Ultrastructure of the prosomal gland complex in unfed larvae of the fresh-water mite Limnesia maculata (Müller, 1776) (Acariformes, Limnesiidae)
Shatrov, Andrey B.1 and Soldatenko, Elena V.2
1✉ Zoological Institute of the Russian Academy of Science, Universitetskaya nab. 1, 199034, St.-Petersburg, Russia.
2Zoological Institute of the Russian Academy of Science, Universitetskaya nab. 1, 199034, St.-Petersburg, Russia.
2020 - Volume: 60 Issue: 1 pages: 45-63
https://doi.org/10.24349/acarologia/20204358Original research
Keywords
Abstract
Introduction
The developed prosomal gland complex is characteristic of Euarthropoda, in particular Arachnida, in which digestion is extra-intestinal (Beklemishev 1964; Cohen 1995, 1998; Alberti and Coons 1999; Farley 1999; Felgenhauer 1999; Schmidt-Rhaese 2007). Typically, this gland complex consists of several pairs of the alveolar podocephalic salivary glands playing a role in digestion, and one pair of the tubular coxal glands functioning in salt-water balance and osmoregulation. In the higher Acariformes, all these glands of each body side sequentially open into the podocephalic canal taking its origin from the excretory canal of the coxal gland. This complex system of glands and their ducts is known as podocephalic system (Evans 1992; Albert and Coons 1999). Importantly, while the alveolar salivary glands are of entodermal origin, the tubular coxal glands are thought to be of mesodermal origin (Alberti et al. 1997), thus representing remnants of the coelom (Beklemishev 1964; Schmidt-Rhaese 2007; Koch et al. 2015). The cuticle lining podocephalic canal is of ectodermal origin, and the latter opens into subcheliceral space of the mouth apparatus on both sides of the mite body (Alberti and Coons 1999).
Though in many groups of Acariformes the number of the acinous podecephalic glands has undergone reduction (Alberti and Coons 1999), mites of the Parasitengonina may retain plesiomorphic number of three pairs. Besides podocephalic glands, these mites frequently possess one pair of the alveolar, so-called infracapitular, glands opening by their own ducts into the subcheliceral space. Among Parasitengonina, the representatives of Trombiculidae and Erythraeidae, both larvae and adults, possess the full prosomal gland complex (plesiomorphy), including infracapitular and coxal glands (Brown 1952; Mitchell 1964; Voigt 1971; Witte 1978; Shatrov 2000; 2015). Microtrombidiid mites demonstrate the full gland complex in adult mites (Shatrov 2005) and highly reduced gland complex consisting of only two pairs of podeocephalic glands opening into the common podocephalic duct in larvae (Shatrov 2004). While adults of Smarididae retain three pairs of the podocephalic glands and are obviously devoid of the infracapitular glands (Witte 1998), a representative of Calyptostomatidae shows four, instead of three pairs of the podocephalic salivary glands, one of which occupies an axial position, whereas the infracapitular glands are lacking (Vistorin-Theis 1977). In water mites, studied mainly by light microscopy, various reduction of the prosomal gland complex has been observed (Croneberg 1878; Michael 1895; Schmidt 1935; Bader 1938; Mitchell 1955). The unpaired, so-called tracheal gland, may be also developed in water mites, as well as in bdellids, (Mitchell 1955; Alberti 1973; Alberti and Coons 1999). The unpaired tracheal and paired silk glands, both alveolar, are additionally present in tetranychids (Alberti and Storch 1974; Mothes and Seitz 1981; Alberti and Coons 1999). Only one ultrastructural investigation of the salivary glands in the larvae of water mite Piona carnea (Koch, 1836) (Pionidae) has shown the presence of only three pairs of the alveolar podocephalic glands, one of which, as in Calyptostomatidae, is located on the axial body line (Shatrov 2012). The infracapitular glands are also absent. Secretion of all these alveolar glands is found to be mainly proteinaceous, in the form of predominantly electron-dense secretory granules owing to the activity of the rough-endoplasmic reticulum and Golgi body system (Alberti and Coons 1999).
As far as coxal glands are concerned, these paired tubular glands constitute an essential component in the podocephalic system forming by their distal portion a common podocephalic duct. Generally, the coxal glands are thought to be homologous, both in the arachnid orders (Woodring 1973; Alberti and Coons 1999) and generally in arthropods (Schmidt-Nielsen et al. 1968) despite some differences in their location and particular anatomy. However, in contrast to the more primitive basal groups, the coxal glands in the derived Parasitengonina mites are devoid of the proximal saccule and composed only of the convoluted tubule (Witte 1991; Alberti and Coons 1999; Shatrov 2000). Typically, these glands possess developed microvilli in the apical cell surface (brush border) and basal plasma membrane infoldings with mitochondria (basal labyrinth) (Alberti and Coons 1999). These morphological characteristics correspond to transporting epithelia, responsible for transport of ions and water across the gland wall, thus playing a role in ion and water regulation (Pease 1956; Anderson and Harvey 1966; Diamond and Tormey 1966; Berridge and Oschman 1969; Berridge 1970; Wall et al. 1970; Kaufman and Phillips 1973; O'Donnel and Maddrell 1983; Beyenbach 2003). These cellular components are well developed in the coxal glands of larvae and adults of trombiculid and microtrombidiid mites (Shatrov 2000, 2006, 2007), whereas in water mites larvae Hydryphantes ruber (de Geer, 1778) only the apical microvilli are present, whereas the basal infoldings are lacking (Shatrov 2017).
To widen our knowledge about principles of organization of the podecephalic gland complex in various groups of Parasitengonina mites, and especially of their larvae, but also to provide their comparative analysis, we have undertaken the present ultrastructural study of the prosomal glands in larvae of the fresh-water mite Limnesia maculata (O.F. Müller, 1776) (Limnesiidae).
Material and Methods
Mites and collecting sites
Several adult mites of Limnesia maculata (Müller, 1776) were collected in the lake Glubokoiye, Smolensk Province, Demidovskiy district, National Park ''Smolenskoye Poozerye,'' near Przhevalskoye town (55°30' N 31°46' E) 11 July 2013 by E. Soldatenko. The mites were placed separately in plastic 100 ml containers with pure bottled artesian water (www.smolvoda.ru) distributed in Smolensk city. Around one week after capture, the mites have started to produce egg masses, from which larvae hatched some time later.
TEM procedures
For TEM study, active unfed larvae were initially fixed and preserved in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2-7.4) for around four months. After immersion into the fixative fluid, integument of the larvae was carefully pierced with sharp insect pins from the dorsal side for a better penetration of the fixative. After the fixation, larvae were then washed in several changes of 0.2 M cacodylate buffer for one hour, postfixed in 2% osmium tetroxide in 0.2 M cacodylate buffer for five hours, dehydrated in ethanol and acetone series, and embedded in an araldite mixture (Fluka, Germany).
Serial ultra-thin sections from araldite blocks both in transversal and longitudinal planes to the long axis of larvae were made on a Leica UC-6 ultramicrotome (Leica Microsystems GmbH Wetzlar, Germany) using a diamond knife (Ultra 45°, Diatome Ltd, Switzerland). Sections were then mounted on copper grids with oval hole provided with a formvar support and, after staining with uranyl acetate and lead citrate (Reynolds 1963), were examined with a TEM Morgagni 268 D (FEI Company) at 80 kV (digital visualization). Control semi-thin sections were made with the same ultramicrotome with glass knives and examined under a light-optical microscope. In total, six larvae were examined.
All instrumental procedures were performed with the equipment of the ''Taxon'' Research Resource Centre, at Zoological Institute of the Russian Academy of Sciences, St-Petersburg, Russia (http://www.ckp-rf.ru/ckp/3038/?sphrase_id=8879024 ![]() ).
).
Results
The prosomal gland complex occupies a relatively narrow space between the large brain and the frontal body wall (Figures 1 a-c and 2 a) as well as the space located partly inside the gnathosoma and laterally to the gnathosomal base (Figure 2 b). The coxal glands extend backward by sides of the brain up to the middle of the body or even further.
The alveolar podocephalic glands
Three pairs of the podocephalic salivary glands are identified, and may be termed, in accordance with their spatial distribution, as medial, ventral and lateral (Figures 1 a-c and 2 a-b). The infracapitular glands are lacking. The medial glands show unusual arrangement one after another along the axial body line, as in water mite larvae Piona carnea (C.L. Koch, 1836) (Shatrov 2012). The posterior medial gland occupies the position above the brain oppressed along its dorsal frontal wall, whereas the anterior medial gland is situated just in front of the brain lower than the posterior gland (Figure 1 a). In the examined larvae, the excretory duct of the posterior medial gland turns right, whereas the duct of the anterior gland turns left. The frontal portion of the anterior gland may enter the gnathosoma.






The medial glands are the largest ones among podocephalic glands with the largest round secretory granules occupying most of the gland cells' cytoplasm (Figure 1 b). The secretory granules contain the homogeneous substance of various, mostly high electron density (Figure 3 a-b). Nevertheless, the single granules showing dense lamellate inclusions may be seen within the lighter matrix (Figure 3 b-c). The very high prismatic cells of these glands are arranged around the expanded intra-alveolar lumen with extremely deep lateral lacunas filled with a fine-granular substance of moderate electron density (Figures 1 b, 2 b and 3 a). The lateral cell margins are highly wavy, and the cell borders frequently disappear among granules of the gland cells. By contrast, the basal plasma membrane facing the adjacent glands and other tissues always remains flat (Figures 3 d-e and 4 b). The basal lamina between the opposite glands are hardly distinguishable, so the cells of the neighboring glands are tightly applied to each other (Figures 3 e and 4 b). From the adjacent tissues, e.g. epidermis, midgut wall, brain, etc., the gland cells are separated by a relatively thin basal lamina of moderate electron density (Figure 3 e). The round nuclei typically occupy the peripheral cell regions, contain a large centrally located nucleolus and heterochromatin granules dispersed uniformly within the nucleoplasm (Figure 3 a-b, d). No prominent cisterns of rough endoplasmic reticulum (RER) and Golgi bodies are observed in the cell cytoplasm which is full of free ribosomes. The cell cytoplasm also contains numerous relatively small electron-lucent vesicles (clear vesicles) (Figure 3 a-b, d). Single ribosomes may attach to the surrounding membrane of these vesicles (Figure 3 d). Small mitochondria are scarce.



The much smaller ventral glands are located ventrad and laterad to the medial glands, generally by sides of the gnathosomal base and the brain (Figures 2 b, 3 d and 4 a). They contact with the sac of the coxal glands (see below) from the medial aspect (Figure 4 a). The cells of the ventral glands are situated as a rosette around an intra-alveolar lumen with large lateral lacunas (Figures 4 a and 5 a-c). The cell borders reveal hardly distinguishable septate junctions. The apical plasma membrane may form short irregular microvilli (Figure 4 b). The basal plasma membrane remains flat. The round nuclei with large nucleolus occupy the basal position within the cells (Figures 4 a and 5 a, c). Round homogeneous secretory granules from moderate to high electron density are smaller and more randomly distributed than those in the medial glands (Figure 5 b-d). Single heterogeneous granules as well as granules of low electron density and larger clear vacuoles may be also present in the cell cytoplasm (Figures 4 a and 5 c). No prominent vesicles, RER cisterns and active Golgi bodies are found in the cells.






The lateral glands occupy the most lateral and dorsal position just below the eyes and above the sac of the coxal glands (see below) (Figures 1 c and 2 a-b). The character of the lateral glands is variable among the larvae studied. The lateral glands may be presented in two variants. The first one is characterized by relatively small size (Figures 2 b and 6 a) with a relatively dense cytoplasm containing short, slightly dilated cisterns of endoplasmic reticulum and irregularly shaped electron-lucent vacuoles (Figure 6 a, b). Some vacuoles may demonstrate a fine-structured matrix of low electron density (Figure 6 b). The intra-alveolar lumen is squeezed among cells showing microvilli penetrating the lumen (Figure 6 a). The second variant is represented by highly enlarged glands (Figure 6 c-d) with giant round electron-lucent vacuoles leaving narrow strips of cytoplasm filled with ribosomes and hardly distinguishable short cisterns of endoplasmic reticulum. The intra-alveolar lumen is greatly dilated (Figure 6 d). In both cases, the round nuclei with a large centrally located nucleolus occupy the peripheral position (Figure 6 a-c) close to the basal plasma membrane. The latter as well as the basal lamina remains flat (Figure 6 b).
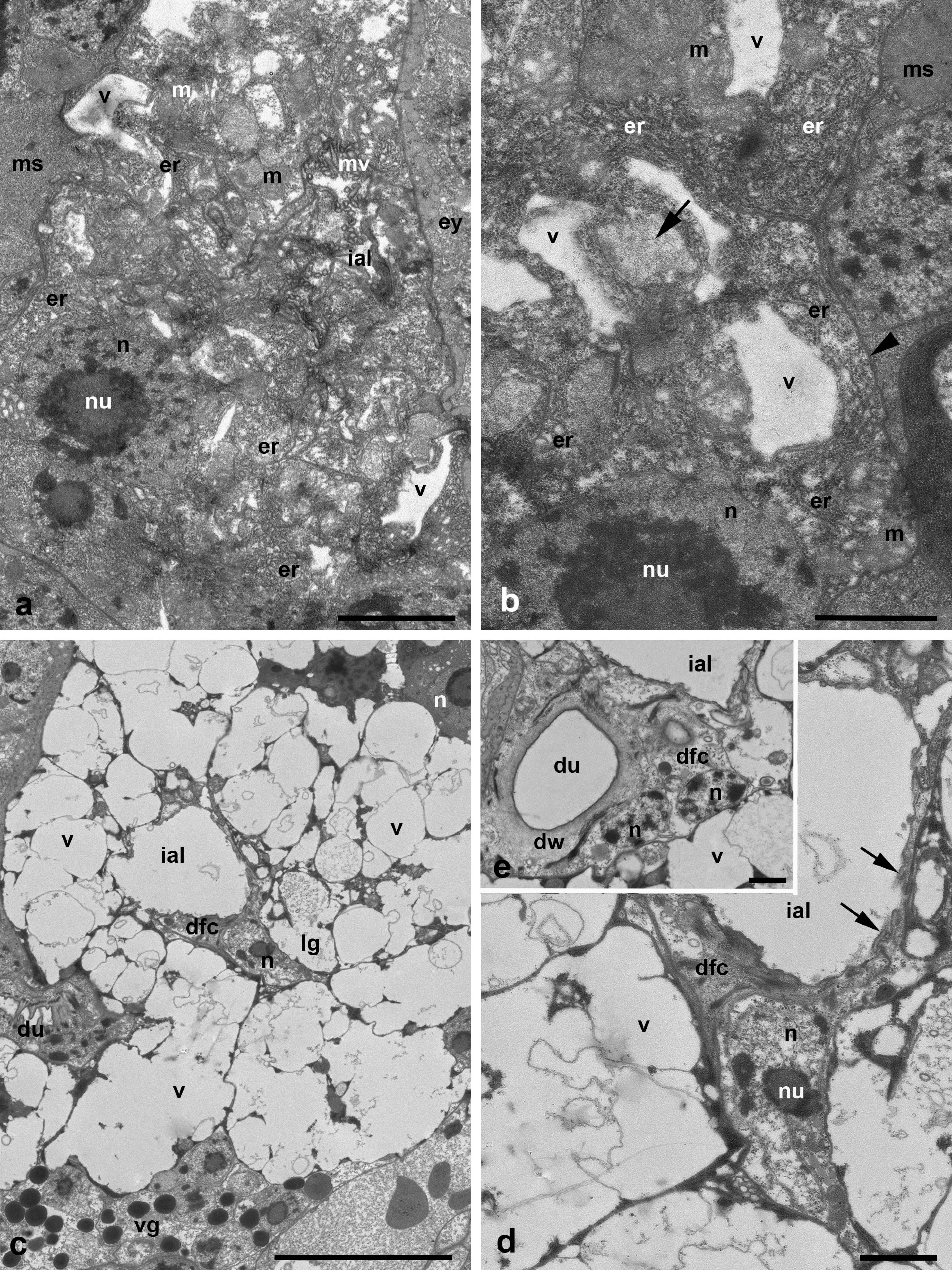


The duct base of the glands is identically organized and is similar to the respective structure of other Parasitengonina (Shatrov 2012). The duct base is formed of the so-called duct-forming ectoderm cells with a clear cytoplasm, small nucleus and few organelles (Figures 3 c, 5 d-e and 6 d-e). They form long extensions flanking lateral lacunas of the intra-alveolar lumen (Figures 1 b and 5 b) as well as chaotically arranged short microvilli on the apical surface (Figures 5 b, d-e and 6 d). In the lumen, microvilli of both glandular cells and duct-forming cells may interdigitate. The duct-forming cells contact to each other and to the gland cells via septate junctions (Figure 6 e). The duct cuticle is formed above the apical plasma membrane of the duct-forming cells. At its base, the cuticle shows a thick electron-light medial lamellate layer provided with bundles of long electron-dense fibers strengthening the duct wall (Figures 3 c and 6 e). The duct lumen is either optical-empty or may contain a fine-granular material of moderate electron density (Figure 3 c).
The coxal glands
These glands are well developed and occupy a relatively large body volume by sides of the brain. They run at an angle to the axial body line, both in vertical and tangential plan. They border the brain, large clusters of nephrocytes and the midgut posteriorly (Figure 7 a). They form at least two loops running back and forth and tightly opposed to each other so they may be identified only by the presence of the axial lumen.
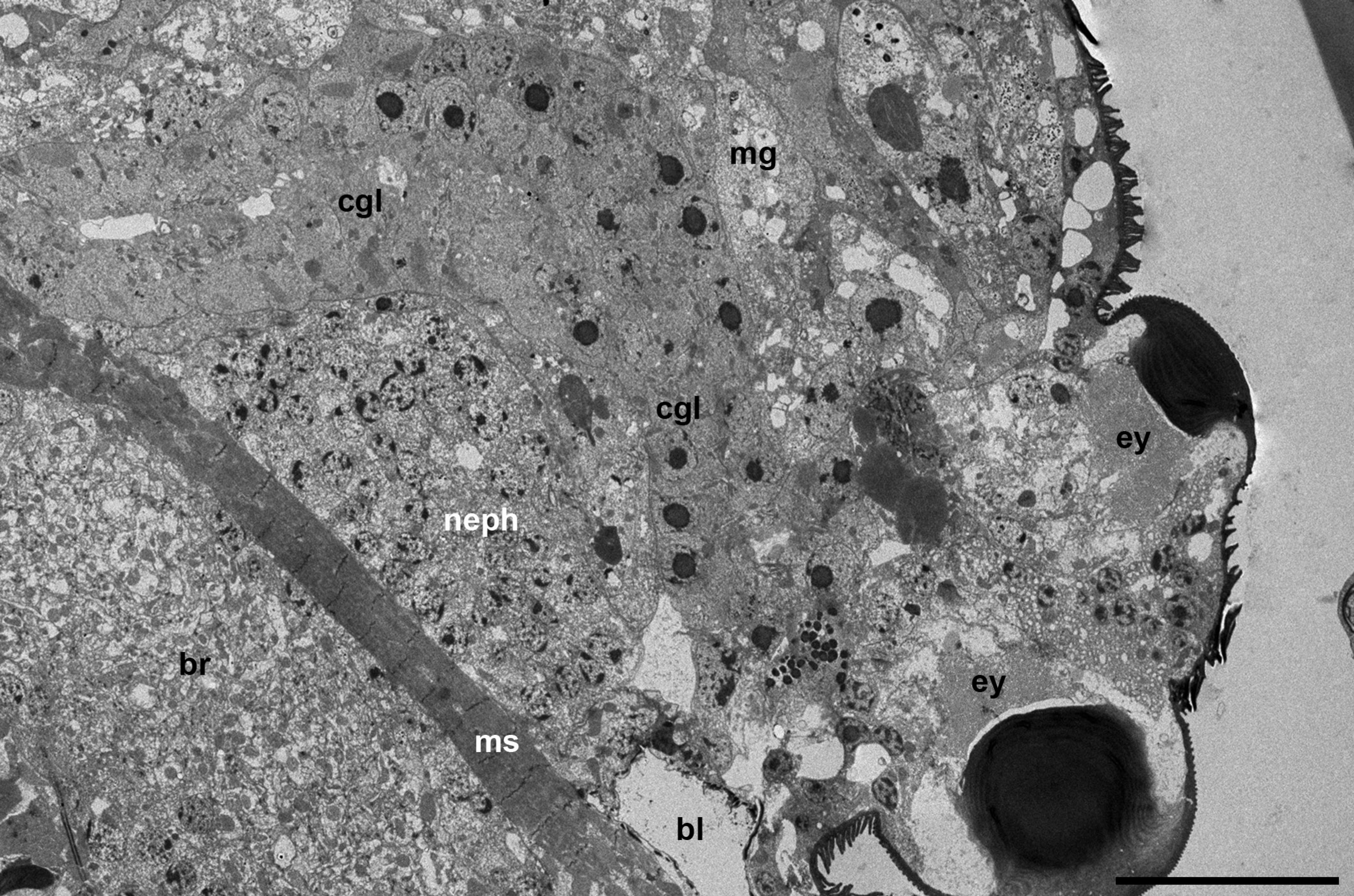


The entire gland, besides the very short frontal/anterior portion (end piece) (see below), is represented by a long curved tube, the so-called labyrinth (Figure 7 a). It is composed of the uniform prismatic cells equipped with microvilli on the apical cell surface (Figures 8 a, d-e, 9 b). The central lumen may be either collapsed (Figure 8 b, e) or filled with dense inclusions and membrane profiles (Figures 8 a, 9 b). The basal plasma membrane always remains flat (Figures 8 b and 9 a-c). However, the basal lamina supporting the gland cells may insert between the cells (Figure 8 b-c). In a subapical zone, the lateral membranes of the adjacent epithelial cells are wavy and interconnected by septate junctions with hardly distinguishable septa (Figure 8 e). Besides, the lateral cell membranes show relatively large semi-circled mutual invaginations (Figure 8 d).
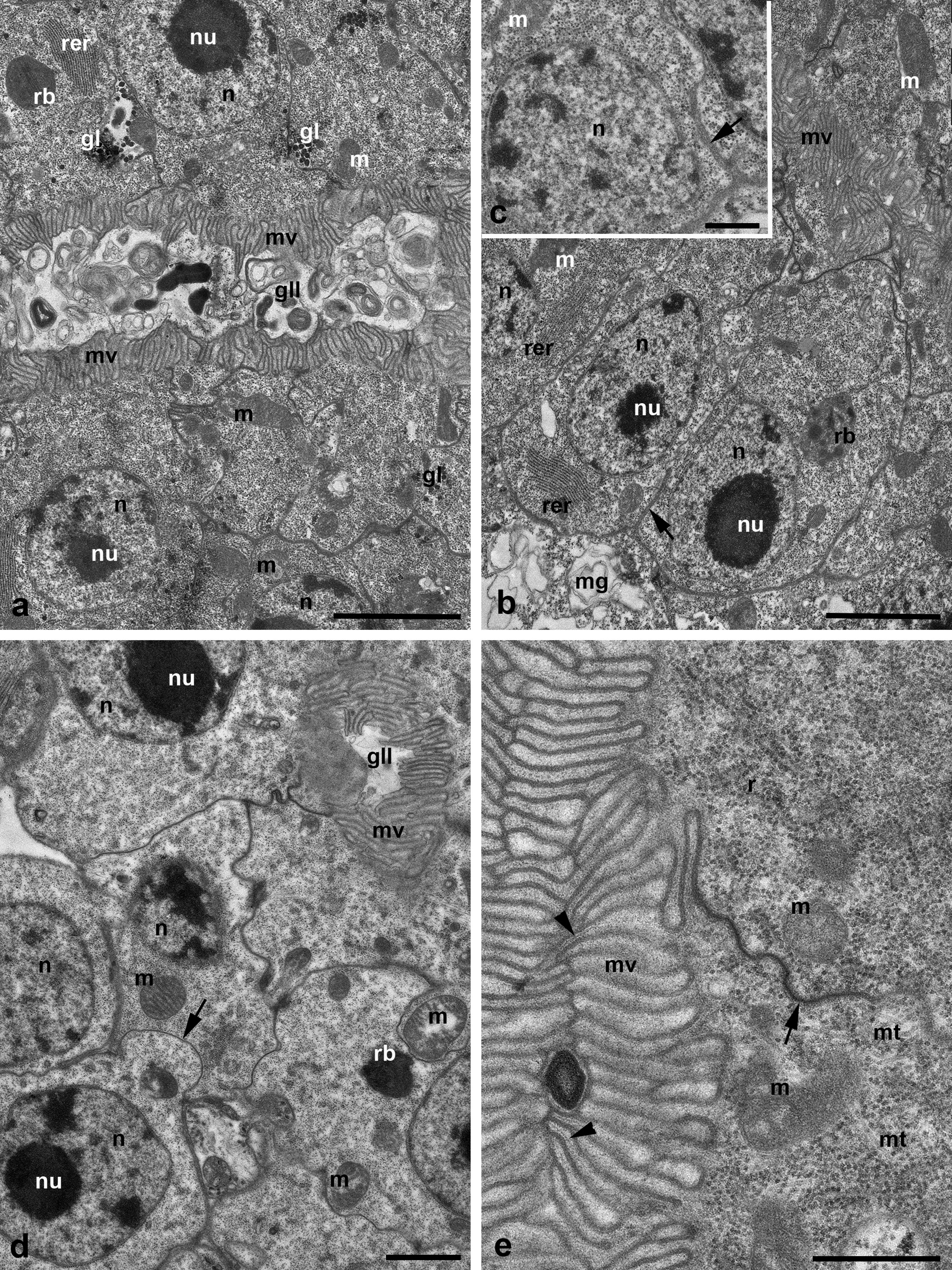





The round nuclei are large with a large eccentrically located nucleolus and occupy the basal cell zones (Figures 7 a and 8 a-b, d). The relatively dense cytoplasm contains scarce elongated mitochondria, single stacks of RER cisterns mostly in the basal cell zones (Figures 8 a-b and 9 a) as well as short curved cisterns, numerous free ribosomes and patches of glycogen particles (Figure 8 a). Separate microtubules penetrate the cell volume in different directions (Figures 8 e and 9 a). The irregularly shaped electron-dense presumably secondary lysosomes/residual bodies containing dense granules and elongated crystal-like inclusions are also present in the cells (Figures 8 b, d and 9 a). Golgi bodies as well as clear vesicles are not discernible. Separate nerve terminations (axons) with dense synaptic vesicles (Figure 9 c) and separate muscle fibers (Figure 9 b) may be seen adjacent to the gland wall.
The anterior/distal gland portion (the so-called end-piece) is short and composed of only one straight tubule. The wall of the tubule consists of simple cuboidal epithelium. The epithelial cells show light cytoplasm with scarce organelles (Figure 9 d). The cells are devoid of both apical microvilli and basal infoldings. However, the basal plasma membrane may insert into the cells to some extent forming irregular profiles (Figure 9 d). The anterior portion turns slightly laterad and transforms into the distal sac/bladder (Figure 10 a). The cuticular lining of the latter is formed similarly to the salivary glands, i.e. by the ectoderm cells above their apical plasma membrane (Figure 10 b). In different individuals, the bladder may be either dilated (Figures 5 a and 7 a) or, otherwise, nearly collapsed (Figures 1 c and 2 b). A spiral cuticular thread reinforces the walls of the bladder (Figures 5 a and 10 a).
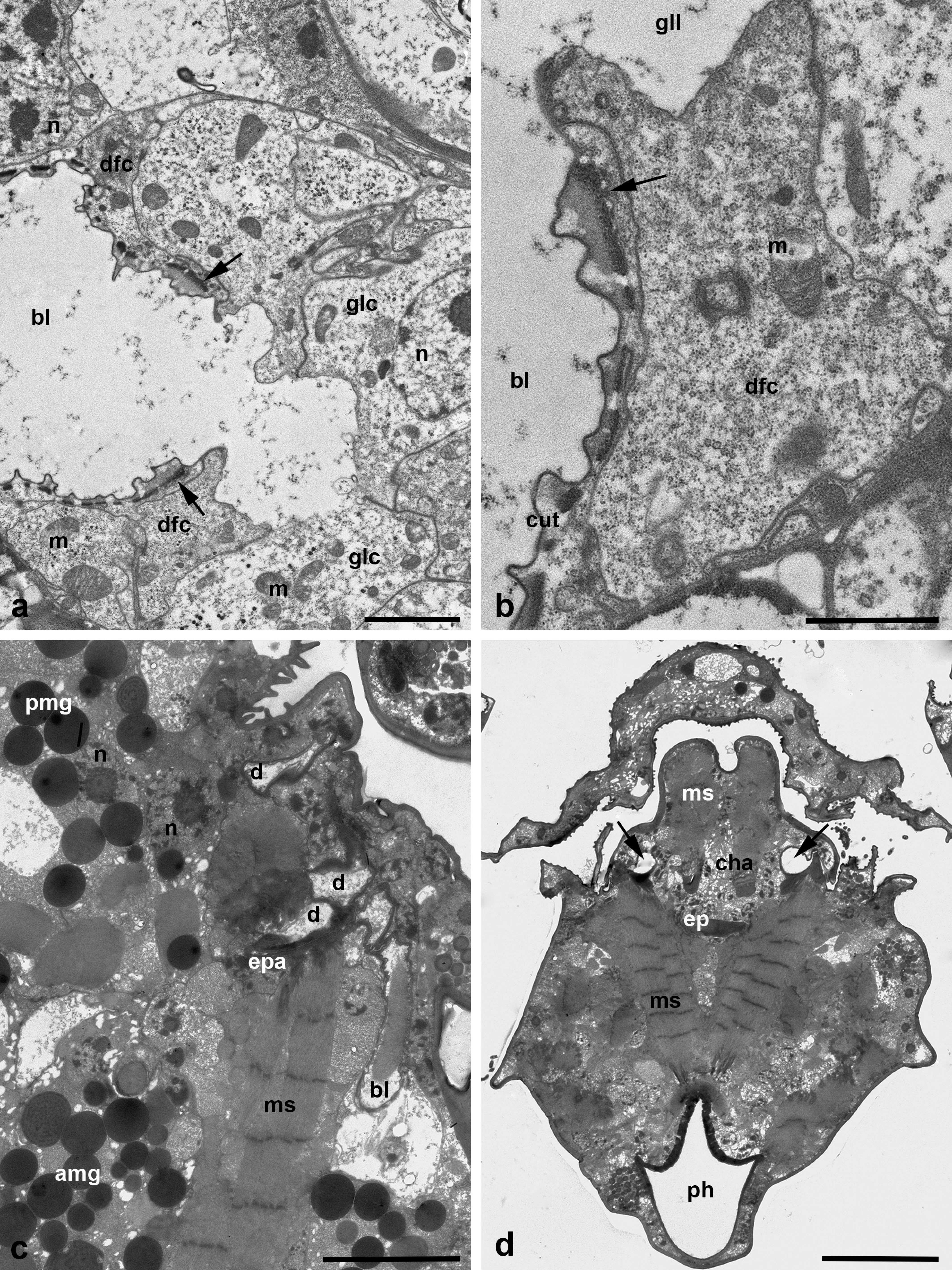


The ducts of all podocephalic glands of each side of the body come together at the upper-frontal side of the distal bladder of the coxal glands (Figure 10 c). They open into this bladder in unknown sequence, thus forming the common podocephalic duct after leaving of the bladder. This duct of each body side opens into the posterior lacunas of the subcheliceral space/atrial cavity (Figure 10 d). The anatomical arrangement of the glands are illustrated in Figure 11.



Discussion
The alveolar salivary glands
Initially, the podocephalic gland complex in actinedid mites and, in particular, in the Parasitengonina, is composed of four gland pairs – three pairs of alveolar/acinar, functioning in saliva production, and one pair of tubular/coxal, functioning in osmoregulation (Alberti and Coons 1999). The excretory ducts of these glands open into the podocephalic/common salivary duct taking its origin from the duct of the coxal glands. In addition to the podocephalic glands, one pair of the separate, so-called infracapitular glands, also alveolar ones, may be identified usually in the ventral position. Both podocephalic ducts and the duct of infracapitular glands open into the subcheliceral space. In fresh-water mites, one of the podocephalic glands and the infracapitular glands are frequently reduced (Croneberg 1878; Michael 1895; Thor 1904; Schmidt 1935; Mitchell 1955; etc.). The plesiomorphic number of the alveolar podocephalic glands may undergo significant reduction in many groups of Acariformes as well (Alberti and Coons 1999). Conversely, in calyptostomatids, the number of the acinous podeocephalic glands increases up to four pairs, probably including the infracapitular glands in their composition (Vistorin-Theis 1977; Alberti and Coons 1999). The full salivary gland complex including podocephalic and infracapitular glands is found in adults of terrestrial trombiculid mites (Trombiculidae) (Brown 1952; Shatrov 2000), microtrombidiids (Microtrombidiidae) (Shatrov 2005) and erythraeids (Erythraeidae) (Witte 1978), whereas in Smarididae (Erythraeoidea) one of the podocephalic glands is reduced (Witte 1998). In all transformation of the alveolar podocephalic glands, the coxal glands are never reduced.
The prosomal glands in the parasitengonine larvae were studied up to now in the terrestrial mites Abrolophus rubipes (Trouessart, 1888) (Erythraeidae) (Witte 1978), Hirsutiella zachvatkini (Schluger, 1948) and Leptotrombidium orientale (Schluger, 1948) (Trombiculidae) (Shatrov 2000, 2015), Platytrombidium fasciatum (Koch, 1836) and Camerotrombidium pexatum (Koch, 1837) (Microtrombidiidae) (Shatrov 2004) and in the fresh-water mite Piona carnea (Koch, 1836) (Pionidae) (Shatrov 2012). Moreover, the coxal glands were studied in larvae of the water mite Hydryphantes ruber (de Geer, 1778) (Hydryphantidae) (Shatrov 2017). Among these larvae, the full prosomal gland complex was found only in trombiculids and erythraeids, whereas in P. carnea the infracapitular glands are reduced. Interestingly, in the latter species the glands of one pair are arranged one after another on the axial body line (Shatrov 2012) as in adult mites of Calyptostoma velutinum (Müller, 1776) (Calyptostomatidae) (Vistorin-Theis 1977) and in the studied larvae L. maculata. In microtrombidiid larvae, the alveolar glands are largely reduced – only two pairs of the podocephalic glands remain.
As is seen from this consideration, reduction of the initial prosomal gland complex within the Parasitengonina (i) is a general tendency, (ii) mostly concerns infracapitular glands and never coxal glands, (iii) is rather irregular among families, and (iv) is thought to depend on combination of the evolutionary position and particular ecological conditions. Generally, larvae may undergo stronger reduction of glands than adults, that may be seen both within the given families and in different evolutionary branches. All alveolar glands studied at present produce mainly protein secretion as a result of RER and Golgi complex activity that may be very pronounced (Alberti and Coons 1999; Shatrov 2004, 2005). Some variation in the character of secretory granules, as in the case of L. maculata, may be influenced by a particular role of the given glands in a feeding process (Mitchell 1970). At the same time, in the alveolar glands of unfed larvae of L. maculata, the activity of a synthetic apparatus is practically inconspicuous, because the secretory granules are already formed. Obviously, the secretion in the medial and ventral glands is ready to evacuate for realization of the feeding process, whereas the dynamics of secretion in the lateral glands is not so clear and may depend on the individual age of the given unfed larva. It may be supposed that the glands produce secretion only through the post-embryonic period, and the large vacuoles (probably partly dissolved during fixation) are mature and ready for evacuation. Distinct cellular composition of these cells comparing to medial and ventral gland cells may indicate that they synthesize other products than proteins, probably rich with glycoprotein components.
The coxal glands
In the body composition of larvae L. maculata, the coxal glands are well developed and occupy a very large space of the body volume. In contrast to other arachnids (Buxton 1913; Rasmont et al. 1958; Rasmont 1959; Groepler 1969; Hecker et al. 1969; Alberti 1979), the coxal glands in the Parasitengonina have progressively lost the proximal sacculus, and the main tubule is, consequently, the region of the primary fluid/urine filtration and formation (Alberti and Coons 1999). While the sacculus is assumed to be of mesodermal origin (Alberti et al. 1997; Alberti and Coons 1999), the origin of tubular region (the so-called labyrinth) has not been ultimately recognized until present. That is why, all debates around the question whether these organs represent coelomoducts (Beklemishev 1964) or nephridia (Bartolomeaus and Quast 2005) in arthropods – remain open. In contrast to many oribatid and actinedid mites studied so far, both adults and larvae (Alberti and Storch 1973, 1974, 1977; Alberti at al., 1997; Alberti and Coons 1999; Shatrov 2006, 2007, 2017; Filimonova 2016), the tubular gland portion in larvae of L. maculata is not clearly divided into certain regions. In other mites studied there may be identified, for instance, proximal tubule, intermediate zone, and distal tubule with different cell organization providing different functions with regard to fluid/ion filtration and osmoregulation. Conversely, the entire gland in L. maculata larvae, apparently consisting of at least 1-1800 bend, shows a uniform cell organization with the well-developed brush-border and without obvious basal infoldings.
Consequently, only one hypothesis concerning the gland functioning may be valid – filtration of fluids across the gland wall is performed, especially after feeding of the liquid substrate, throughout the entire gland length. This filtration is expected to occur in a passive manner due to variation of the body pressure (Manton 1958). The reverse ion resorption/reabsorption, extremely important in the fresh-water organisms, obviously takes place in the same gland portion via the active mode with the energy consumption in the microvillous compartment. This hypothesis resulted from the observed gland organization, which is similar to that of the fresh-water oribatids (except for the presence of the sacculus in the latter group), in which the labyrinth with the same proposed function is developed much better than in the terrestrial oribatid mites (Woodring 1973).
The above-mentioned functional morphology of the coxal glands in larvae of L. maculata is not identical to that of larvae of the terrestrial Parasitengonina mites (Witte 1978, 1998; Shatrov 2006, 2007, 2017). In the latter group, different regions of the typical coxal glands are well developed. By contrast, the coxal glands studied in this work are represented by the single enormously developed region composed of the uniformly organized epithelium. The cells of this epithelium show no signs of secretion, which, in contrast, may be well presented in coxal glands of different mite groups (Alberti and Coons 1999, Filimonova 2004, 2016; Shatrov 2006, 2007). Contrary to unfed larvae of Hydryphantes ruber (De Geer, 1778) (Shatrov 2017), the coxal glands of L. maculata contain large secondary/autophagic lysosomes, or residual bodies that suggests the intensive metabolic processes within the cells. Similar lysosome-like bodies are also contained in the coxal gland cells of Arrenurus (Alberti and Storch 1977), adult trombiculids (Shatrov 2000) and syringophilids (Filimonova 2016). In each case, the role of the coxal glands in the process of the saliva formation, especially if the podocephalic canal opens into the subcheliceral space, is thought to be efficient (Mitchell 1970, Wharton and Furumizo 1977; Alberti and Coons 1999; Shatrov 2017). The large distal bladder is highly characteristic of water mites (Alberti and Coons 1999; Shatrov 2017), which additionally indicates the transportation of large fluid masses through the gland. The volume of the bladder may vary in different specimens which probably suggests its periodic functioning and the intensive fluid transport across the gland even in unfed larvae.
This study has proved the previous findings that larvae of the fresh-water mites undergo reduction of the infracapitular glands, and that one pair of the podocephalic glands tends to be lined-up one after another in the axial body line. The coxal glands responsible for osmoregulation, are well developed but have lost the proximal sacculus and basal infoldings. No secretion is evident in the coxal glands of unfed larvae.
Acknowledgements
This study is supported by a grant N 18-04-00075-a from the Russian Foundation for Fundamental Research and by the State Federal Scientific Programs \#\# АААА-А19-119020790133-6 and AAAA-A19-119020690072-9. We are also grateful to P.A. Smirnov, engineer of the ''Taxon'' Resource Research Center, for his assistance in TEM.
References
Alberti G. 1973. Ernährungsbiologie und Spinnvermögender Schnabelmilben (Bdellidae, Trombidiformes). Z. Morph. Tiere, 76: 285-338. doi:10.1007/BF00281815 ![]()
Alberti G. 1979. Light- und elektronenmikroskopische Untersuchungen an Coxaldrüsen von Walzenspinnen (Arachnida: Solifugae). Zool. Anz., 203: 48-64.
Alberti G., Coons L.B. 1999. Acari: Mites. In: Harrison F.W., Foelix R.F., (Eds). Microscopic Anatomy of Invertebrates, Vol. 8C. New York: Wiley-Liss. p. 515-1217.
Alberti G., Kaiser T., Klauer A.-K. 1997. New ultrastructural observations of the coxal glands (nephridia) of Acari. In: Mitchell R., Horn D.J., Needham G.R., Welbourn W.C. (Eds). Acarology IX. Columbus: Ohio Biol. Survey. p. 309-318.
Alberti G., Storch V. 1973. Zur Feinstruktur der "Munddrüsen" von Schnabelmilben (Bdellidae, Trombidiformes). Zeitschr. Wiss. Zool., 186: 149-160.
Alberti G., Storch V. 1974. Über Bau und Funktion der Prosoma-Drüsen von Spinnmilben (Tetranychidae, Trombidiformes). Z. Morph. Tiere., 79: 133-153. doi:10.1007/BF00298779 ![]()
Alberti G., Storch V. 1977. Zur Ultrastruktur der Coxaldrüsen actinotricher Milben (Acari, Actinotrichida). Zool. Jahrb. Abt. Anat., 98: 394-428.
Anderson E., Harvey W.R. 1966. Active transport by the cecropia midgut. II. The fine structure of the midgut epithelium. J. Cell Biol., 31: 107-134. doi:10.1083/jcb.31.1.107 ![]()
Bader C. 1938. Beiträge zur Kenntnis der Verdanungsvergänge bei Hydracarinen. Rev. Suisse. Zool., 45: 721-806.
Bartolomeaus T., Quast B. 2005. Structure and development of nephridia in Annelida and related taxa. Hydrobiologia, 535/536: 139-165. doi:10.1007/s10750-004-1840-z ![]()
Beklemishev V.N. 1964. Bases of comparative anatomy of invertebrates. Vol. 2. Organologia. Moscow: Nauka. 444 pp. [In Russian].
Berridge M.J. 1970. Osmoregulation in terrestrial arthropods. In: Florkin M., Scheer B.T. (Eds). Chemical zoology. Vol. 5. New York - London: Academic Press. p. 287-319. doi:10.1016/B978-0-12-395538-8.50037-9 ![]()
Berridge M.J., Oschman J.L. 1969. A structural basis for fluid secretion by Malpighian tubules. Tissue & Cell, 1: 247-272. doi:10.1016/S0040-8166(69)80025-X ![]()
Beyenbach K.W. 2003. Transport mechanisms of diuresis in Malpighian tubules of insects. J. Exp. Biol., 206: 3845-3856. doi:10.1242/jeb.00639 ![]()
Brown J.R.C. 1952. The feeding organs of the adult of the common "chigger". J. Morph., 91: 15-52. doi:10.1002/jmor.1050910103 ![]()
Buxton B.H. 1913. Coxal glands of the Arachnids. Zool. Jahrb. Suppl., 14: 231-282.
Cohen A.C. 1995. Extra-oral digestion in predaceous terrestrial Arthropoda. Ann. Rev. Entomol., 40: 85-103. doi:10.1146/annurev.en.40.010195.000505 ![]()
Cohen A.C. 1998. Solid-to-liquid feeding: The inside(s) story of extra-oral digestion in predaceous Arthropoda. Amer. Entomol., 44: 103-116. doi:10.1093/ae/44.2.103 ![]()
Croneberg A.I. 1878. On the structure of Eylaïs extendens (O.F. Müller). With notes on some related forms. Izv. Imp. Obzch. Lubit. Estestv. Antropol. Etnogr., 29: 1-37. [In Russian].
Diamond J.M., Tormey J.McD. 1966. Studies on the structural basis of water transport across epithelial membranes. Fed. Proc., 25: 1458-1463.
Evans G.O. 1992. Principles of Acarology. Wallingford (UK): C.A.B. International. pp. 563.
Farley R.D. 1999. Scorpiones. In: Harrison F.W., Foelix R.F. (Eds). Microscopic Anatomy of Invertebrates, Vol. 8A. Chelicerate Arthropoda. New York: Wiley-Liss. p. 117-222.
Felgenhauer B.E. 1999. Araneae. In: Harrison F.W., Foelix R.F. (Eds). Microscopic Anatomy of Invertebrates, Vol. 8A. Chelicerate Arthropoda. New York: Wiley-Liss. p. 223-66.
Filimonova S.A. 2004. The fine structure of the coxal glands in Myobia murismusculi (Schrank) (Acari: Myobiidae). Arthr. Str. Dev., 33: 149-160. doi:10.1016/j.asd.2004.01.002 ![]()
Filimonova S.A. 2016. Morpho-functional variety of the coxal glands in cheyletoid mites (Prostigmata). I. Syringophilidae. Arthr. Str. Dev., 45: 356-367. doi:10.1016/j.asd.2016.06.005 ![]()
Groepler W. 1969. Feinstruktur der Coxalorgane bei der Gattung Ornithodorus (Acari: Argasidae). Z. Wiss. Zool., 178: 235-275.
Hecker H., Diehl P.A., Aeschlimann A. 1969. Recherches sur l'ultrastructure et l'histochemie de l'organe coxal d'Ornithodoros moubata (Murray) (Ixodoidea: Argasidae). Acta Trop., 26: 346-360.
Kaufman W.R., Phillips J.E. 1973. Ion and water balance in the ixodid tick Dermacentor andersoni. I. Routes of ion and water excretion. J. Exp. Biol., 58: 523-536.
Koch M., Quast B., Bartolomaeus T. 2015. Chapter 11. Coeloms and nephridia in annelids and arthropods. In: Wägele W., Bartolomaeus. T. (Eds). Deep Metazoan Phylogeny: The Backbone of the Tree of Life. Amsterdam: De Gruyter. pp. 173-284.
Manton S.M. 1958. Hydrostatic pressure and leg extension in arthropods with special reference to arachnids. Ann. Mag. Nat. Hist. Ser. 13, 1: 161-182. doi:10.1080/00222935808650934 ![]()
Michael A.D. 1895. A study of the internal anatomy of Thyas petrophilus, an unrecorded Hydrachnid found in Cornwall. Proc. Zool. Soc. London: 174-209. doi:10.1111/j.1469-7998.1895.tb00003.x ![]()
Mitchell R.D. 1955. Anatomy, life history and evolution of the mites parasitizing fresh water mussels. Misc. Publ. Mus. Zool. Univ. Michigan, 89: 1-41.
Mitchell R.D. 1964. The anatomy of an adult chigger mite Blankaartia acuscutellaris (Walch). J. Morph., 114: 373-391. doi:10.1002/jmor.1051140302 ![]()
Mitchell R.D. 1970. The evolution of a blind gut in trombiculid mites. J. Nat. Hist., 4: 221-229. doi:10.1080/00222937000770211 ![]()
Mothes U., Seitz K.-A. 1981. Fine structure and function of the prosomal glands of the two-spotted spider mite, Tetranychus urticae (Acari, Tetranychidae). Cell Tiss. Res., 221: 339-349. doi:10.1007/BF00216738 ![]()
O'Donnell M.J., Maddrell S.H.P. 1983. Paracellular and transcellular routes for water and solute movements across insect epithelia. J. Exp. Biol., 106: 231-253.
Pease D.C. 1956. Infolded basal plasma membranes found in epitheliae noted for their water transport. J. Biophys. Biochem. Cytol., 2 (2 suppl.): 203-208. doi:10.1083/jcb.2.4.203 ![]()
Rasmont R. 1959. Structure et ultrastructure de la glande coxale d'un scorpion. Ann. Soc. Roy. Zool. de Belgique, 89: 239-272.
Rasmont R., Vandermeersche G., Castiaux P. 1958. Ultrastructure of the coxal glands of the scorpion. Nature, 182: 328-329. doi:10.1038/182328a0 ![]()
Reynolds E.S. 1963. The use of lead citrate at high pH an electron-opaque stain in electron microscopy. J. Cell Biol., 17: 208-212. doi:10.1083/jcb.17.1.208 ![]()
Schmidt U. 1935. Beiträge zur Anatomie und Histologie der Hydrachniden, besonders von Diplodontus despiciens O.F. Müller. Z. Morph. Ökol. Tiere, 30: 99-176. doi:10.1007/BF00406228 ![]()
Schmidt-Nielsen B., Gertz K.H., Davis L.E. 1968. Excretion and ultrastructure of the antennal gland of the fiddler crab Uca mordax. J. Morph., 125: 473-495. doi:10.1002/jmor.1051250406 ![]()
Schmidt-Raese A. 2007. The evolution of organ systems. New York: Oxford University Press Ltd. pp. 385.
Shatrov A.B. 2000. Trombiculid mites and their parasitism on vertebrate hosts. St.-Petersburg: St.-Petersburg Univ. Publ. p. 276. [In Russian, with extensive English summary].
Shatrov A.B. 2004. Ultrastructure of the salivary gland complex in unfed larvae of Platytrombidium fasciatum (C.L. Koch, 1836) and Camerotrombidium pexatum (C.L. Koch, 1837) (Acariformes: Microtrombidiidae). Acarologia, 44: 219-229. doi:10.1016/S1467-8039(03)00044-6 ![]()
Shatrov A.B. 2005. Ultrastructural investigations of the salivary glands in adults of the microtrombidiid mite Platytrombidium fasciatum (C.L. Koch, 1836) (Acariformes: Microtrombidiidae). Arthr. Str. Dev., 34: 49-61. doi:10.1016/j.asd.2004.09.001 ![]()
Shatrov A.B. 2006. Ultrastructure of coxal glands in the unfed microtrombidiid larvae, Platytrombidium fasciatum (C.L. Koch, 1836) and Camerotrombidium pexatum (C.L. Koch, 1837) (Acariformes: Microtrombidiidae). Abh. Ber. Naturkundemus. Görlitz, 78: 55-76.
Shatrov A.B. 2007. Ultrastructure of coxal glands in the adult microtrombidiid mite Platytrombidium fasciatum (C.L. Koch, 1836) (Acariformes: Microtrombidiidae). Acarologia, 47: 63-78.
Shatrov A.B. 2012. Anatomy and ultrastructure of the salivary (prosomal) glands in unfed water mite larvae Piona carnea (C.L. Koch, 1836) (Acariformes: Pionidae). Zool. Anz., 251: 279-287. doi:10.1016/j.jcz.2011.12.008 ![]()
Shatrov A.B. 2015. Comparative morphology and ultrastructure of the prosomal salivary glands in the unfed larvae Leptotrombidium orientale (Acariformes, Trombiculidae), a possible vector of tsutsugamushi disease agent. Exp. Appl. Acarol., 66: 347-367. doi:10.1007/s10493-015-9912-5 ![]()
Shatrov A.B. 2017. Comparative ultrastructure of coxal glands in unfed larvae of Leptotrombidium orientale (Schluger, 1948) (Trombiculidae) and Hydryphantes ruber (de Geer, 1778) (Hydryphantidae). J. Morph., 278: 1551-1569. doi:10.1002/jmor.20730 ![]()
Thor S. 1904. Recherches sur l'anatomie compare des Acariens prostigmatiques. Ann. Sci. Nat. Zool., 19: 1-190.
Vistorin-Theis G. 1977. Anatomische Untersuchungen an Calyptostomiden (Acari, Trombidiformes). Acarologia, 19: 242-257.
Voigt B. 1971. Anatomie und Histologie der Drüsen bei Trombiculiden-Milbenlarven. Zool. Anz., 186: 403-417.
Wall B.J., Oschman J.L., Schmidt-Nielsen B. 1970. Fluid transport: concentration of the intercellular compartment. Science, 167: 1497-1498. doi:10.1126/science.167.3924.1497 ![]()
Wharton G.W., Furumizo R.T. 1977. Supracoxal gland secretion as a source of fresh water for Acaridei. Acarologia, 19: 112-116.
Witte H. 1978. Die postembryonal Entwicklung und die funktionnel Anatomie des Gnathosoma in der Milbenfamilie Erythraeidae (Acarina: Prostigmata). Zoomorph., 91: 157-189. doi:10.1007/BF00993859 ![]()
Witte H. 1991. The phylogenetic relationships within the Parasitengona. In: Dusbábek F., Bukva V. (Eds). Modern Acarology. Vol. 2. Prague: Academia, The Hague: SPB Academic Publishing bv. pp. 171-182.
Witte H. 1998. On the internal organization of smaridid mites (Acari, Erythraeoidea), and on the role of organismal properties for determining the course of evolutionary change. In: Ebermann E. (Ed). Arthropod Biology: Contributions to Morphology, Ecology and Systematics. Wien: Biosystematics and Ecology Series, 14: 245-289.
Woodring J.P. 1973. Comparative morphology, function and homologies of the coxal glands of Oribatid mites (Arachnida, Acari). J. Morph., 139: 407-429. doi:10.1002/jmor.1051390404 ![]()



2019-09-19
Date accepted:
2019-12-26
Date published:
2020-01-17
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Shatrov, Andrey B. and Soldatenko, Elena V.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




