengths of the chelicerae and longer spermatodactyl in La Réunion Island specimens.
Phytoseius intermedius Evans & Macfarlane
Phytoseius (Dubininellus) intermedius Evans & Macfarlane 1961: 587; Denmark 1966: 70; Gupta 1977: 636.
Phytoseius (Phytoseius) intermedius, Ehara 1972: 170; Prasad 1974: 171; Ehara 1975: 27; Blommers 1976: 82; Moraes et al. 1986: 222, 2004a: 242; Chant & McMurtry 2007: 129.
Phytoseius( Phytoseius) yira Pritchard & Baker 1962: 227 (synonymy according to Denmark 1966).
This species belongs to the horridus species group as setae J2 and R1 are absent (Chant and McMurtry 1994). It was recorded in several countries of Asia, Africa, Madagascar (Demite et al. 2019) and was already known from La Réunion (Quilici et al. 2000; Demite et al. 2019). If all details of collections were provided in previous papers, measurements of specimens collected and identified were not given. Measurements of specimens collected during this study are provided in table 33. The biology of this species remains totally unknown.



Specimens examined: 7 ♀♀ + 1 im. in total, 5 ♀♀ measured. Saint-Joseph – Manapany, SCEA Multiplantes (aasl 404 m, Long 55°35'37'' E, Lat 21°22'9'' S), 2 ♀♀ + 1 im. on Mangifera indica L., 1 ♀ on Rivina humilis L., 14/2/2017; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 2 ♀♀ on A. viridis, 1 ♀ on A. heterophyllus, 27/2/2017; 1 ♀ on L. leucocephala, 30/3/2017.
Remarks: measurement values of morphological characters of specimens from La Réunion (Table 33) and specimens from neighbouring countries are very close, especially for specimens from Africa. Some values for specimens from Asia are slightly lower.
Phytoseius punicae Chinniah & Mohanasundaram
Phytoseius punicae Chinniah & Mohanasundaram, 2001: 526.
This species belongs to the plumifer species group as setae J2 and R1 are present (Chant and McMurtry 1994).
This species was recorded in India, in Tamil Nadu state (Demite et al. 2019). Its biology is totally unknown.
This is the first record of this species in La Réunion Island.
Specimens examined: A single ♀ in total, measured. Le Tampon – Bras de Pontho, Aldo Grace farm (aasl 661 m, Long 55°29'48" E, Lat 21°14'33" S), 1 ♀ on D. incanum, 21/2/2017.
Remarks: measurement values of morphological characters (Table 34) were compared to those obtained for specimens of India, the only country in which Phytoseius punicae was recorded and specimens measured. Values of measurements are very close except for seta j3, z3 and st1-st3 length of sternal shield that are smaller compared to specimens of India. Macrosetae StIV is however longer in the single specimen of La Réunion Island.



Phytoseius woodburyi De Leon
Phytoseius (Phytoseius) woodburyi De Leon 1965b: 130; Muma & Denmark 1968: 236; Kreiter & Moraes 1997: 380.
Phytoseius (Dubininellus) woodburyi, Denmark 1966: 64.
Phytoseius woodburyi, Moraes et al. 1986: 229; 2004a: 258; Chant & McMurtry 2007: 131.
This species belongs to the horridus species group as setae J2 and R1 are absent (Chant and McMurtry 1994).
This species was recorded in several countries of the Caribbean area, South America, Hawaii and India (Demite et al. 2019). Its biology is totally unknown.
This is the first record of this species in La Réunion.
Specimens examined: 2 ♀♀ in total, both measured. Montvert-les-Hauts – EARL Le Mont Vert farm (aasl 582 m, Long 55°32'19'' E, Lat 21°19'42'' S), 1 ♀ on C. annuum, 15/5/2015; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 1 ♀ on D. acutangula, 23/6/2016.
Remarks: measurements of morphological characters of P. woodburyi female specimens from La Réunion (Table 35) are very close from measurements for specimens from other countries, even very far from Indian Ocean. The only two specimens of La Réunion Island have however some longer setae, especially s4, Z4, and macrosetae SgeIV.



Genus Platyseiella Muma
Platyseiella Muma 1961a: 280; Chant 1965a: 370; Muma & Denmark 1970: 56; Chant & McMurtry 1994: 233; 2007: 131.
Amblyseius (Platyseiella) Muma, van der Merwe 1968: 168.
Phytoseius (Platyseiella) Muma, Wainstein 1970: 1726.
Proprioseiopsis (Platyseiella) Muma, Karg 1983: 302.
Platyseiella eliahui Ueckermann
Platyseiella eliahui Ueckermann 1992: 20; Moraes et al. 2004a: 259; Chant & McMurtry 2007: 131.
This species belongs to the platypilis species group as setae r3 are inserted on the dorsal shield and setae JV1 are off the ventrianal shield (Chant and McMurtry 1994). It was only recorded from South Africa (Demite et al. 2019). Its biology is totally unknown.
This is the first record of this species in La Réunion Island.
Specimens examined: 2 ♀♀ in total, both measured. Le Tampon – Grand Tampon, Janick Bénard farm (aasl 1100 m, Long 55°34'12" E, Lat 21°16'48" S), 2 ♀♀ on P. aquilinum, 7/1/2016.
Remarks: measurements of morphological characters (Table 36) agree well with those obtained for specimens from South Africa and Zambia.
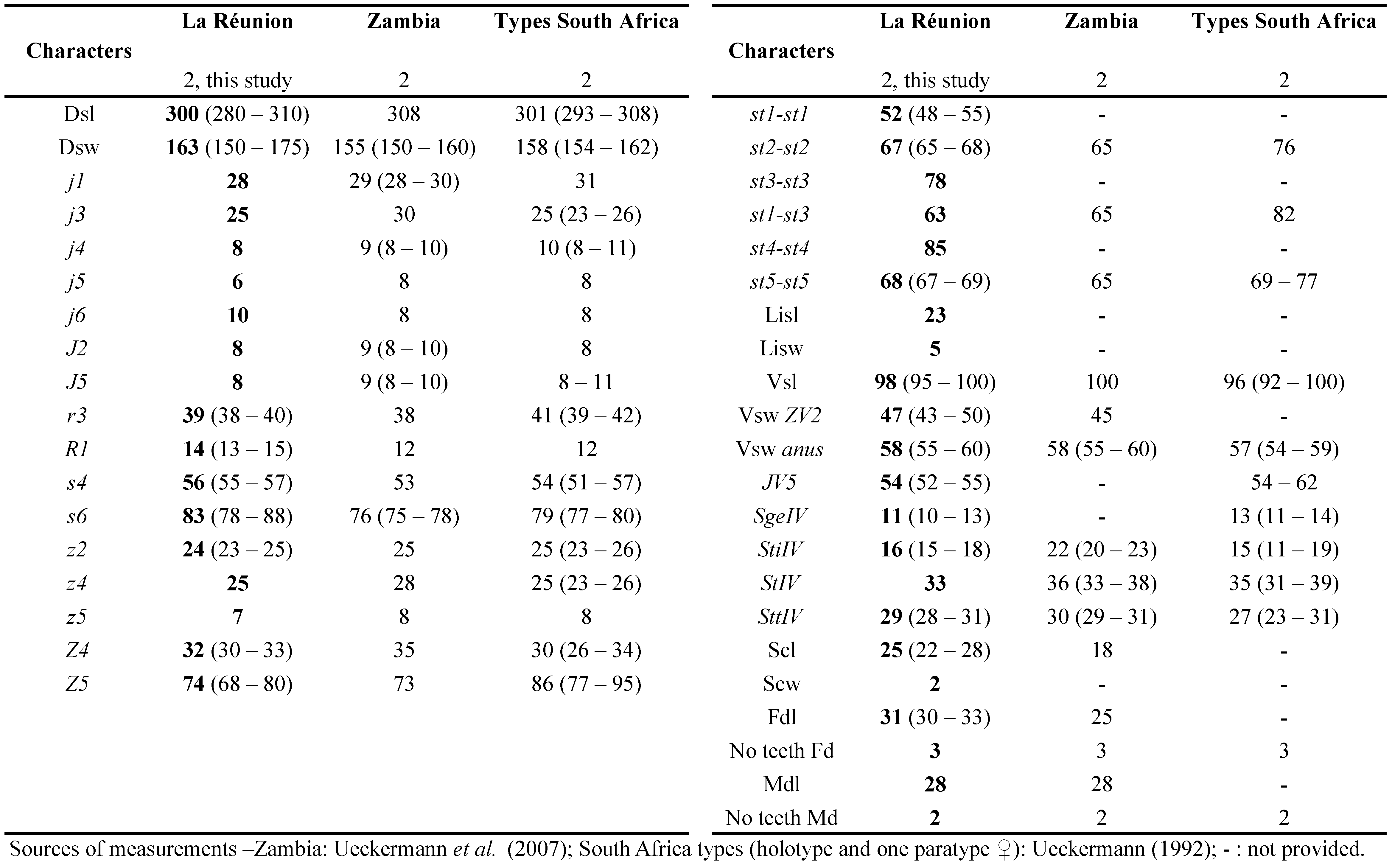


Sub-family Typhlodrominae Wainstein
Typhlodromini Wainstein 1962: 26; Typhlodrominae, Chant & McMurtry 1994: 235.
Tribe Paraseiulini Wainstein
Paraseiulini Wainstein 1976: 697; Chant & McMurtry 1994: 243; 2007: 141.
Genus Kuzinellus
Kuzinellus Wainstein 1976: 699.
Paraseiulus (Kuzinellus), Karg 1983: 322.
Typhlodromus ecclesiasticus group, Chant & Yoshida-Shaul 1986: 447.
Paraseiulus Muma 1961: 299 (part).
Kuzinellus scytinus (Chazeau)
Typhlodromus scytinus Chazeau 1970: 6.
Paraseiulus scytinus, Moraes et al. 1986: 206.
Kuzinellus scytinus Moraes et al. 2004a: 274; Chant & McMurtry 2007: 144.
This species belongs to the ecclesiasticus species group that contains 36 species (Chant and McMurtry 20017) as setae of the dorsal shield are setiform.
This species was recorded in Burundi, Rwanda, Australia, Madagascar (Demite et al. 2019) and it was already recorded in previous survey (Quilici et al. 2000; Demite et al. 2019). If all details of collections were provided in previous papers, measurements of specimens collected and identified were not given. Measurements of specimens collected during this study are provided in table 37. The biology of this species remains totally unknown.



Specimens examined: 2 ♀♀ in total, both measured. Le 19e– Plaine des Caffres, JL Robert farm (aasl 1000 m, Long 55°32'9'' E, Lat 21°14'16'' S), 1 ♀ on E. sonchifolia, 15/12/2015; Le Tampon – Bras de Pontho, Aldo Grace farm (aasl 661 m, Long 55°29'48" E, Lat 21°14'33" S), 1 ♀ on Ageratina riparia (Regel) R.M.King & H.Rob., 21/2/2017.
Remarks: measurement values s (Table 37) for 2 females of K. scytinus from La Réunion Island collected during this study are very similar from those obtained for females from Madagascar in the original description of Chazeau (1970) and from females collected from Burundi and Rwanda (Moraes et al. 2008).
Tribe Typhlodromini Wainstein
Typhlodromus Scheuten, Evans 1953: 449.
Typhlodromus (Typhlodromus), Chant 1957c: 528.
Typhlodromini Wainstein 1962b: 26; Chant & McMurtry 1994: 246; 2007: 144.
Genus Typhlodromus (Anthoseius) Scheuten
Typhlodromus (Anthoseius) De Leon, van der Merwe 1968: 20; Karg 1982: 194; Chant & McMurtry 1994: 250; 2007: 149.
Typhlodromus (Anthoseius) ndibu Pritchard & Baker
Typhlodromus ndibu Pritchard & Baker 1962: 221.
Amblydromella ndibu, Moraes et al. 1986: 168; Denmark & Welbourn 2002.
Typhlodromus (Anthoseius) ndibu, Moraes et al. 2004a: 339; Chant & McMurtry 2007: 155.
This species belongs to the large rhenanus species group as setae S4, JV3 and JV4 are present, the setae on dorsal shield are setiform, approximately equal in length except Z4/Z5, setae r3 and R1 are inserted on lateral integument and setae on dorsal shield in the z-Z and s-S series shorter than distances between their bases.
In the literature, Pritchard described this species and Baker (1962) from Congo and Rwanda, recorded than from Nigeria (Matthysse and Denmark 1981), from Indonesia (Oomen 1982), from Kenya (El-Banhawy et al. 2009) and our specimens are very similar to the original description based on drawings. This species has very original characters.
Surprisingly, no measurements were given in all papers mentioning this species, even the original description. Measurements are given in the table 38 for the first time for the three adult female specimens and for the single adult male specimen.
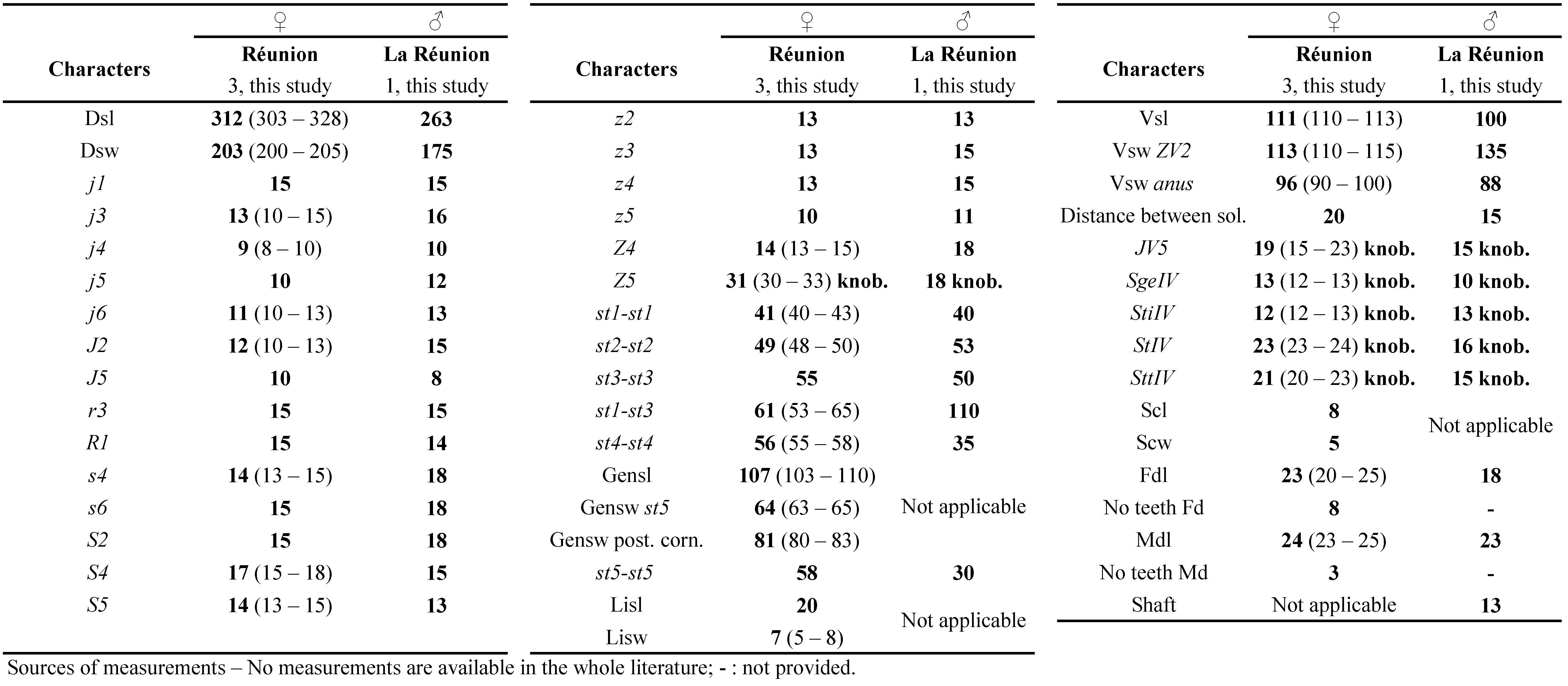


This species if mentioned for the first time from La Réunion, from primary forest of medium altitude. Its biology is totally unknown.
Specimens examined: 3 ♀♀ + 1 ♂ in total, all measured. Forêt de Bélouve – Gite (aasl 1500m, Long 55°33'36" E, Lat 21°6'0" S), 3 ♀♀ on C. borbonica, 28/1/2017; Forêt de Bélouve – Trou de fer (aasl 1300 m, Long 55°33'36" E, Lat 21°2'24" S), 1 ♂ on A. heterophylla, 28/1/2017.
Remarks: this species has short subequal setae except Z5 in females which are around the double length of all other dorsal setae. The ventrianal shield of the female quadrangular, almost square, and as large as wide. Setae Z5, JV5 and the four macrosetae of the leg IV are knobbed (Table 38).
Typhlodromus (Anthoseius) transvaalensis (Nesbitt)
Kampimodromus transvaalensis Nesbitt 1951: 55.
Neoseiulus transvaalensis, Muma 1961: 295.
Clavidromus transvaalensis, Muma & Denmark 1968: 238; Muma & Denmark 1970: 128; Moraes et al. 1986: 182.
Typhlodromus transvaalensis, Chant & Baker 1965: 5; Schicha 1981a: 36; Moraes et al. 2004a: 355; Chant & McMurtry 1994: 252, 2007: 157.
Typhlodromus jackmickleyi, De Leon 1958: 75; van Der Merwe 1968: 23 (synonymy according to Muma & Denmark 1968).
Typhlodromus pectinatus, Athias-Henriot 1958a: 179 (synonymy according to Muma & Denmark 1968).
This species has elongate serrated dorsal setae, setae Z1 and JV3 absent, an elongate calyx of the spermatheca, leg IV with 3 macrosetae and few teeth on chelicerae. It belongs to the transvaalensis species group of the subgenus Anthoseius of the genus Typhlodromus (Chant and McMurtry 1994).
According to McMurtry et al. (2013), T. transvaalensis is a type III phytoseiid and a generalist predator that feeds on mites, insects and pollen. It completed its life cycle when fed on the eriophyid mites Eriophyes dioscoridis Soliman and Abou-Awad and Eriophyes olive Zaher and Abou-Awad, eggs of the scale insect Parlatoria zizyphus (Lucas) and pollen of R. communis in experimental conditions. The percentage of individuals attaining maturity was less than 20% when nymphs of the tetranychid mite, T. urticae Koch, were provided. The development was faster and reproduction was higher when T. transvaalensis fed on eriophyid mites. T. urticae was an unsuitable feeding and reproduction substrate. The daily reproduction was as low as 0.4 and 0.8 egg/ female/ day when females were maintained on pollen grains of R. communis and eggs of P. zizyphus. The adult female daily consumed 126, 97 and 6 individuals of E. olivi, E. dioscoridis and T. urticae, respectively (Momen and Hussein 1999). Adult female T. transvaalensis were more efficient at predating all stages of P. latus (Banks) than Tetranychus bastosi Tuttle, Baker and Sales. The T. transvaalensis life cycle was shorter with diets including R. communis pollen, but Zea mays pollen was also suitable for reproduction. The results indicate that T. transvaalensis is a generalist predator with high potential for controlling P. latus in Jatropha curcas plantations and that the presence of R. communis and Z. mays crops boosts its development and reproduction (Canarte et al. 2017). This species is widely distributed all over the world (Demite et al. 2019). It was already recorded from La Réunion in previous surveys (Quilici et al. 2000). If all details of collections were provided in the previous paper, measurements of specimens collected and identified were not given. Measurements of specimens collected during this study are provided in table 39.



Specimens examined: 70 ♀♀ + 4 im. in total, 20 ♀♀ measured. Saint-Paul – Savannah (aasl 61 m, Long 55°29'43'' E, Lat 21°20'41'' S), 5 ♀♀ in Frankliniothrips sp. rearings, 16/6/2015; Petite Île – Les bas, Doris Morel farm (aasl 230 m, long 55°34'1" E, lat 21°21'22" S), 1 ♀ on P. lanceolata, 31/5/2016; Saint-Pierre – Bassin-Plat CIRAD Research Station (aasl 153 m, Long 55°29'18'' E, Lat 21°19'25'' S), 1 ♀ in plot CC, 16/8/2016; 1 ♀ in plot BM, 23/8/2016; Petite Île – Piton Bloc, Yébo Luguy farm (aasl 973 m, Long 55°34'64'' E, Lat 21°18'64'' S), 1 ♀ on B. catharticus, 18/10/2016; Saint-Pierre – Ligne Paradis, La Coccinelle Inc. (aasl 164 m, Long 55°28'59'' E, Lat 21°18'55'' S), 22 ♀♀ + 3 im. in rearings of beneficial insects, 22/2/2017; 4 ♀♀ in rearings of beneficial insects, 11/6/2017; 32 ♀♀ in rearings of beneficial insects, 1/8/2017; 3 ♀♀ + 1 im. in rearings of beneficial insects, 11/8/2017.
Remarks: all measurement values (Table 39) agree well with those already published on this species with only very slight variations. Measurement values of female specimens of La Réunion are very similar with values for specimens of Kenya and South Africa. Despite the large number of females collected, sometimes from a single location, we did not find any males in our study, suggesting that this predatory mite can reproduce through thelytokous parthenogenesis, as observed by Kishimoto (2015).
Conclusion
Before this 10th paper, the fauna of Phytoseiidae of La Réunion Island was limited to 33 recorded species including 24 Amblyseiinae, 5 Phytoseiinae and 4 Typhlodrominae, among which 8 species that had been described as new.
This paper reports on results of surveys done recently (2015-2018) and add 19 newly recorded species among which 3 are new to Science and 21 already known species but with additional data. The number of species for La Réunion Island now reached to 52 among which 11 have been described only from this Island (P. haroldi was found in Mauritius recently).
Among these 19 species newly recorded, at least nine are already well known as biological control agents (BCA). This must be underlined as new regulations on importation of macro-organisms are proposed in a lot of countries and specifically for countries like France that have very far over-sea territories. Therefore, it is impossible to import and of course to sell and use exotic species if they are not indigenous in La Réunion Island that is considered as a territory. An importation permit must be requested, but it is expensive and chances to obtain are generally very low (Kreiter et al. 2016). The knowledge of the biodiversity, especially of efficient biological control agents from overseas territories, not only for fundamental biodiversity knowledge or conversation purposes but also for agricultural and economical ones, is so of a considerable importance.
Acknowledgements
Acknowledgements are due to the institution that have granted travel and accommodations to the senior author in La Réunion during more than 3 months: Montpellier SupAgro, CIRAD (UMR PVBMT and UR Hortsys), Armeflhor and La Coccinelle.
Part of the present study was realised within the framework of the project Agrum'Aide (2014-2018) managed by CIRAD (the leader was Fabrice Le Bellec). This study was financially supported by the French Agency of Biodiversity (formerly ONEMA), in the framework of the tender `Biodiversité-Ecophyto'.
Many thanks are also due to FDGDON La Réunion (23 Rue Jules Thirel – Cour de l'Usine de Savannah, 97460 Saint Paul), ANSES (Laboratoire de la Santé des Végétaux, Unité Ravageurs et Agents Pathogènes tropicaux, Site de La Réunion, 7 Chemin de l'Irat, 97410 Saint-Pierre), and UMR PVBMT (same address) which have provided with several samples.
We are grateful to the growers for permission to collect in their farms: Pierre and Ingrid Avril, EARL Montvert in Le Montvert-les-Hauts, Jean-Louis Robert, in Le 19e, Plaine des Caffres, Janick Bénard in Le Tampon, Grand Tampon, Yebo Lugy in Petite-Île, Piton-Bloc, Doris Morel in Petite-Île, Les Bas, Delaunay Jean-Max in Vincendo and Aldo Grace in Le Tampon, Bras de Pontho.
Huge thanks to Maëva Labouyrie, Inès Seka, András Kovács, and Hélder Silva, students of Master of Montpellier SupAgro, who have worked enthusiastically for a master project with the senior author on the material of La Réunion Island from March to July 2018.
We are very grateful to Professor Gilberto J. de Moraes (University of Sao Paulo, Escuala Superior de Agricultura Luiz de Queiroz, Departamento Entomologia and Acarologia, Piracicaba, Brasil) for organising a loan of the holotype, paratypes and some additional materials of Neoseiulus recifensis Gondim Jr and Moraes and Drs Danuta Knihinicki and Lauren Drysdale (NSW Department of Primary Industries, Orange Agricultural Institute, Biosecurity Collections, Orange NSW, Australia) for organising a loan of the holotype of Neoseiulus houstoni (Schicha).
I would like also to thank my colleague Professor Marie-Stéphane Tixier who have examined the synonymy of the three species of Neoseiulus with a polytomous key we are developing in UMR CBGP under her supervision and has reached the same conclusion than the senior author on the synonymy of the three species impossible to distinguish on the basis of morphological characters.
We are finally very grateful to Professor Francisco Ferragut who has given his opinion on specimens of Amblyseius neoankaratrae from la Réunion Island after the publication of his paper on Phytoseiidae of Mauritius (2019) in which this species is mentioned and thanks to whom this paper was greatly improved.
I finally would like to thank hearthfully the two reviewers for very valuable constructive comments and great improvements of this paper.
References
Abo-Shnaf R.I.A., Moraes G.J. de 2014. Phytoseiid mites (Acari: Phytoseiidae) from Egypt, with new records, descriptions of new species, and a key species. Zootaxa, 3865: 1-71. doi:10.11646/zootaxa.3865.1.1 ![]()
Abo-Shnaf R.I.A., Sánchez L., Moraes G.J. de 2016. Plant inhabiting Gamasina mites (Acari: Mesostigmata) from the Dominican Republic, with description of four new species of Lasioseius (Blattisociidae) and complementary description of other species. Syst. Appl. Acarol., 21(5): 607-646. doi:10.11158/saa.21.5.5 ![]()
Akyazi R., Ueckermann E.A., Soysal M. 2016. The new distribution of Amblyseius herbicolus in Turkey (Parasitiformes, Phytoseiidae) with a key of Amblyseius species found in Turkey. Acarologia, 56(2): 237-244. doi:10.1051/acarologia/20162241 ![]()
Amitai S., Swirski E. 1966. Illustrations of spermathecae in several previously described phytoseiid mites (Acarina) from Hong Kong and Israel. Isr. J. Agric. Res., 16: 19-24.
Athias-Henriot C. 1957. Phytoseiidae et Aceosejidae (Acarina, Gamasina) d'Algérie. I. Genres Blattisocius Keegan, Iphiseius Berlese, Amblyseius Berlese, Phytoseius Ribaga, Phytoseiulus Evans. Bul. Soc. Hist. Nat. Afrique du Nord, 48: 319-352.
Athias-Henriot C. 1958. Phytoseiidae et Aceosejidae (Acarina: Gamasina) d'Algérie. II. Phytoseiidae. Clé des genres Amblyseius Berlese (Suite) et Seiulus Berlese. Bul. Soc. Hist. Nat. Afrique du Nord, 49: 23-43.
Athias-Henriot C. 1961. Mésostigmates (Urop. excl.) édaphiques Méditerranéens (Acaromorpha, Anactinotrichida). Acarologia, 3: 381-509.
Athias-Henriot C. 1966. Contribution à l'étude des Amblyseius paléarctiques (Acariens anactinotriches, Phytoseiidae). Bul. Scientif. Bourgogne, 24: 181-230.
Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. Le relevé organotaxique de la face dorsale adulte (Gamasides protoadéniques, Phytoseiidae). Acarologia, 17(1): 20-29.
Athias-Henriot C. 1977. Nouvelles notes sur les Amblyseiini. III. Sur le genre Cydnodromus: Redéfinition, composition (Parasitiformes, Phytoseiidae). Entomophaga, 22: 61-73. doi:10.1007/BF02372991 ![]()
Auger P., Tixier M.-S., Kreiter S., Fauvel G. 1999. Factors affecting ambulatory dispersal in the predaceous mite Neoseiulus californicus. Exp. Appl. Acarol., 23: 235-250. doi:10.1023/A:1006019014708 ![]()
Beaulieu F., Beard J.J. 2018. Acarine biocontrol agents Neoseiulus californicus sensu Athias-Henriot (1977) and N. barkeri Hughes (Mesostigmata: Phytoseiidae) redescribed, their synonymies assessed, and the identity of N. californicus (McGregor) clarified based on examination types. Zootaxa, 4500(4): 451-507. doi:10.11646/zootaxa.4500.4.1 ![]()
Beard J.J. 2001. A review of Australian Neoseiulus Hughes and Typhlodromips De Leon (Acari: Phytoseiidae: Amblyseiinae). Invert. Taxon., 15: 73-158. doi:10.1071/IT99017 ![]()
Berlese A. 1913. Systema Acarorum genera in familiis suis disposita. Acaroteca Italica, 1-2: 3-19.
Berlese A. 1914. Acari nuovi. Manipulus IX. Redia, 10: 113-150.
Bhattacharyya S.K. 1968. Two new phytoseiid mites from eastern India (Acarina: Phytoseiidae). J. Bombay Nat. Hist. Soc., 65(3): 677-680.
Blommers L. 1974. Species of the genus Amblyseius Berlese, 1914, from Tamatave, east Madagascar (Acarina: Phytoseiidae). Bul. Zool. Mus. Univ. Amster., 3: 143-155.
Blommers L. 1976. Some Phytoseiidae (Acarina: Mesostigmata) from Madagascar, with descriptions of eight new species and notes on their biology. Bijd. Dierk., 46(1): 80-106. doi:10.1163/26660644-04601005 ![]()
Blommers L., Chazeau J. 1974. Two new species of predator mites of the genus Amblyseius Berlese (Acarina: Phytoseiidae) from Madagascar. Zeit. Angew. Entomol., 75: 308-315. doi:10.1111/j.1439-0418.1974.tb01856.x ![]()
Boller H.F. 1984. Eine ainfache Ausschwemm-methode zur schellen Erfassung von Raumilben, Trips und anderen Kleinarthropoden imbWeinbau. Z. Obst- und Weinbau, 120: 249-255.
Broodsgaard H.F., Stengaard Hansen L. 1992. Effect of Amblyseius cucumeris and Amblyseius barkeri as Biological Control Agents of Thrips tabaci on Glasshouse Cucumbers. Biocont. Sc. Technol. 2(3): 215-223. doi:10.1080/09583159209355235 ![]()
Castagnoli M., Simoni S. 2003. Neoseiulus californicus (McGregor) (Acari Phytoseiidae): Survey of Biological and Behavioural Traits of a Versatile Predator. Redia, 86: 153-164.
Cañarte E., Sarmento R.A., Venzon M., Neto M.P., Ferreira D.F. Jr, Santos F.A., Pallini A. 2017. Suitability and nutritional requirements of the predatory mite Typhlodromus transvaalensis, a potential biological control agent of physic nut pest mites. Biol. Control, 115: 165-172. doi:10.1016/j.biocontrol.2017.10.008 ![]()
Cavalcante A.C.C., Demite P.R, Amaral F.S.R., Lofego A.C., Moraes G.J. de 2017. Complementary description of Neoseiulus tunus (De Leon) (Acari: Mesostigmata: Phytoseiidae) and observation on its reproductive strategy. Acarologia, 57(3): 591-599. doi:10.24349/acarologia/20174178 ![]()
Chant D.A. 1957. Descriptions of some phytoseiid mites (Acarina, Phytoseiidae). Part I. Nine new species from British Columbia with keys to the species of British Columbia. Part II. Redescriptions of eight species described by Berlese. Can. Entomol., 89(7): 289-308. doi:10.4039/Ent89289-7 ![]()
Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. Can. Entomol., 61(12): 1-166. doi:10.4039/entm9112fv ![]()
Chant D.A., Baker E.W. 1965. The Phytoseiidae (Acarina) of Central America. Mem. Entomol. Soc. Can., 41: 1-56. doi:10.4039/entm9741fv ![]()
Chant D.A., McMurtry J.A. 1994. A review of the subfamilies Phytoseiinae and Typhlodrominae (Acari: Phytoseiidae). Intern. J. Acarol., 20(4): 223-310. doi:10.1080/01647959408684022 ![]()
Chant D.A., McMurtry J.A. 2003a. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part I. Neoseiulini new tribe. Intern. J. Acarol., 29(1): 3-46. doi:10.1080/01647950308684319 ![]()
Chant D.A., McMurtry J.A. 2003b. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part II. The tribe Kampimodromini Kolodochka. Intern. J. Acarol., 29(3): 179-224. doi:10.1080/01647950308684331 ![]()
Chant D.A., McMurtry J.A. 2004. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part III. The tribe Amblyseiini Wainstein, subtribe Amblyseiina n. subtribe. Intern. J. Acarol., 30(3): 171-228. doi:10.1080/01647950408684388 ![]()
Chant D.A., McMurtry J.A. 2005a. A review of the subfamily Amblyseiina Muma (Acari: Phytoseiidae): Part V. Tribe Amblyseiini, subtribe Proprioseiopsina Chant and McMurtry. Intern. J. Acarol., 31(1): 3-22. doi:10.1080/01647950508684412 ![]()
Chant D.A., McMurtry J.A. 2005b. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae) Part VI. The tribe Euseiini n. tribe, subtribes Typhlodromalina n. subtribe, Euseiina n. subtribe, and Ricoseiina n. subtribe. Intern. J. Acarol., 31(3): 187-224. doi:10.1080/01647950508684424 ![]()
Chant D.A., McMurtry J.A. 2005c. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae) Part VII. Typhlodromipsini n. tribe. Intern. J. Acarol., 31(4): 315-340. doi:10.1080/01647950508683673 ![]()
Chant D.A., McMurtry J.A. 2006a. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part VIII. The tribes Macroseiini Chant, Denmark and Baker, Phytoseiulini n. tribe, Afroseiulini n. tribe and Indoseiulini Ehara and Amano. Intern. J. Acarol., 32(1): 13-25. doi:10.1080/01647950608684439 ![]()
Chant D.A., McMurtry J.A. 2006b. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part IX. An overview. Intern. J. Acarol., 32(2): 1-27. doi:10.1080/01647950608684453 ![]()
Chant D.A., McMurtry J.A. 2007. Illustrated keys and diognoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, 219 pp.
Chant D.A., Yoshida-Shaul E. 1986. A world review of the ecclesiasticus species group in the genus Typhlodromus Scheuten (Acarina: Phytoseiidae). Can. J. Zool., 64(2): 447-466. doi:10.1139/z86-069 ![]()
Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Intern. J. Acarol., 17(3): 187-199. doi:10.1080/01647959108683906 ![]()
Chant D.A., Yoshida-Shaul E. 1992. Adult idiosomal setal patterns in the family Phytoseiidae (Acari: Gamasina). Intern. J. Acarol., 18(3): 177-193. doi:10.1080/01647959208683949 ![]()
Chaudhri W.M. 1968. Six new species of mites of the genus Amblyseius (Phytoseiidae) from Pakistan. Acarologia, 10: 550-562.
Chazeau J. 1970. Typhlodromus scytinus, n. sp., nouveau phytoseiide de Madagascar (Acariens, Gamasides, Phytoseiidae). Cahiers ORSTOM, Série Biologie, 12: 3-14.
Chinniah C., Mohanasundaram M. 2001. Five new predatory mites (Acarina: Phytoseiidae) from Kerala, India. Entomon, 26(1): 65-77.
Corpuz L.A., Rimando L. 1966. Some Philippine Amblyseiinae (Phytoseiidae: Acarina). Philip. Agric., 50: 114-136.
Daneshvar H., Denmark H.A. 1982. Phytoseiids of Iran (Acarina: Phytoseiidae). Intern. J. Acarol., 8(1): 3-14. doi:10.1080/01647958208683272 ![]()
De Leon D. 1957 Three new Typhlodromus from southern Florida (Acarina: Phtyoseiidae). Fla Entomol., 40: 141-144. doi:10.2307/3492041 ![]()
De Leon D. 1958. Four new Typhlodromus from southern Florida (Acarina: Phytoseiidae). Fla Entomol., 41(2): 73-76. doi:10.2307/3492363 ![]()
De Leon D. 1965. A note on Neoseiulus Hughes 1948 and new synonymy (Acarina: Phytoseiidae). Proceed. Entomol. Soc. Washington, 67(1): 23.
De Leon D. 1966. Phytoseiidae of British Guyana with keys to species (Acarina: Mesostigmata). Stud. Fauna Suriname and other Guyanas, 8: 81-102.
De Leon D. 1967. Some mites of the Caribbean Area. Part I. Acarina on plants in Trinidad, West Indies. Allen Press Inc., Lawrence, Kansas, USA: 1-66.
Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2019. Phytoseiidae Database. Available from: www.lea.esalq.usp.br/phytoseiidae (last access 01/08/2019).
Denmark H.A. 1966. Revision of the genus Phytoseius Ribaga, 1904 (Acarina: Phytoseiidae). Fla Dep. Agric. Bul., 6: 1-105.
Denmark H.A., Evans G.A. 2011. Phytoseiidae of North America and Hawaii (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, USA, 451 pp.
Denmark H.A., Evans G.A., Aguilar H., Vargas C., Ochoa R. 1999. Phytoseiidae of Central America (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, Michigan, USA, 125 pp.
Denmark H.A., Muma M.H. 1973. Phytoseiid mites of Brazil (Acarina: Phytoseiidae). Rev. Bras. Biol., 33(2): 235-276.
Denmark H.A., Muma M.H. 1989. A revision of the genus Amblyseius Berlese, 1914 (Acari: Phytoseiidae). Occas. Pap. Fla State Coll. Arthropods, USA, 4, 149 pp.
Denmark H.A., Welbourn W.C. 2002. Revision of the genera Amblydromella Muma and Anthoseius De Leon (Acari: Phytoseiidae). Intern. J. Acarol., 28(4): 291-316. doi:10.1080/01647950208684308 ![]()
Dosse G. 1958. Uber einige neue Raubmilbenarten (Acari: Phytoseiidae). Pflanzen. Ber., 21: 44-61.
Duarte M.V.A, Venzon M., Bittencourt M.C.de S., Rodriguez-Cruz F.A., Pallini A., Janssen A. 2015. Alternative food promotes broad mite control on chilli pepper plants. BioControl, 60: 817-825. doi:10.1007/s10526-015-9688-x ![]()
Easterbrook M.A., Fitzgerald J.D., Solomon M.G. 2001. Biological control of strawberry tarsonemid mite Phytonemus pallidus and two-spotted spider mite Tetranychus urticae on strawberry in the UK using species of Neoseiulus (Amblyseius) (Acari: Phytoseiidae). Exp. Appl. Acarol., 25: 25-36
Ehara S. 1958. Three predatory mites of the genus Typhlodromus from Japan (Phytoseiidae). Annot. Zool. Japon., 31: 53-57.
Ehara S. 1964. Some mites of the families Phytoseiidae and Blattisocidae from Japan (Acarina: Mesostigmata). J. Fac. Science Hokkaido Univ., Ser. VI, Zoology, 15(3): 378-394.
Ehara S. 1966. A tentative catalogue of predatory mites of Phytoseiidae known from Asia, with descriptions of five new species from Japan. Mushi, 39: 9-30.
Ehara S. 1967. Phytoseiid mites from Okinawa Island (Acarina: Mesostigmata). Mushi, 40(6): 67-82.
Ehara S. 1972. Some phytoseiid mites from Japan, with descriptions of thirteen new species (Acarina: Mesotigmata). Mushi, 46(12): 137-173.
Ehara S. 1975. List and keys to Phytoseiidae of Japan. In: Yasumatsu, K. and Mori, H. (Eds.), JIBP Synthesis, 7, Approaches to biological control, University of Tokyo Press, Japan: 25-37.
Ehara S. 2002. Some phytoseiid mites (Arachnida: Acari: Phytoseiidae) from west Malaysia. Species Div., 7: 29-46. doi:10.12782/specdiv.7.29 ![]()
Ehara S., Amano H. 1998. A revision of the mite family Phytoseiidae in Japan (Acari: Gamasina), with remarks on its biology. Species Div., 3(1): 25-73. doi:10.12782/specdiv.3.25 ![]()
Ehara S., Amano H. 2002. Some Japanese phytoseiid mites (Acari: Phytoseiidae) mostly from Ishigaki and Taketomi Islands. Entomol. Sc., 5(3): 321-329.
Ehara S., Amano H. 2004. Checklist and keys to Japanese Amblyseiinae (Acari: Gamasina: Phytoseiidae). J. Acarol. Soc. Japan, 13(1): 1-30. doi:10.2300/acari.13.1 ![]()
Ehara S., Bhandhufalck A. 1977. Phytoseiid mites of Thailand (Acarina: Mesotigmata). J. Fac. Educ. Tottori University, Nat. Sc., 27(2): 43-82.
Ehara S., Kishimoto H. 2007. The occurrence of Typhlodromus (Anthoseius) transvaalensis (Nesbitt) (Acari: Phytoseiidae) in Japan. J. Acarol. Soc. Japan, 16(2): 139-143. doi:10.2300/acari.16.139 ![]()
Ehara S., Okada Y., Kato H. 1994. Contribution to the knowledge of the mite family Phytoseiidae in Japan (Acari: Gamasina). J. Fac. Educ. Tottori University, Nat. Sc., 42(2): 119-160.
El-Banhawy E.M. 1984. Description of some phytoseiid mites from Brazil (Acarina: Phytoseiidae). Acarologia, 25(2): 125-144.
El-Banhawy E.M., Irungu L., Mugo H. 2009. Survey of predacious mites (Acari: Phytoseiidae) inhabiting coffee trees in Kenya with description of some new species. Acarologia, 49(3-4): 121-137.
El-Banhawy E.M., Knapp M. 2011. Mites of the family Phytoseiidae Berlese from Kenya (Acari: Mesostigmata). Zootaxa, 2945: 1-176. doi:10.11646/zootaxa.2945.1.1 ![]()
Evans G.O. 1952. A new typhlodromid mite predaceous on Tetranychus bimaculatus Harvey in Indonesia. Ann. Mag. Nat. Hist., 5: 413-416. doi:10.1080/00222935208654311 ![]()
Evans G.O. 1953. On some mites of the genus Typhlodromus Scheuten, 1857, from S. E. Asia. Ann. Mag. Nat. Hist., 6: 449-467. doi:10.1080/00222935308654444 ![]()
Evans G.O., Macfarlane D. 1962. A new mites of the genus Phytoseius Ribaga (Acari: Mesostigmata). Ann. Mag. Nat. Hist., 13 (4): 587-588. doi:10.1080/00222936108651183 ![]()
Fadamiro H.Y., Xiao Y., Hargroder T., Nesbitt M., Childers C.C. 2009. Diversity and seasonal abundance of predacious mites in Alabama Satsuma citrus. Ann. Entomol. Soc. Am., 102 (4): 617-628. doi:10.1603/008.102.0406 ![]()
Famah Sourassou N., Hanna R., Zannou I., Moraes G.J. de, Breeuwer J.A.J., Sabelis M.W. 2012. Morphological, molecular and cross-breeding analysis of geographic populations of coconut-mite associated predatory mites identified as Neoseiulus baraki: evidence for cryptic species? Exp. Appl. Acarol., 57: 15-36. doi:10.1007/s10493-012-9534-0 ![]()
Famah Sourassou N., Hanna R., Zannou I., Moraes G.J. de, Negloh K., Sabelis M.W. (2011) Morphological variation and reproductive incompatibility of three coconut-mite-associated populations of predatory mites identified as Neoseiulus paspalivorus (Acari: Phytoseiidae). Exp. Appl. Acarol., 53: 323-338. doi:10.1007/s10493-010-9413-5 ![]()
Fan Y.Q., Petitt F.L. 1994a. Biological Control of Broad Mite, Polyphagotarsonemus latus (Banks), by Neoseiulus barkeri Hughes on Pepper. Biol. Cont., 4(4): 390-395. doi:10.1006/bcon.1994.1049 ![]()
Fan Y.Q., Petitt F.L. 1994b. Parameter estimation of the functional response. Environ. Entomol., 23: 785-794. doi:10.1093/ee/23.4.785 ![]()
Fernando L.C.P., Aratchige N.S., Peiris T.S.G. 2003. Distribution patterns of cocounut mite, Aceria guerreronis, and its predator Neoseiulus aff. paspalivorus in coconut palms. Exp. Appl. Acarol., 31: 71-78. doi:10.1023/B:APPA.0000005126.16574.3b ![]()
Ferragut F., Baumann J. 2019. New phytoeiid mites (Mesostigmata: Phytoseiidae) of Mauritius, with the description of two new species. Syst. Appl. Acarol., 24(5): 825-856. doi:10.11158/saa.24.5.8 ![]()
Ferragut F., Pérez Moreno I., Iraola V., Escudero A. 2010. Ácaros depredadores em las plantas cultivadas. Família Phytoseiidae. Ediciones Agrotécnicas, Madrid, 202 pp.
Furtado I.P., Kreiter S., Moraes G.J. de, Tixier M.-S., Flechtmann C.H.W., Knapp M. 2005. Plant mites (Acari) from Northeastern Brazil, with description of two new species of the family Phytoseiidae (Mesostigmata). Acarologia, 45(2-3): 131-143.
Furtado I.P., Moraes G.J. de, Kreiter S., Flechtmann C.H.W., Tixier M.-S., Knapp M. 2014. Plant inhabiting phytoseiid predators of Midwestern Brazil, with emphasis on those associated with the tomato red spider mite, Tetranychus evansi (Acari: Phytoseiidae, Tetranychidae). Acarologia, 54(4): 425-431. doi:10.1051/acarologia/20142138 ![]()
Garman P. 1958. New species belonging to the genera Amblyseius and Amblyseiopsis with keys to Amblyseius, Amblyseiopsis, and Phytoseiulus. Ann. Entomol. Soc. Amer., 51: 69-79. doi:10.1093/aesa/51.1.69 ![]()
Gondim Jr. M.G.C., Moraes G.J. de 2001. Phytoseiid mites (Acari: Phytoseiidae) associated with palm trees (Arecaceae) in Brazil. Syst. Appl. Acarol., 6: 65-94. doi:10.11158/saa.6.1.11 ![]()
Gomez-Moya C.A., Ferragut F. 2009. Spatial distribution pattern and efficacy of Neoseiulus californicus and Phytoseiulus persimilis (Acari: Phytoseiidae) in the control of red spider mites on vegetables under semi-field conditions. Bol. Sanid. Veget. Plagas, 35: 377-390.
Guanilo A.D., Moraes G.J. de, Knapp M. 2008a. Phytoseiid mites (Acari: Phytoseiidae) of the subfamily Amblyseiinae Muma from Peru, with description of four new species. Zootaxa, 1880: 1-47. doi:10.11646/zootaxa.1880.1.1 ![]()
\biblio{
Guanilo A.D., Moraes G.J. de, Toledo S., Knapp M. 2008b. Phytoseiid mites (Acari: Phytoseiidae) from Argentina, with description of a new species. Zootaxa, 1884: 1-35. doi:10.11646/zootaxa.1884.1.1 ![]() }
}
Gupta S.K. 1977. Some undescribed and little-known species of Amblyseius (Acarina: Phytoseiidae) from western and northern India. Ind. J. Acarol., 1: 28-37.
Gupta S.K. 1981. Phytoseiidae (Acari: Mesostigmata) from Jammu and Kashmir, India, with descriptions of five new species. Ind. J. Acarol., 5: 37-49.
Gupta S.K. 1986. Fauna of India (Acari: Mesostigmata) Family Phytoseiidae. Zoological Survey of India, Calcutta, India, 350 pp.
Gutierrez J., Etienne J. 1986. Les Tetranychidae de I'île de la Réunion et quelques-uns de leurs prédateurs. L'Agronomie Tropicale, 41(1): 84-91.
Hirschmann W. 1962. Gangystematik der Parasitiformes. Acarologie Schriftenreihe fur Vergleichende Milbenkunde, Hirschmann-Verlag, Furth/Bay, 5(5-6), 80 pp.+ 32 plates.
Ho C.C., Lo K.C. 1989. Contribution to the knowledge of the genus Paraphytoseius Swirski and Shechter (Acarina: Phytoseiidae) in Taiwan. J. Agric. Res. China, 38(1): 88-99.
Hughes A.M. 1948. The mites associated with stored food products. Ministry of Agriculture and Fisheries, H. M. Stationary Office, London, 168 pp.
Hughes A.M. 1961. The mites of stored food. Ministry of Agriculture, Fishery and Food Technical Bulletin, First Edition, London, 9: 1-287.
Huyen L.T., Tung N.D., Lan D.H., Chi C.V., De Clercq P., Dinh N.V. 2017. Life table parameters and development of Neoseiulus longispinosus (Acari: Phytoseiidae) reared on citrus red mite, Panonychus citri (Acari: Tetranychidae) at different temperatures. Syst. Appl. Acarol., 22(9): 1316-1326. doi:10.11158/saa.22.9.3 ![]()
Kade N., Gueye-Ndiaye A., Duverney C., Moraes G.J. de 2011. Phytoseiid mites (Acari: Phytoseiidae) from Senegal. Acarologia, 51(1): 133-138. doi:10.1051/acarologia/20112001 ![]()
Karg W. 1970. Neue Arten der Raubmilbenfamilie Phytoseiidae Berlese, 1916 (Acarina: Parasitiformes). Deut. Entomol. Zeit. N. F., 17: 289-301. doi:10.1002/mmnd.4810170402 ![]()
Karg W. 1982. Diagnostic and systematics of predatory mites of the family Phytoseiidae Berlese in orchards. Zool. Jahrb. Syst., 109: 188-210.
Karg W. 1983. Systematische untersuchung der Gattungen und Untergattungen der Raubmilbenfamilie Phytoseiidae Berlese, 1916, mit der beschreibung von 8 neuen Arten. Mitt. Zool. Mus. Berlin, 59(2): 293-328. doi:10.1002/mmnz.4830590203 ![]()
Karg W. 1989. Neue Raubmilbenarten der Gattuig Proprioseiopsis Muma, 1961 (Acarina, Parasitiformes) mit Bestimmungsschlusseln. Zool. Jahrb. Syst., 116(2): 199-216.
Karg W. 1991. Die Raubmilbenarten der Phytoseiidae Berlese (Acarina) Mitteleuropas sowie angrenzender Gebiete. Zool. Jahrb. Syst., 118(1): 1-64.
Karg W., Oomen-Kalsbeek F. 1987. Neue Raubmilbenarten der Gattung Amblyseius Berlese (Acarina, Parasitiformes, Phytoseiidae) Antagonisten der unechten Spinnmilbe Brevipalpus phoenicis Geijskes. Zool. Jahrb. Syst., 114(1): 131-140.
Kaźmierski A. 1996. A revision of the subfamilies Pretydeinae and Tydeinae (Acari: Actinedida: Tydeidae). Part III. Seven new genera and some new species of the Tydeinae, with a generic key. Mitt. Hamburg. Zool. Mus. Inst., 93: 199-227.
Kennett C.E., Caltagirone L.E. 1968. Biosystematics of Phytoseiulus persimilis Athias-Henriot (Acarina: Phytoseiidae). Acarologia, 10(4): 563-577.
Kishimoto H. 2015. Development and oviposition of eight native phytoseiid species (Acari: Phytoseiidae) reared on eggs of the mediterranean flour Moth, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). J. Acarol. Soc. Japan, 24: 71-76. doi:10.2300/acari.24.71 ![]()
Kolodochka L.A. 1998. Two new tribes and the main results of a revision of Paleartic phytoseiid mites (Parasitiformes, Phytoseiidae) with the family system concept. Vest. Zool., 32(1-2): 51-63 [in Russian].
Kostiainen T., Hoy M.A. 1996. The Phytoseiidae as biological control agents of pest mites and insects. A bibliography. Monograph 17. University of Florida, Agricultural Experiment Station, Institute of Food and Agricultural Sciences, USA, 355 pp.
Kreiter S. Amiri K., Douin M. Bohinc T., Trdan S., Tixier M.-S. 2020a. Phytoseiid mites of Slovenia (Acari: Mesostigmata): new records and first description of the male of Amblyseius microorientalis. Acarologia 60(2): 203-242 doi:10.24349/acarologia/20204364 ![]()
Kreiter S., Bopp M.-C, Douin M., Nguyen D.T., Wyckhuys K. 2020b. Phytoseiidae of Vietnam (Acari: Mesostigmata) with description of anew species. Acarologia 60(1): 75-110. doi:10.24349/acarologia/20204362 ![]()
Kreiter S., Fontaine O., Payet R.-M. 2018a. New records of Phytoseiidae (Acari: Mesostigmata) from Mauritius. Acarologia, 58(4): 773-785. doi:10.24349/acarologia/20184273 ![]()
Kreiter S., Mailloux J., Tixier M.-S., Le Bellec F., Douin M., Guichou S., Etienne J. 2013. New phytoseiid mites of the French West Indies, with description of a new species, and new records (Acari: Mesostigmata). Acarologia, 53(3): 285-303. doi:10.1051/acarologia/20132095 ![]()
Kreiter S., Moraes G.J.de 1997. Phytoseiidae mites (Acari: Phytoseiidae) from Guadeloupe and Martinique. Fla Entomol., 80(3): 376-382. doi:10.2307/3495770 ![]()
Kreiter S., Payet R.-M., Fillâtre J., Abdou Azali H. 2018b. First records of Phytoseiidae from one island of the Comoros Archipelago. Acarologia, 58(3): 529-545. doi:10.24349/acarologia/20184256 ![]()
Kreiter S., Tixier M.-S., Etienne J. 2006. New records of phytoseiid mites (Acari: Mesostigmata) from the French Antilles, with description of Neoseiulus cecileae sp. nov. Zootaxa, 1294: 1-27. doi:10.11646/zootaxa.1294.1.1 ![]()
Kreiter S., Ueckermann E.A., Quilici S. 2002. Seven new phytoseiid species, with a new generic assignement and a key to the species of La Reunion Island (Acari: Mesostigmata). Acarologia, 42(4): 335-350.
Kreiter S., Vicente V., Tixier M.-S., Fontaine O. 2016a. An unexpected occurrence of Amblyseius swirskii Athias-Henriot in La Reunion Island (Acari: Phytoseiidae). Acarologia, 56(2): 175-181. doi:10.1051/acarologia/20162254 ![]()
Kreiter S., Vicente V., Tixier M.-S., Fontaine O., Avril I., Cottineau J.-S., Fillâtre J. 2016b. Présence inattendue d'Amblyseius swirskii Athias-Henriot à l'Île de La Réunion. Phytoma-La santé des végétaux, 695: 38-42.
Kreiter S., Zriki Z., Ryckewaert P., Pancarte C., Douin M., Tixier M.-S. 2018c. New phytoseiid mites of Martinique, with redescription of four species and new records. Acarologia, 58(2): 366-407. doi:10.24349/acarologia/20184248 ![]()
Lawson-Balagbo L.M., Gondim Jr. M.G.C., Moraes G.J. de, Hanna R., Schausberger P. 2008. Compatibility of Neoseiulus paspalivorus and Proctolaelaps bickleyi, candidate biocontrol agents of the coconut mite Aceria guerreronis: spatial niche use and intraguild predation. Exp. Appl. Acarol., 45: 1-13.
Lindquist E.E. 1994. Some observations on the chaetotaxy of the caudal body region of gamasine mites (Acari: Mesostigmata), with a modified notation for some ventrolateral body setae. Acarologia, 35: 323-326.
Lindquist E.E., Evans G.W. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entomol. Soc. Canada, 47: 1-64. doi:10.4039/entm9747fv ![]()
Lofego A.C., Demite P.R., Moraes G.J., Kishimoto R.G. 2009. Phytoseiid mites on grasses in Brazil (Acari: Phytoseiidae). Zootaxa, 2240: 41-59. doi:10.11646/zootaxa.2240.1.3 ![]()
Lombardini G. 1959. Acari Nuovi. XXXVII. Bol. Ist. Entomol. Agr. Univ. Palermo ed Osserv. Reg. Mal. Piante, 21: 163-167.
Luong T.H., Nguyen D.T., Dang H.L., Cao V.C., De Clercq P., Nguyen V.D. 2017. Life table parameters and development of Neoseiulus longispinosus (Acari: Phytoseiidae) reared on citrus red mite, Panonychus citri (Acari: Tetranychidae) at different temperatures. Syst. Appl. Acarol., 22(9): 1316-1326. doi:10.11158/saa.22.9.3 ![]()
Mailloux J., Le Bellec F., Kreiter S., Tixier M.-S., Dubois P. 2010. Influence of ground cover management on diversity and density of phytoseiid mites (Acari: Phytoseiidae) in Guadeloupean citrus orchards. Exp. Appl. Acarol., 52: 275-290. doi:10.1007/s10493-010-9367-7 ![]()
Massaro M., Montrazi M., Melo J.W.S., Moraes G.J. 2018. Production of Amblyseius tamatavensis with Thyreophagus crasentiseta (Acari: Phytoseiidae, Acaridae). Intern. J. Pest Manag. (in press).
Massaro M. Moraes G.J. de. 2019. Predation and oviposition potential of Brazilian populations of the predatory mite Amblyseius tamatavensis (Acari: Phytoseiidae) on eggs of Bemisia tabaci (Insecta: Hemiptera). Acarologia, 59(1): 120-128. doi:10.24349/acarologia/20194314 ![]()
Matthysse J.G., Denmark H.A. 1981. Some phytoseiids of Nigeria (Acarina: Mesostigmata). Fla Entomol., 64: 340-357. doi:10.2307/3494585 ![]()
McGregor E.A. 1954. Two new mites in the genus Typhlodromus (Acarina: Phytoseiidae). South. Calif. Acad. Sc. Bul., 53: 89-92.
McMurtry J.A., Badii M.H. 1989. Reproductive compatibility in widely separated populations of three species of phytoseiid mites (Acari: Phytoseiidae). Pan-Pacific Entomol., 65(4): 397-402.
McMurtry J.A., Croft B.A. 1997. Life-styles of phytoseiid mites and their roles in biological control. Ann. Rev. Entomol., 42: 291-321. doi:10.1146/annurev.ento.42.1.291 ![]()
McMurtry J.A., Moraes G.J.de 1984. Some phytoseiid mites from the South Pacific, with descriptions of new species and a definition of the Amblyseius largoensis species group. Intern. J. Acarol., 10: 27-37. doi:10.1080/01647958408683347 ![]()
McMurtry J.A., Moraes G.J.de 1989. Some phytoseiid mites from Peru with descriptions of four new species (Acari: Phytoseiidae). Intern. J. Acarol., 15(3): 179-188. doi:10.1080/01647958908683843 ![]()
McMurtry J.A., Moraes G.J. de 1991. Two new Phytoseiidae (Acari: Mesostigmata) from Zimbabwe with new records of other species. Intern. J. Acarol., 17(1): 21-27. doi:10.1080/01647959108683882 ![]()
McMurtry J.A., Moraes G.J. de, Sourassou N.F. 2013. Revision of the life styles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. doi:10.11158/saa.18.4.1 ![]()
McMurtry J.A., Sourassou N.F., Demite P.R. 2015. The Phytoseiidae (Acari: Mesostigmata) as Biological Control Agents. Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms: 133-149. doi:10.1007/978-3-319-15042-0\_5 ![]()
Mégevand B., Klay A., Gnanvossou D., Paraiso G. 1993. Maintenance and mass rearing of phytoseiid predators of the cassava green mite. Exp. Appl. Acarol., 17: 115-128.
Meyer M.K.P., Rodrigues M. da C. 1966. Acari associated with Cotton in Southern Africa. References to other plants. Garcia de Orta, Rev. Junta Investig., 13: 27-31.
Momen F., Hussein H. 1999. Relationships between food substances, developmental success and reproduction in Typhlodromus transvaalensis (Acari: Phytoseiidae). Acarologia, 40(2): 107-111.
Moraes G.J. de, Castro T.M.G. de, Kreiter S., Quilici S., Gondim Jr. M.G.C., Sá L.A. 2012. Search for natural enemies of Raoiella indica Hirst in Réunion Island (Indian Ocean). Acarologia, 52(2): 129-134. doi:10.1051/acarologia/20122043 ![]()
Moraes G.J. de, Kreiter S., Lofego A.C. 2000. Plant mites (Acari) of the French Antilles. 3. Phytoseiidae (Gamasida). Acarologia, 40(3): 237-264.
Moraes G.J. de, Lopes P.C., Fernando C.P. 2004b. Phytoseiid mite (Acari: Phytoseiidae) of coconut growing areas in Sri Lanka, with descriptions of three new species. J. Acarol. Soc. Japan, 13(2): 141-160. doi:10.2300/acari.13.141 ![]()
\biblio{
Moraes G.J. de, McMurtry J.A. 1983. Phytoseiid mites (Acarina) of northeastern Brazil with descriptions of four new species. Intern. J. Acarol., 9(3): 131-148. doi:10.1080/01647958308683326 ![]() }
}
Moraes G.J. de, McMurtry J.A., Denmark H.A. 1986. A catalog of the mite family Phytoseiidae. References to taxonomy, synonymy, distribution and habitat. EMBRAPA - DDT, Brasilia, Brazil, 353 pp.
Moraes G.J. de, McMurtry J.A., Denmark H.A., Campos C.B. 2004a. A revised catalog of the mite family Phytoseiidae. Zootaxa, 434: 1-494. doi:10.11646/zootaxa.434.1.1 ![]()
Moraes G.J. de, McMurtry J.A., van den Berg H., Yaninek J.S. 1989a. Phytoseiid mites (Acari: Phytoseiidae) of Kenya, with descriptions of five new species and complementary descriptions of eight species. Intern. J. Acarol., 15(2): 79-93. doi:10.1080/01647958908683829 ![]()
Moraes G.J. de, McMurtry J.A., Yaninek, J.S. 1989b. Some phytoseiid mites (Acari, Phytoseiidae) from tropical Africa with description of a new species. Intern. J. Acarol., 15(2): 95-102. doi:10.1080/01647958908683830 ![]()
Moraes G.J. de, Ueckermann E.A., Oliveira A.R., Yaninek J.S. 2001. Phytoseiidae mites of the genus Euseius (Acari: Phytoseiidae) from Sub-Saharan Africa. Zootaxa, 3: 1-70. doi:10.11646/zootaxa.3.1.1 ![]()
Moraes G.J. de, Zannou I.D., Oliveira A.R., Yaninek J.S., Hanna R. 2006. Phytoseiid mites of the subtribes Typhlodromalina and Euseiina (Acari: Phytoseiidae: Euseiini) from sub-Saharan Africa. Zootaxa, 1114: 1-52. doi:10.11646/zootaxa.1114.1.1 ![]()
\biblio{
Moraes G.J. de, Zannou I.D., Ueckermann E.A., Oliveira A.R., Hanna R., Yaninek J.S. 2008. Phytoseiid mites of the tribe Paraseiulini Wainstein (Acari: Phytseiidae) from sub-Saharan Africa. Zootaxa, 1687: 1-34. doi:10.11646/zootaxa.1687.1.1 ![]() }
}
Moraes G.J. de, Zannou I.D., Ueckermann E.A., Oliveira A.R., Hanna R., Yaninek J.S. 2007a. Species of the subtribes Arrenoseiina and Proprioseiopsina (Tribe Amblyseiini) and the tribe Typhlodromipsini (Acari: Phytoseiidae) from sub-Saharan Africa. Zootaxa, 1448: 1-39. doi:10.11646/zootaxa.1448.1.1 ![]()
Moraes G.J. de, Zannou I.D., Ueckerman E.A., Oliveira A.R., Yaninek J.S., Hanna R. 2007b. Phytoseiid mites of the tribes Afroseiulini, Kampimodromini and Phytoseiulini, and complementary notes on mites of the tribes Euseiini and Neoseiulini (Acari: Phytoseiidae) from sub-Saharan Africa. Zootaxa, 1628: 1-22. doi:10.11646/zootaxa.1628.1.1 ![]()
Muma M.H. 1961. Subfamiles, genera, and species of Phytoseiidae (Acarina: Mesostigmata). Fla St. Mus. Bul., 5(7): 267-302
Muma M.H., Denmark H.A. 1968. Some generic descriptions and name changes in the family Phytoseiidae (Acarina: Mesostigmata). Fla Entomol., 51: 229-240. doi:10.2307/3493424 ![]()
Muma M.H., Denmark H.A. 1970. Phytoseiidae of Florida. Arthropods of Florida and neighboring land areas, 6. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville, USA, 150 pp.
Mwangi E., Kiarie A., Wainwright H. 2015. Typhlodromalus spinosus as a potentially new biological control agent for Western Flower Thrips. Glob. Adv. Res. J. Agric. Sc., 4 (3): 162-165.
Myers N. 1988. Threatened biotas: hostspots in tropical forests. Environmentalist, 8: 187-208. doi:10.1007/BF02240252 ![]()
Myers N., Mittermeier R.A., Mittermeier C.G., Da Fonseca G.A., Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. doi:10.1038/35002501 ![]()
Narayanan E.S., Kaur R.B.N., Ghai S. 1960. Importance of some taxonomic characters in the family Phytoseiidae., with new records and descriptions of species. Proceed. Nat. Inst. Sc. India, 26B: 384-394.
Navasero M.M., Navasero M.V. 2016. Biology of Paraphytoseius orientalis (Narayanan et al.) reared on the broad mite, Polyphagotarsonemus latus (Banks) (Acari: Phytoseiidae, Tarsonemidae) in the Philippines. Philip. Entomol., 30 (1): 21-28.
Nesbitt H.H.J. 1951. A taxonomic study of the Phytoseiinae (Family Laelaptidae) predaceous upon Tetranychidae of economic importance. Zool. Verhandel., 12: 1-96.
Nusartlert N., Vichitbandha P., Baker G., Chandrapatya A. 2011. Pesticide-induced mortality and preydependent life history of the predatory mite Neoseiulus longispinosus (Acari: Phytoseiidae). Trends in Acarology: 495-498. doi:10.1007/978-90-481-9837-5\_83 ![]()
Nwilene F.E., Nachman G. 1996. Functional responses of Iphiseius degenerans and Neoseiulus teke (Acari: Phytoseiidae) to changes in the density of the cassava green mite, Mononychellus tanajoa (Acari: Tetranychidae). Exp. Appl. Acarol., 20: 259-271. doi:10.1007/BF00052876 ![]()
Oliveira D.C., Charanasri V., Kongchuensin M., Konvipasruang P., Chandrapatya A., Moraes G.J. de 2012. Phytoseiidae of Thailand (Acari: Mesostigmata), with a key for their identification. Zootaxa, 3453: 1-24. doi:10.11646/zootaxa.3453.1.1 ![]()
Okassa M., Tixier M.-S., Kreiter S. 2010. Morphological and molecular diagnostics of Phytoseiulus persimilis and Phytoseiulus macropilis (Acari: Phytoseiidae). Exp.Appl. Acarol., 52: 291-303. doi:10.1007/s10493-010-9364-x ![]()
Oomen P. A. 1982. Studies on population dynamics of the scarlet mite. Brevipalpus phoenicis, a pest of tea in Indonesia. Meded. Land. Wagen., 82(1): 1-88.
Prasad V. 1968. Amblyseius mites from Hawaii. Ann. Entomol. Soc. Amer., 61(6): 1514-1521. doi:10.1093/aesa/61.6.1514 ![]()
Prasad V. 1974. A catologue of mites of India. Indira Acarology Publishing House, Ludhiana, Punjab, India, 320 pp.
Pritchard A.E., Baker E.W. 1962. Mites of the family Phytoseiidae from Central Africa, with remarks on the genera of the world. Hilgardia, 33(7): 205-309. doi:10.3733/hilg.v33n07p205 ![]()
Quilici S., Kreiter S., Ueckermann E. A., Vincenot D. 1997. Predatory mites (Acari) from various crops on Réunion Island. Intern. J. Acarol., 23(4): 283-291. doi:10.1080/01647959708683578 ![]()
Quilici S., Ueckermann E. A., Kreiter S., Vayssières J.-F. 2000. Phytoseiidae (Acari) of La Réunion Island. Acarologia, 41(1-2): 97-108.
Quilici S., Verbizier B., Trahais B., Manikom R. 1988. Note sur les ravageurs du litchi à la Réunion. Fruits, 43 (7-8): 459-464.
Ragusa S., Athias-Henriot C. 1983. Observations on the genus Neoseiulus Hughes (Parasitiformes, Phytoseiidae). Redefinition. Composition. Geography. Description of two new species. Rev. Suisse Zool. 90(3): 657-678. doi:10.5962/bhl.part.82005 ![]()
Reis P.R., Teodoro A.V., Pedro Neto M., Da Silva E.A. 2007. Life history of Amblyseius herbicolus (Chant) (Acari: Phytoseiidae) on coffee plants. Neotrop. Entomol., 36(2): 282-287. doi:10.1590/S1519-566X2007000200016 ![]()
Ribaga C. 1904. Gamasidi planticoli. Riv. Patol. Veget., 10: 175-178.
Rhodes L.M., Liburd O.E. 2006. Evaluation of predatory mites and Acramite for control of twospotted spider mites in strawberries in north central Florida. J. Econ. Entomol., 99(4): 1291-1298. doi:10.1093/jee/99.4.1291 ![]()
Rodriguez-Cruz F.A., Venzon M., Pinto C.M.F. 2013. Performance of Amblyseius herbicolus on broad mites and on castor bean and sunnhemp pollen. Exp. Appl. Acarol., 60: 497-507. doi:10.1007/s10493-013-9665-y ![]()
Rodriguez-Reina J.M., Garcia-Mari F., Ferragut F. 1992. Predatory activity of phytoseiid mites on different developmental stages of theWestern flower thrips Frankliniella occidentalis. Bol. Sanid. Veget. Plagas, 18: 253-263.
Rowell H.J., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). Can. Entomol., 110: 859-876. doi:10.4039/Ent110859-8 ![]()
Ryu M.O., Lee W.K., Cho S.R. 2001. Phytoseiid mites (Acari: Phytoseiidae) from the pear field of Naju District in Korea. Kor. J. Soil Zool., 6(1-2): 7-9.
Schicha E. 1975. A new predacious species of Amblyseius Berlese from strawberry in Australia, and A. longispinosus (Evans) redescribed (Acari: Phytoseiidae). J. Austral. Entomol. Soc., 14: 101-106. doi:10.1111/j.1440-6055.1975.tb02010.x ![]()
Schicha E. 1977. Two new species of Amblyseius Berlese from Australia (Acari: Phytoseiidae). J. Austral. Entomol. Soc., 16: 393-396. doi:10.1111/j.1440-6055.1977.tb00126.x ![]()
Schicha E. 1981a. Five known and five new species of phytoseiid mites from Australia and the South Pacific. Gen. Appl. Entomol., 13: 29-46.
Schicha E. 1981b. A new species of Amblyseius (Acari: Phytoseiidae) from Australia compared with ten closely related species from Asia, America and Africa. Intern. J. Acarol., 7: 203-216. doi:10.1080/01647958108683262 ![]()
Schicha E. 1981c. Two new species of Amblyseius Berlese from Queensland and New Caledonia compared with A. largoensis (Muma) from the South Pacific and A. deleoni Muma and Denmark from New South Wales (Acari: Phytoseiidae). J. Austral. Entomol. Soc., 20: 101-109. doi:10.1111/j.1440-6055.1981.tb01008.x ![]()
Schicha E. 1987. Phytoseiidae of Australia and neighboring areas. Indira Publishing House, West Bloomfield, Michigan, USA, 187 pp.
Schicha E., Corpuz-Raros L.A. 1985. Contribution to the knowledge of the genus Paraphytoseius Swirski and Shechter (Acarina: Phytoseiidae). Intern. J. Acarol., 11(2): 67-73. doi:10.1080/01647958508683398 ![]()
Schuster R.O., Pritchard A.E. 1963. Phytoseiid mites of California. Hilgardia, 34: 191-285. doi:10.3733/hilg.v34n07p191 ![]()
Souza I.V. de, Argolo P.S., Gondim Jr. M.G.C., Moraes G.J. de, Bittencourt M.A.L., Oliveira A.R. 2015. Phytoseiid mites from tropical fruit trees in Bahia State, Brazil (Acari, Phytoseiidae). Zookeys, 533: 99-131. doi:10.3897/zookeys.533.5981 ![]()
Specht H.B. 1968. Phytoseiidae (Acarina: Mesostigmata) in the New Jersey apple orchard environment with descriptions of spermathecae and three new species. Can. Entomol., 100: 673-692. doi:10.4039/Ent100673-7 ![]()
Swirski E., Amitai S. 1966. Descriptions of the males of four phytoseiid mites (Acarina) from Hong Kong. Isr. J. Agric. Res., 16: 11-18.
Swirski E., Ragusa S. 1978. Three new species of phytoseiid mites from Kenya (Mesostigmata: Phytoseiidae). Zool. J. Linn. Soc., 63: 397-409. doi:10.1111/j.1096-3642.1978.tb02101.x ![]()
Swirski E., Shechter R. 1961. Some phytoseiid mites (Acarina: Phytoseiidae) of Hong-Kong, with a description of a new genus and seven new species. Isr. J. Agric. Res., 11: 97-117.
Takahashi F., Chant D.A. 1993. Phylogenetic relationships in the genus Phytoseiulus Evans (Acari: Phytoseiidae). II. Taxonomic review. Intern. J. Acarol., 19(1): 23-37. doi:10.1080/01647959308683535 ![]()
Tixier M.-S. 2012. Statistical approaches to assess intraspecific variations of morphological continuous characters: the case study of the family Phytoseiidae (Acari: Mesostigmata). Cladistics, 28: 489-502. doi:10.1111/j.1096-0031.2012.00394.x ![]()
Tixier M.-S., Guichou S., Kreiter S. 2008. Morphological variation in the biological control agent Neoseiulus californicus (McGregor) (Acari: Phytoseiidae): consequences for diagnostic reliability and synonymies. Invert. Syst., 22: 453-469. doi:10.1071/IS07052 ![]()
Tixier M.-S., Otto J., Kreiter S., Santos V.V. dos, Beard J. 2014. Is Neoseiulus wearnei the Neoseiulus californicus of Australia? Exp. Appl. Acarol., 62: 267-277. doi:10.1007/s10493-013-9740-4 ![]()
Tseng Y.H. 1976. Systematics of the mite family Phytoseiidae from Taiwan, with a revised key to genera of the world (II). J. Agric. Ass. China New Series, 94: 85-128.
Tseng Y.H. 1983. Further study on phytoseiid mites from Taiwan (Acarina: Mesostigmata). Chin. J. Entomol., 3: 33-74.
Ueckermann E.A. 1992. Notes on the genus Platyseilella Muma (Acari: Phytoseiidae) with a description of a new species, P. eliahui, from South Africa. Isr. J. Entomol., 25-26: 19-22.
Ueckermann E.A., Loots G.C. 1985. Trochoseius Pritchard and Baker, a new subgenus of Amblyseius Berlese with notes on its former genus Iphiseius Berlese (Acari: Phytoseiidae). Phytophylactica, 17: 129-137.
Ueckermann E.A., Loots G.C. 1987. Two species of Amblyseius (Paraphytoseius) Swirski and Shechter from Africa (Acari: Phytoseiidae). Acarologia, 28(3): 221-226.
Ueckermann E.A., Loots G.C. 1988. The African species of the subgenera Anthoseius De Leon and Amblyseius Berlese (Acari: Phytoseiidae). Entomol. Mem., Dep. Agric. Water Supply, Rep. South Africa 73, 168 pp.
Ueckermann E.A., Meyer M.K.P.S. 1988. South African Acari. IV. Some mites of the Addo Elephant National Park. Koedoe, 31: 31-51. doi:10.4102/koedoe.v31i1.483 ![]()
Ueckermann E.A., Zannou I.D., Moraes G.J. de, Oliveira A.R. de, Hanna R., Yaninek J.S. 2007. Phytoseiidd mites of the subfamily Phytoseiinae (Acari: Phytoseiidae) from sub-Saharan Africa. Zootaxa, 1658: 1-20. doi:10.11646/zootaxa.1658.1.1 ![]()
\biblio{
Ueckermann, E.A., Zannou, I.D., Moraes, G.J. de, Oliveira, A.R. de, Hanna, R., Yaninek J.S. 2008. Phytoseiid mites of the tribe Typhlodromini (Acari: Phytoseiidae) from sub-Saharan Africa. Zootaxa, 1901: 1-122. doi:10.11646/zootaxa.1901.1.1 ![]() }
}
Ullah M.S., Gotoh T. 2014. Life-table attributes of Neoseiulus womersleyi (Schicha) feeding on five tetranychid mites (Acari: Phytoseiidae, Tetranychidae). Intern. J. Acarol., 40(4): 337-348. doi:10.1080/01647954.2014.920045 ![]()
van der Merwe G.G. 1965. South African Phytoseiidae (Acarina). I. Nine new species of the genus Amblyseius Berlese. J. Entomol. Soc. South Afr., 28: 57-76.
van der Merwe G.G. 1968. A taxonomic study of the family Phytoseiidae (Acari) in South Africa with contributions to the biology of two species. Entomol. Mem. South Africa Dep. Agric. Techn. Serv., 18: 1-198.
Vitzthum H. von 1941. Acarina. In: Bronns, H.G. (Ed.), Klassen und Ordnungen des Tierreichs 5, Akademischer Verlag, Leipzig, Germany, pp. 764-767.
Wainstein B.A. 1962. Révision du genre Typhlodromus Scheuten, 1857 et systématique de la famille des Phytoseiidae (Berlese 1916) (Acarina: Parasitiformes). Acarologia, 4: 5-30.
Wainstein B.A. 1970. On the system of the genus Phytoseius Ribaga (Parasitiformes, Phytoseiidae). Zool. Zh., 49: 1726-1728 [in Russian].
Wainstein B.A. 1976. A new tribe of the family Phytoseiidae (Parasitiformes). Zool. Zh., 55: 696-700 [in Russian].
Womersley H. 1954. Species of the subfamily Phytoseiinae (Acarina: Laelaptidae) from Australia. Austral. J. Zool., 2: 169-191. doi:10.1071/ZO9540169 ![]()
Wu W.N., Chou F.W. 1981. A new species of Amblyseius (Acarina: Phytoseiidae) from Guangdong Province. Zool. Res., 2: 273-274 [in Chinese].
Wu W.N., Ou J.F. 1999. A new species group of the Amblyseius, with descriptions of two new species (Acari: Phytoseiidae) from China. Syst. Appl. Acarol., 4: 103-110. doi:10.11158/saa.4.1.15 ![]()
Xin J.L., Liang LR., Ke L.S. 1981. A new species of the genus Amblyseius from China (Acarina: Phytoseiidae). Intern. J. Acarol., 7: 75-80.
Zannou I.D., Moraes G.J. de, Ueckermann E.A., Oliveira A.R., Yaninek J.S., Hanna R. 2006. Phytoseiid mites of the genus Neoseiulus Hughes (Acari: Phytoseiidae) from sub-Saharan Africa. Intern. J. Acarol., 32 (3): 241-276. doi:10.1080/01647950608684467 ![]()
Zannou I.D., Moraes G.J. de, Ueckermann E.A., Oliveira A.R., Yaninek J.S., Hanna R. 2007. Phytoseiid mites of the subtribe Amblyseiina (Acari: Phytoseiidae: Amblyseiini) from sub-Saharan Africa. Zootaxa, 1550: 1-47. doi:10.11646/zootaxa.1550.1.1 ![]()



2019-08-06
Date accepted:
2020-01-14
Date published:
2020-02-28
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Kreiter, Serge; Payet, Rose-My; Douin, Martial; Fontaine, Olivier; Fillâtre, Jacques and Le Bellec, Fabrice
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




