A further modification of Dioni’s mounting media to allow staining, clearing and mounting of Acari
Chick, Andrew I. R.1
1✉ School of Human Science, University of Derby, Kedleston Rd, Derby DE22 1GB, United Kingdom.
2018 - Volume: 58 Issue: 4 pages: 795-800
https://doi.org/10.24349/acarologia/20184286Keywords
Abstract
The optical properties of the Acari mean they are not suitable to be mounted in microscopical mounting media such as Canada balsam, and as such are traditionally mounted in the gum chloral media, such as Hoyers or Berlese (Chick, 2016). Chick (2011) also mentions the use of the gum chloral media, Faure’s modification of Berlese, as a mountant for Acari. Singer (1967) states that for Acari, mounting media need to be of a refractive index lower than 1.50 or greater than 1.55. Neuhaus et al. (2017) list the refractive index of Canada Balsam as between 1.5181 and 1.5447, Brown (1997) lists the refractive index of Canada Balsam as 1.532, whereas Gum Chloral media such as Hoyers has a refractive index of 1.419, Berlese of 1.485 and Faure’s of 1.437 (Neuhaus et al., 2017). Faraji and Bakker (2008) presented a method of staining and mounting Acari stating that traditional Acarological mounting media, may in the long term over clear specimens making them harder to identify. A number of stains have been recommended for the Acari in the past, Hughes (1973) recommended using lignin pink in a mixture of lactic acid and glycerol to clear and stain Acari prior to mounting. Evans et al. (1961) stated that acid fuchsin can be used to stain Acari as an alternative to lignin pink, Krantz and Walter (2009) suggested the use of rose bengal to stain inconspicuous mites, however Horobin and Kiernan (2002) state that both acid fuchsin and rose bengal have poor light fast properties. The method of Faraji and Bakker (2008) incorporated iodine into Hoyers mounting media to act as a stain. This method was also used by de Lillo et al. (2010) as a recommended protocol for Eriophyoidea. Iodine was one of the first microscopic stains, with the Gram staining protocol dating back to 1884 (Gurr, 1952).
However, the obtaining of traditional gum chloral mounting media such as Hoyer’s can be problematic due to the restrictions placed on chloral hydrate, which is illegal to obtain in some areas without a prescription (Chick, 2010). Clark and Morishita (1950) previously suggested that lactic acid can be used to clear Acari, and incorporated it in their C-M Medium however, Dioni (2003) presented a gum arabic mounting media for the amateur microscopist that used a commercial artists grade solution of gum arabic and lactic acid as a clearing agent replacing chloral hydrate. Chick (2010) presented a modification of Dioni’s fluid suitable for professional entomological applications using purer laboratory grade reagents noting that it behaved in a similar fashion to commercially available Berlese fluid, being of a similar colour, composition and having a gentle clearing action, with less bubble formation than commercial Berlese media. Chick (2011) further went on to recommend the use of Modified Dioni’s as a mounting media for the Acari. This paper aims to evaluate further modifying Dioni’s fluid to act as a one stage clearing, stain, and mounting media for the Acari.
Gum Arabic 3 g
Distilled Water 10 mL
Glycerine 5 mL
Liquid Glucose 2.5 mL
Lactic Acid 6 mL
Antiseptic* 1 mL
*The antiseptic is used to prevent the formation of mould. Chick (2010) used a commercial antiseptic called T.C.P.
Chick (2010) remarks that Dioni’s mounting media behaves similar to that of commercially available Berlese mounting media, with the presence of lactic acid aiding to clear the specimen overtime, but it is less prone to bubble formation.
The current paper suggests substituting the Antiseptic with a tincture of iodine to provide an antiseptic and staining action giving the following formula:
Gum Arabic 3 g
Distilled Water 10 mL
Glycerine 5 mL
Liquid Glucose 2.5 mL
Lactic Acid (80%) 6 mL
Tincture of Iodine 3 mL*
*The Tincture of iodine used was British Pharmacopeia (BP) grade and the ingredients listed are: iodine BP 2.5% w/v, potassium iodide BP 2.5% w/v, ethanol BP 89% v/v, purified water BP to 100%. This would make the active stain a 0.3% solution in the mounting media. An initial test was performed with a 0.1% solution of stain in the media.
The ingredients are combined and warmed at between 30°C and 40°C over night to mix. Chick (2010) recommended using a vivarium heat mat. Dioni’s media is normally straw coloured (like Berlese/Hoyers), whereas this modification has a more orange-brown colour due to the presence of the iodine. The media can be filtered and decanted into a fresh jar to prevent the impurities that are common in gum arabic such as woody debris and small gels (Henshaw, 1981) affecting the mounts made.
Lightly sclerotized specimens can be mounted directly in the mounting media, or from alcohol. More pigmented specimens can be gently warmed in lactic acid prior to mounting to clear the specimen.
Specimens are mounted in the same manner as that of traditional gum chloral media such as Hoyers. The current author leaves his slides in a slide warming oven at between 35-40°C for a week to make such the media is fully set and seals his slides once the media has set using a turntable and model maker’s enamel paint.
The test mites used for this investigation were Poecilochirus spp. (Acari: Parasitidae) collected from a burying beetle (Nicrophorus humator) prior to mounting specimens were warmed in 80% lactic acid for seven days to replicate the effects of over clearing. Specimens where mounted in plain Dioni’s mountant (Chick’s 2010 formula) as a negative control, a 0.1% solution of iodine stain in Dioni’s and a 0.3% solution of iodine in Dioni’s. A further specimen was mounted without a preclearing step in Dioni’s media (with 0.3% iodine) as a positive control. After the media had set for a number of days the slides were sealed with a model makers enamel paint and the specimens were examined using bright field and phase contrast microscopy.
Phase contrast Specimens were photographed using a Vickers instruments Patholette phase contrast microscope using a x40 Vickers PH objective. Brightfield photographs were taken using a Vickers instruments M15C Trinocular microscope (with a x1.6 magnification factor) and a x20 Vickers objective. Photomicrographs were captured using a Nikon D7100 DSLR camera with an x2.5 photo eyepiece. The hind pulvillus of the specimens was the area photographed in all cases. Scale bars were added using Fiji-ImageJ (Schindelin et al., 2012), white balance was auto corrected using Adobe Lightroom 5, the plate was compiled in Photoshop Creative Suite 5.
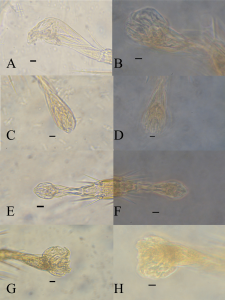
The images presented in Figure 1 A–H shows the hind pulvillus of the test specimens under varying treatments. As can be seen from Figure 1 A–B tradition Dioni’s media (negative control), provides a contrast between the specimen and the media however the action of preclearing causes a lack of clarity, even when phase contrast is used to enhance the specimen. Figure 1 C–D show how the addition of iodine aids with contrast even in the specimen has been over cleared. The definition appears best with a 0.3% solution of iodine in the Dioni’s media. With Figure 1 E showing a greater level of fine detail using bright field illumination, with features around 1μm in size. Further details are visible using phase contrast (figure 1 F). Figures G–H show the uptake of stain and clearing of a fresh specimen without preclearing (positive control) to simulate the effects of long term storage. As can be seen the specimen still takes up a large amount of stain, and detail is improved.
Modified Dioni’s has a number of advantages over other mounting media such as PVA and some of the Gum Chloral media.
As previously stated the substitution of chloral hydrate with lactic acid makes Dioni’s media suitable for places where chloral hydrate is restricted, in places such as the UK many amateur entomologists undertake monitoring of various invertebrates, and preservation of slides could be an issue. Chick (2010) remarked that when compared to “Berlese” fluid from an entomological supplier Dioni’s fluid without iodine was less prone to bubble formation in the mount. The present author notes that the addition of iodine does not affect bubble formation in the mount compared to plain Dioni’s media.
Brown (1997) remarks gives the formula of PVA as a mixture of PVA acetone, glycerine and lactic acid, and states that the related media Polyvinyl lactophenol is not used at the NHM due to PVA’s tendency to continue to shrink, damaging the specimen. As well as the slides crystallising and turning opaque. Due to the lack of archival properties of PVA based media, Dioni’s was not compared directly to PVA.


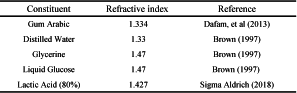
While an exact determination of the refractive index of Dioni’s media was beyond the current resources of the present author, the late Walter Dioni (pers com) estimated it as lower than 1.5. Looking at the relevant constitutes one can see that there are no constituents that are higher than 1.5 (Table 1).


As can be seen in figure 1 the pulvillus is an area of the specimens prone to over clearing, the addition of 0.3% iodine improves the level of contrast sufficiently in both bright field and phase contrast images, the current author did not have access to DIC as recommended in Baker (1999). However, as stated by Faraji and Bakker (2008) while phase contrast is preferable, many acarologists, particularly students or those in forensic, medical or veterinary laboratories do not have access to phase contrast, as the majority of laboratories only have bright field. The comments of Faraji and Bakker (2008) regarding the longevity of gum arabic based slide is echoed here, the current author has been using Dionis mountant for just under 10 years with no issues with crystallisation as long as the coverslip is properly sealed. Such slides were ringed with nail varnish (as suggested by Disney, 1983) or model makers enamel paint. All slides were stored flat (as suggested by Brown, 1998) in commercially made card slide boxes in a temperate environment. Most gum arabic based media are classified as “semi-permanent” (Upton, 1993). However, as advocated by Brown (1997) a watch brief should be observed and slides should be checked regularly for any signs of degradation. In summary the mounting media presented in this paper is a suitable substitute for the one step clearing, staining and mounting solution presented in Faraji and Bakker (2008), with the added benefit that Dioni’s media can be prepared in areas where chloral hydrate is difficult to obtain. It aides with the visualisation of features under both bright field and phase contrast illumination and as such makes for a suitable bench safe media for use in the teaching and research of acarology.
Baker A.S. 1999. Mites and ticks of domestic animals: an identification guide and information source. HMSO., 240 pp.
Brown P.A. 1997. A Review of Techniques Used in the Preparation, Curation and Conservation of Microscope Slides at the Natural History Museum, London. The Biology Curator, 10 Special Supplement 1-33.
Brown P.A. 1998. Microscope Slide Collections Storage, the Horizontal or vertical? How should slide collections be housed? Natural Sciences Conservation Group Newsletter. 9: 43-46.
Chick A.I.R. 2010. A modification of Dioni's mountant as a substitute for Berlese mountant. Entomologists Monthly Magazine. 146 (2): 117-118.
Chick A.I.R. 2011. The mounting of mites (Acari) in modified Dioni's mountant. Micscape 194.
Chick A.I.R. 2016. Insect Microscopy. Crowood Press., 128 pp.
Clark E.W., Morishita, F. 1950. C-M Medium: A mounting media for small insects, mites and other whole mounts. Science 112 (2922): 789-790. doi:10.1126/science.112.2922.789 ![]()
de Lillo E., Craemer C., Amrine Jr, J.W., Nuzzaci G. 2010. Recommended procedures and techniques for morphological studies of Eriophyoidea (Acari: Prostigmata). Exp. Appl. Acarol., 51: 283-307. doi:10.1007/s10493-009-9311-x ![]()
Dafam D.G., Abubakar M.S., Nuhu H., Ajima U., Okwori V. 2013. Quantitative evaluation of some physical and chemical properties of the gum-mucilage of Anacardium occidentale L. (Anacardiaceae) International journal of pharmaceutical science invention., 2(9): 46-48.
Dioni W. 2003. Safe microscopic techniques for amateurs. I – Mounting microscopic subjects, Part 3c – The mixed formulae gum arabic media, Micscape, 91.
Disney R.H.L. 1983. Scuttle Flies, Diptera Phoridae except Megaselia. Handbooks for the Identification of British Insects 10 (6) Royal Entomological Society., 81 pp.
Evans G.O., Sheals J.G., Macfarlane D. 1961. The terrestrial Acari of the British Isles: an introduction to their Morphology, Biology and Classification. Volume 1 Introduction and Biology. Trustees of the British Museum., 219 pp.
Faraji F., Bakker F. 2008. A modified method for clearing, staining and mounting plant-inhabiting mites. Eur. J. Entomol., 105: 793-795. doi:10.14411/eje.2008.105 ![]()
Gurr G.T. 1952. Microscopical stains. Quekett Journal of Microscopy, 3: 419-426.
Henshaw D.J. de C. 1981. Observations on the preparations of Berlese's fluid. Entomologists Monthly Magazine 116 (3): 206.
Horobin R.W., Kiernan J.A. 2002. Conn's Biological Stains: A handbook of Dyes, Stains and Fluorochromes for use in Biology and Medicine. 10th ed. Bios Scientific Publications. pp. 576.
Hughes A.M. 1976. The Mites of stored food and houses. M.A.F.F Technical Bulletin 9 H.M.S.O., 400 pp.
Krantz G.W., Walter D.E. 2009. A Manual of Acarology, 3rd ed., Texas University Press., 816 pp.
Neuhaus B., Schmid T., Riedel J. 2017. Collection management and study of microscope slides: Storage, profiling, deterioration, restoration procedures, and general recommendations. Zootaxa, 4322 (1): 001–173. doi:10.11646/zootaxa.4322.1.1 ![]()
Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J., White D.J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A. 2012. Fiji: an open-source platform for biological-image analysis, Nature methods 9(7): 676-682. doi:10.1038/nmeth.2019 ![]()
Sigma Aldrich: L-(+)-Lactic acid solution [internet] 2018. Available from: https://www.sigmaaldrich.com/catalog/product/sigma/199257?lang=en®ion=GB ![]() accessed 30/07/2018
accessed 30/07/2018
Singer G. 1967. A comparison between different mounting techniques commonly employed in Acarology. Acarologia 9: 475–484.
Upton M.S. 1993. Aqueous gum-chloral slide mounting media: an historical review. Bulletin of entomological research 83: 267-274. oibibhttps://doi.org/10.1017/S0007485300034763



2018-06-05
Date accepted:
2018-08-14
Date published:
2018-09-10
Edited by:
Faraji, Farid

This work is licensed under a Creative Commons Attribution 4.0 International License
2018 Chick, Andrew I. R.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



