First record of Poecilochirus mrciaki Mašán, 1999 (Acari, Parasitidae) and its phoretic carriers in the Iberian peninsula
Saloña Bordas, Marta I.1 and Perotti, M. Alejandra2
1✉ Dpto de Zoología y Biología Celular Animal, Facultad de Ciencia y Tecnología, UPV/EHU, Barrio de Sarriena s/n, 48940 Bilbao, Spain.
2Ecology and Evolutionary Biology Section, School of Biological Sciences, University of Reading, Whiteknights Campus, Reading RG6 6AS, United Kingdom.
2019 - Volume: 59 Issue: 2 pages: 242-252
https://doi.org/10.24349/acarologia/20194328ZooBank LSID: 36F2E895-9345-409F-B655-17FDB5F8C741
Original research
Keywords
Abstract
Introduction
Poecilochirus mrciaki Mašán, 1999 is a necrophilous Parasitidae (Mesostigmata). Its phoretic deutonymphs are easily recognised due to the stout club-shaped setae on the gnathosoma and coxae II and III, and the dark band surrounding completely the sternal shield. Deutonymphs of the species of the genus Poecilochirus are phoretic on burying and carrion beetles (Silphidae). P. mrciaki was first found and described from South-West Slovakia (in 1999), and first collected on the silphid species Necrodes littoralis (L., 1758), Nicrophorus humator (Gleditsch, 1767) and Oiceoptoma thoracica (L., 1758) (Mašán 1999). More recently, it has been found in Poland transported by O. thoracica (misnamed as Silpha thoracica) (Haitlinger 2008). Carrion beetles may arrive to a carcass or corpse during the first days of decomposition (Díaz-Martín and Saloña-Bordas 2015; Grassberger and Frank 2004) especially with high temperatures (Matuszewski 2011). In the original description of P. mrciaki (Mašán 1999) no details on habitat, e.g. carcass type, are given.
Burying beetles are attracted to animal remains, as they need animal tissues to feed their offspring (Milne and Milne, 1976). They excavate hollows underneath or nearby carrion to build a nest or crypt, to breed (Pukowski 1933), while parasitid mites keep the nest clean from competitors, usually fly maggots (Perotti and Braig 2009; Schwarz and Müller 1992).
As a dead body decomposes, fluids and volatile substances will attract a specific sarcosaprohagous community with different species arriving at different stages of decomposition. Scavenger colonisation follows a model of succession. Jean Pierre Mégnin (1828-1905) described for the first time this model and proposed up to eight different waves of colonisation by arthropods (Mégnin 1894). At present, the succession model proposed by Mégnin has been reorganised into 5 stages of decomposition such as fresh, bloated, active decay, advanced decay, and skeleton or dried remains (Payne 1965; Anderson and VanLaerhoven 1996; Goff 2009).
Poecilochirus species are phoretic (Hyatt 1980; Milne and Milne 1976; Neuman 1943; Perotti and Braig 2009; Saloña-Bordas and Perotti, 2014; Schwarz and Müller 1992). Phoresy is the interaction between a host or carrier where a phoront uses an organism to be transported to a new environment and to a new food source. Some mite species arrive in carrion on the first necrophagous insects, becoming early colonisers of carcasses (Leclercq and Verstraeten 1993; Perotti and Braig 2009; Perotti et al. 2010). Mites might synchronise their life cycle with the phoront (Houck and O'Connor 1991; Neuman 1943; Schwarz and Müller 1992), especially those having high specificity with the carrier (Camerik 2010; Perotti and Braig 2009). In the case of carrion associated parasitid mites, the presence of the mite is critical for the reproductive success of the beetle carrier, by preventing the survival of competitors, as for example blowflies (Springett 1968).
Parasitidae might be used as indicators of post mortem interval (Perotti et al, 2010; González Medina et al. 2012; Saloña-Bordas and Perotti, 2014). Poecilochirus deutonymphs are obligate phoronts on necrophagous beetles (Baker and Schwarz 1997, García-Guerrero et al. 2014, Perotti and Braig 2009; Schwarz and Müller 1992, Schwarz and Walzl, 1996; Springett 1968). A recent review on phoretic mites underlines the presence of Parasitidae mites on carcasses at different stages of decomposition, mainly associated to black putrefaction, butyric fermentation or advance decay (Braig and Perotti 2009; Perotti et al. 2010).
High phoretic specificity has been reported between Poecilochirus mites and Nicrophorus beetles (Silphidae) (Milne and Milne 1976, Neuman 1943; Perotti et al. 2010; Schwarz and Müller 1992; Schwarz and Walzl 1996; Springett 1968). However, P. carabi has been collected at an early stage of decomposition (fresh) of a mice carcass, reported as P. necrophori by Wilson (1983), and from the soil underneath a hung corpse at active decay by Saloña-Bordas and Perotti (2014). P. austroasiaticus has been also reported on corpses at advanced stage of decomposition (González Medina et al. 2012). The correct identification of the mite species is crucial for an accurate interpretation of circumstances surrounding death, like the estimation of the post-mortem interval.
The present work confirms the presence of P. mrciaki associated with carcasses, its arrival from the beginning of the decomposition process, fresh stage, as well as its phoresy on blowflies and carrion beetles.
Material and methods
Ten piglet carcasses (Sus scrofa Linnaeus, 1758) were placed in Aiako Harria natural Park (UTM: 30TWN91458860), North Spain. The park environment is a mixed deciduous forest with pine trees. Five carcasses were placed on 6 Aug. 2009, and 5 new replicates were placed one year later (30 Jul. 2010) under similar environmental conditions (Figure 1).
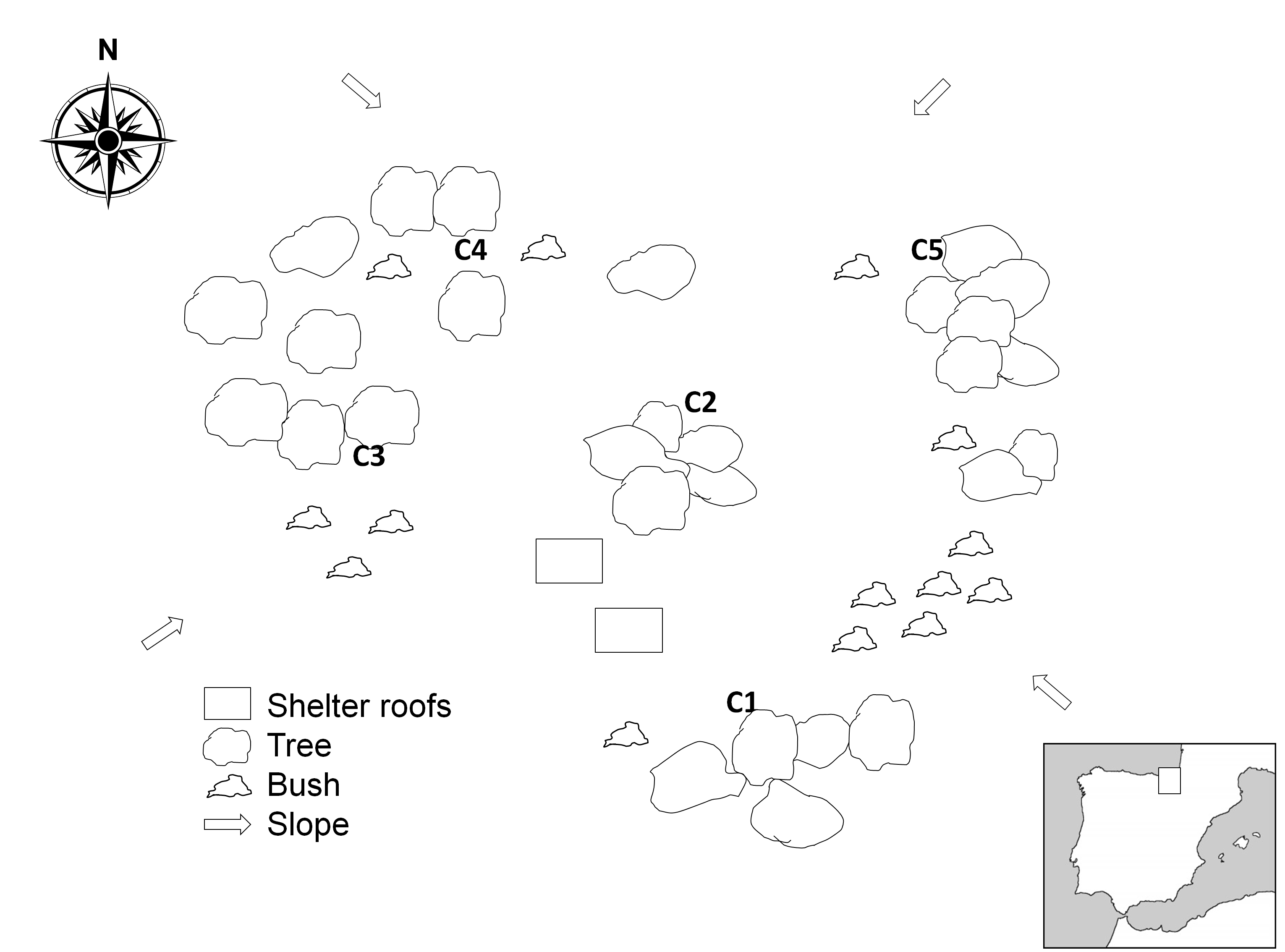


Carcasses were daily observed during the first weeks, taking notes on morphological changes of the remains (following the decomposition process). Insects arriving to the carcasses were collected with insect nets during 85 days in 2009 and 88 days in 2010. A representative sample of the arthropods observed on the remains was collected with forceps or with a brush, aiming to reduce disturbance of the decomposition process during the collection of the samples (Díaz-Martín and Saloña-Bordas 2015). Collected insects and mites were stored in 70% ethanol and labelled for future identification. Mites sampled on the surface of the carcass were taken without altering the decay. A complete list of the insect species collected during this project was published in Díaz-Martín and Saloña-Bordas (2015). While identifying the insects, attached mites were separated, cleared with lactic acid and mounted in Hoyer's fluid for identification (Krantz, 1971). A close inspection of the remains also indicated mites detached from carriers, and these were carefully collected with a small brush, stored in 70% ethanol and processed for further identification.
Statistical analyses
Data of both the number of mites per carrier and per stage of decomposition did not follow a normal distribution. Non-parametric analyses were conducted on mite numbers on beetles. Medians of mite numbers were compared and tested using Kruskal Wallis, followed by post hoc pairwise comparisons (Dunn's post hoc, uncorrected values). Exploratory data analysis used Corresponding Analysis (based on Correlation), especially for the association of carriers with stages of decomposition. All analyses were performed in PAST3 (Hammer et al. 2001).
Results
A total of 48 specimens identified as Poecilochirus mrciaki were collected from flies (12%) and beetles (88%), while 3 specimens were found on remains (Table 1).



All mites were phoretic, therefore, at the deutonymphal stage. In 2009, P. mrciaki was only recorded between day 3 and day 15 after the death; while in 2010, it was collected between day 4 and day 15. Slight differences in the duration of each stage of decomposition were observed for each carcass (Table 2).



Poecilochirus mrciaki specimens collected in Aiako Harria natural park presented little intraspecific variation of shield lengths, with podonotal shield length varying between 404-441 µm, and opisthonotal shield length ranging from 240 to 263 µm (Figure 2A) (Number of mites measured = 6). Shape of other diagnostic characteristics is unique to this population, such as the irregular tectum with lateral teeth, well defined in some specimens (Figure 2B) and the granulated metapodal plate (Figure 2C). The peritreme has the same shape than in the original description.



The majority of specimens where collected from insects visiting the carcasses during bloat, active and advanced decay (Figure 3), with the highest abundance observed in the active decay phase (Figure 4); fewer specimens where sampled from fresh and dry remains. The list of insects comprises: unidentified Calliphoridae and other Diptera, and the beetles, Anoplotrupes stercorosus, Creophilus maxilosus, Necrodes littoralis, Nicrophorus vespilloides and Trypocopris pyrenaeus (Table 1). The insect species transporting most deutonymphs was by far N. littoralis (Figure 5), and its prevalence increased towards active decay; showing a similar behaviour of colonisation as other beetles, like A. stercorosus, N. littoralis, N. vespilloides and T. pyrenaeus (Figure 6).
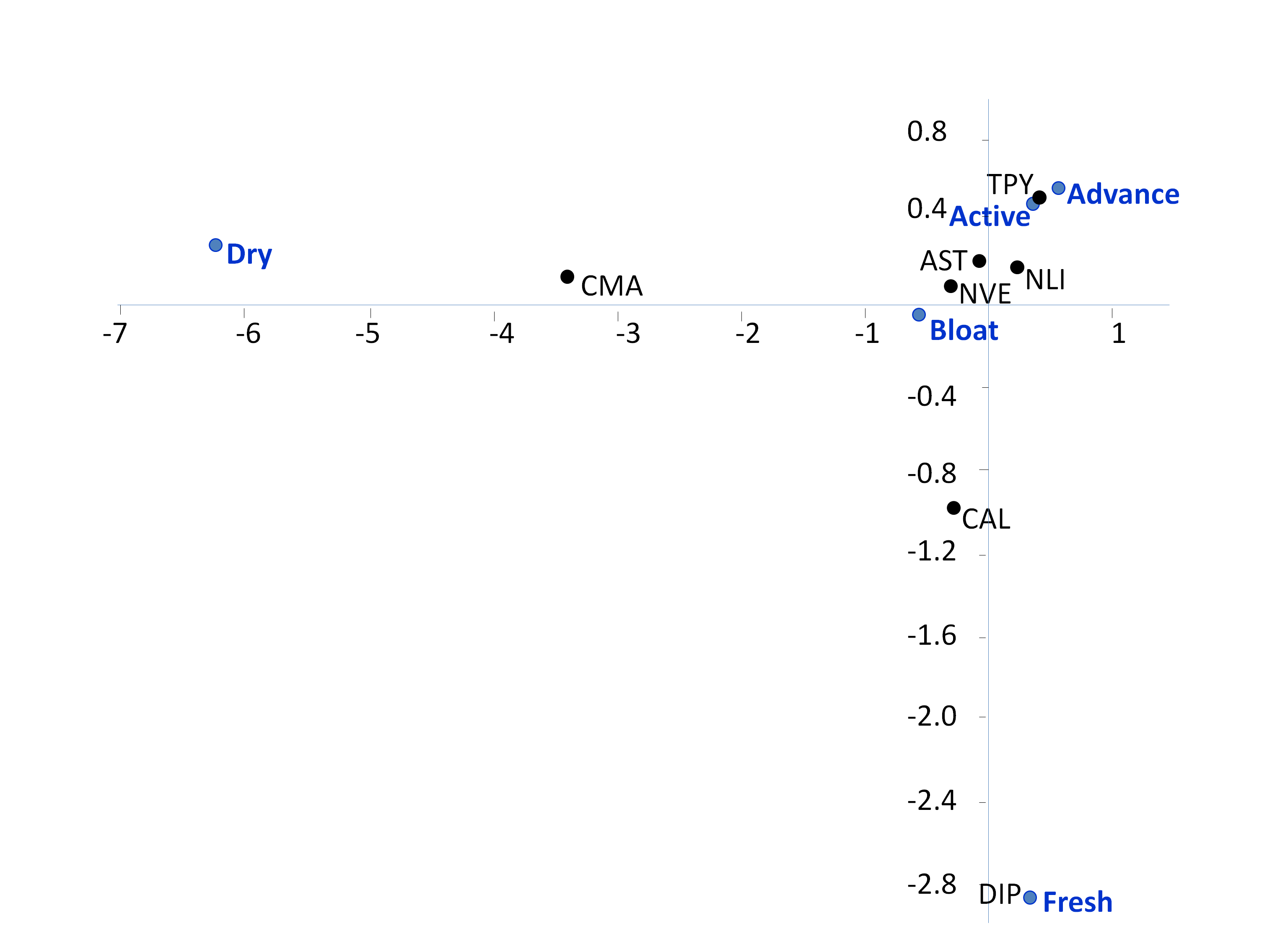











The number of mites was not significantly different between the seven carriers (pooling the data for all the stages of decomposition) (Kruskal-Wallis, P = 0.273); however, pairwise post-hoc Dunn's comparisons showed significant difference between N. littoralis with Creophilus maxilosus, and with Diptera (Table 3 Bonferroni), confirming the pattern observed in the multivariate analysis (Figure 3).



The median number of mites was not significantly different between the stages of decomposition (Kruskal-Wallis, P = 0.148).
This study brings new knowledge on the carriers of P. mrciaki, such as the beetles N. vespilloides (Silphidae, Coleoptera), T. pyrenaeus, A. stercorosus (Geotrupidae, Coleoptera), C. maxilosus, (Staphylinidae, Coleoptera) and the flies, Lucilia caesar and/or Calliphora vomitoria (Diptera, Calliphoridae).
It was not possible to identify the specific Diptera carriers. Two deutonymphs were found from unidentified Diptera and, three more specimens were collected directly from carcass remains on days 9 (D9) to fifteen (D15) (Table 1).
Discussion
In decomposition, carcass or corpse `environments', most of the data gathered from Parasitidae mites refer to the mites in their deutonymphal stage and, adult descriptions are missing for some species of forensic interest. In this work, adult Parasitidae specimens were collected (data not presented); however, their identification is uncertain, as Poecilochirus mrciaki were described only as phoretic deutonynmphs (Mašán 1999). Further research on this species will help clarify the identity of adults. The present results confirm that P. mrciaki travel on Silphidae. It might have achieved a closed association with some of them. Poecilochirus mites interaction with beetles is well known for P. carabi s. l. and P. austroasiaticus, which are considered symbiotic mites of a few silphids (Baker and Schwarz 1997, Schwarz and Müller 1992, Schwarz and Walzl 1996, González Medina et al., 2012).
Morphological differences between this population of P. mrciaki and the original description refer to the length of dorsal shields, where the podonotal shield (podonotum) is shorter in the questioned specimens. The tectum and the metapodal plate are particularly unique for these specimens.
The occurrence of P. mrciaki associated with carrion insects and carcass decomposition is reported here for the first time. This is also the first record of this species in the Iberian peninsula (Aiako Harria Natural Park, Gipuzkoa, Basque Country, North Spain). A link between this mite species and necrophagous beetles of the family Silphidae can be suspected, with 68% of the specimens collected on silfid beetles. Especially for the large carrion beetle Necrodes littoralis, which was its main transporter, with 60% of the mites on this species. The density of mites was lower on Creophilus maxillosus, Anoplotrupes stercorosus and Trypocopris pyrenaeus (all of which accounted for 18% of the specimens collected). This preference for or strong interaction with silphids and perhaps with geotrupids too, also arises from the comparison of medians, with a significant difference only observed in the case of the staphylinid Creophilus maxilosus or flies.
This work also highlights a diversity of insect families used by the phoretic deutonymphs, such as, in addition to Silphidae, Geotrupidae (Trypocopris pyrenaeus 8%) and Staphylinidae (C. maxillosus 4%), as well as blowflies (Calliphoridae) and other unidentified flies (Diptera). Poecilochirus has been reported associated to Drosophilidae (Diptera) during butiric fermentation and dry stage (Perotti et al. 2010). It is also well documented that Poecilochirus deutonymphs use blowflies to abandon a carcass under conditions of phoretic saturation that is when their main phoronts or carriers are absent (Perotti and Braig 2009). During this research, the mites were isolated from the blowflies during the first days after decease, being the carcass fresh or bloated. This observation suggests that Poecilochirus mrciaki may use flies both to colonise and to abandon a carcass.
All the aforementioned insects are early colonisers of carcasses and corpses (Grassberger and Frank 2004) and showed an uninterrupted arrival to the carcasses along the different stages of decomposition, as reported by Schoenly and Reid (1987). Although the insects seem to peak at different stages of decomposition (Díaz Martín 2010). Following the insect dynamics of carcass colonisation, the first P. mrciaki mites arrived on blowflies Calliphoria vomitoria or Lucilia caesar and on the beetle Necrodes littoralis on day 3 after death. Some carcasses were still fresh in day 3 in 2009 and bloated in day 5 in 2010. The highest abundance of mites was recorded in active decay (days 5 to 9). The last deutonymph was observed on day 15 on Creophilus maxillosus, in 2009, and on remains in 2010. P. mrciaki deutonymphs were only collected during the first two weeks after death, although carcasses were sampled for up to 3 months (Díaz-Martín and Saloña-Bordas 2015). Consequently, this mite species seems to be an early coloniser of corpses and carcasses. Further studies on the life history of P. mrciaki may clarify its value as a marker of time, potentially complementing estimations of post-mortem intervals, PMI, or periods of insect activity on corpses, PIA.
Acknowledgements
Special thanks to Beatriz Diaz-Martin for carrying out technical field and lab work (collecting and mounting mites) and, to Pablo Bahillo de la Puebla for his assistance in the identification of the beetle species.
This research was founded by the PhD program of the Basque Government (Departamento de Educación, Becas e Investigación; Gobierno Vasco – Eusko Jaurlaritza 2009) and the Central Government (Ministerio de Educación Cultura y Deporte, Programa Salvador de Madariaga 2016).
References
Anderson G.S., VanLaerhoven S.L. 1996. Initial studies on insect succession on carrion in southwestern British Columbia. J. Foren. Sci., 41: 617-625. doi:10.1520/JFS13964J ![]()
Baker, A.S., Schwarz, H.H. 1997. Morphological differences between sympatric populations of the Poecilochirus carabi complex (Acari: Mesostigmata: Parasitidae) associated with burying beetles (Silphidae: Nicrophorus). Syst. Parasitol., 37: 179-185, doi:10.1023/A:1005822702267 ![]()
Brown J.M., Wilson D.S. 1992. Local specialization of phoretic mites on sympatric carrion beetle hosts. Ecology, 73: 463-478. doi:10.2307/1940753 ![]()
Camerik A.M. 2010. Phoresy revisited In: Sabelis M.W., Bruin J. (Eds). Trends in Acarolology. Netherlands: Springer. p. 333-336. doi:10.1007/978-90-481-9837-5\_53 ![]()
Díaz-Martín B. 2010. Entomofauna associated with domestic pig (Sus scrofa) decomposition in an Atlantic environment. [PhD Thesis]. Leioa: University of the Basque Country. pp. 212.
Díaz-Martín B., Saloña-Bordas M.I. 2015. Arthropods of forensic interest associated to pig carcasses in Aiako-Harria Natural Park (Basque Country, north of Spain). Ciencia Forense, 12: 207-228.
García-Guerrero D.A., Monjarás-Barrera J.I., Hernandez-Juarez A., Aguirre-Uribe L.A. 2014. Asociación del ácaro Poecilochirus sp. (Acari: Parasitidae) y Nicrophorus marginatus (Coleoptera: Silphidae) en Saltillo, Coahuila, México. Entomol. Mex., 1: 69-73.
Goff L.M. 2009. Early post-mortem changes and stages of decomposition in exposed cadavers. Exp. Appl. Acarol., 49: 21-36. doi:10.1007/s10493-009-9284-9 ![]()
González Medina A., González Herrera L., Perotti M.A., Jimenez Ríos G. 2012. Occurrence of Poecilochirus austroasiaticus (Acari: Parasitidae) in forensic autopsies and its application on postmortem interval estimation. Exp. Appl. Acarol., 59: 297-305. doi:10.1007/s10493-012-9606-1 ![]()
Grassberger M., Frank. C. 2004. Initial study of arthropod succession on pig carrion in a central European urban habitat. J. Med. Entomol., 41: 511-523. doi:10.1603/0022-2585-41.3.511 ![]()
Haitlinger R. 2008. Mites associated with insects in Poland. In: Gwiazdowiczd J. (Ed). Selected problems of acarological research in forests. Poznań: Wydawnictwo Uniwersytetu Przyrodniczego, p. 113-125.
Hammer Ø., Harper D.A.T. and Ryan P.D. 2001. Paleontological statistics software package for education and data analysis. Palaeontol. Elect., 4: 1-9.
Houck M.A., OConnor B.M. 1991. Ecological and evolutionary significance of phoresy in the Astigmata. Ann. Rev. Entomol., 36: 611-636. doi:10.1146/annurev.en.36.010191.003143 ![]()
Hyatt K.H. 1980 Mites of the subfamily Parasitinae (Mesostigmata: Parasitidae) in the British Isles. Bull. Brit. Mus. (Nat Hist) Zool., 38: 237-378.
Krantz G.W. 1971. A Manual of Acarology. Oregon, U. S. A.: O. S. U. Book Stores. pp. 335.
Leclercq M., Verstraeten C. 1993. Entomologie et médecine légale. L'entomofaune des cadavres humains: sa succession par son interprétation, ses résultats, ses perspectives. J. Med. Leg. Droit. Med., 36: 205-222.
Mašán P. 1999. Mites (Acarina) associated with burying and carrion beetles (Coleoptera, Silphidae) and description of Poecilochirus mrciaki sp.n. (Mesostigmata, Gamasina). Biologia, 54: 515-524.
Matuszewski S. 2011. Estimating the pre-appearance interval from temperature in Necrodes littoralis L. (Coleoptera: Silphidae). Forensic Sci. Int., 212: 180-188. doi:10.1016/j.forsciint.2011.06.010 ![]()
Mégnin P. 1894. La Faune des Cadavres. Application de l'Entomologie à la Médecine Légale. Paris: Masson and Gauthier-Villars et Fils. pp. 214.
Milne L.J., Milne M. 1976. The social behavior of burying beetles. Sc. American, 235: 84-89 doi:10.1038/scientificamerican0876-84 ![]()
Neuman K.W. 1943. Die Lebensgeschichte der Käfermilbe Poecilochirus necrophori Vitzt. nebst Beschreibung aller Entwicklungsstufen. Zool. Anz., 142: 1-21.
Payne J.A. 1965. A summer carrion study of the baby pig Sus scrofa Linnaeus. Ecology, 46: 592-602. doi:10.2307/1934999 ![]()
Perotti M.A., Braig H.R. 2009. Phoretic mites associated with animal and human decomposition. Exp. Appl. Acarol., 49: 85-124. doi:10.1007/s10493-009-9280-0 ![]()
Perotti M.A., Braig H.R., Goff M.L. 2010. Phoretic mites and carcasses: Acari transported by organisms associated with animal and human decomposition in: Amendt J., Campobasso C. P., Grassberger M., Goff M.L. (Eds). Current concepts in forensic entomology. New York: Springer, p. 69-91. doi:10.1007/978-1-4020-9684-6\_5 ![]()
Pukowski E. 1933. Ökologische Untersuchungen an Necrophorus F. Z. Morphol. Ökol. Tiere, 27: 518-586. doi:10.1007/BF00403155 ![]()
Saloña M., Moraza M.L., Carles-Tolrá M., Iraola V., Bahillo P., Yélamos T., Outerelo R., Alcaraz R. 2010. Searching the soil: forensic importance of edaphic fauna after the removal of a corpse. J. Forensic Sci., 55: 1652-1655. doi:10.1111/j.1556-4029.2010.01506.x ![]()
Saloña-Bordas M.I., Perotti M.A. 2014. First contribution of mites (Acari) to the forensic analysis of hanged corpses: A case study from Spain. Forensic Sci. Int., 244: e6-e11. doi:10.1016/j.forsciint.2014.08.005 ![]()
Schoenly K., Reid W. 1987. Dynamics of heterotrophic succession in carrion arthropod assemblages: discrete seres or a continuum of change? Oecologia, 73: 192-202. doi:10.1007/BF00377507 ![]()
Schwarz H.H., Müller J.K. 1992. The dispersal behaviour of the phoretic mite Poecilochirus carabi (Mesostigmata, Parasitidae): Adaptation to the breeding biology of its carrier Necrophorus vespilloides (Coleoptera, Silphidae). Oecologia, 89: 487-493. doi:10.1007/BF00317154 ![]()
Schwarz H.H., Walzl M.G. 1996. Pairing, oviposition and development in two sibling species of phoretic mites (Acari: Mesostigmata: Parasitidae: Poecilochirus spp.) associated with burying beetles (Coleoptera: Silphidae: Nicrophorus spp.). J. Nat. Hist., 30: 1337-1348. doi:10.1080/00222939600771251 ![]()
Smith K.G.V. 1986. A Manual of Forensic Entomology. The trustees of the British Museum (Natural History). London: British Museum (Natural History) and Cornell University Press, pp. 205.
Scott M.P. 1998. The ecology and behavior of burying beetles. Annu. Rev. Entomol., 43: 595-618. doi:10.1146/annurev.ento.43.1.595 ![]()
Springett B.P. 1968. Aspects of the relationship between burying beetles, Necrophorus spp. and the mite, Poecilochirus necrophori Vitz. J. Anim. Ecol., 37: 417-424. doi:10.2307/2957 ![]()
Wilson D.S. 1983. The Effect of Population Structure on the Evolution of Mutualism: A Field Test Involving Burying Beetles and Their Phoretic Mites. Am. Nat. 121: 851-870. doi:10.1086/284108 ![]()



2018-12-13
Date accepted:
2019-06-26
Date published:
2019-07-09
Edited by:
Tixier, Marie-Stéphane

This work is licensed under a Creative Commons Attribution 4.0 International License
2019 Saloña Bordas, Marta I. and Perotti, M. Alejandra
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



